Abstract
Objective
To determine if the levels of circulating myeloid-derived suppressor cells increase with progression of prostate cancer (PCa); to determine if such cells could contribute to the relative inefficiency of PCa immunotherapy.
Materials and Methods
We analyzed peripheral blood mononuclear cells isolated from untreated PCa patients (uPCa; N = 18; mean age ± SD: 72.1 ± 6.9 years), tPCa (N = 22; 72.8 ± 9.8 years) and age matched controls (AMC; N = 12; 68.8 ± 7.5 years). We quantified surface marker phenotype, differentiation potential, effects on T cell proliferation and intracellular cytokines.
Results
We observed an unexpectedly high percentage of a type of myeloid-derived suppressor cells, CD14+HLA-DRlow/− monocytes, in tPCa (30.7 ± 15.0% of CD14+ cells) relative to AMC (4.1 ± 6.5%, P < 0.0001) and uPCa (10.6 ± 14.3%, P = 0.0001). The levels of CD14+ HLA-DRlow/− cells were significantly correlated with circulating PSA levels and treatment with LHRH-agonist leuprolide in combination with either an antiandrogen or dexamethasone. Monocytes from tPCa inhibited autologous T cell proliferation statistically significantly more effectively than AMC monocytes and were defective in their ability to differentiate into phenotypically mature dendritic cells. Isolated CD14+HLA-DRlow/− cells expressed higher levels of intracellular interleukin-10 and suppressed T cell proliferation more effectively than isolated CD14+HLA-DR+ cells.
Conclusions
This is the first report of CD14+ cells exhibiting reduced expression of HLA-DR molecules in PCa patients. These cells suppress immune cell function in vitro and, plausibly, in vivo, a finding that must be factored into the design of immunotherapy protocols for PCa patients.
Keywords: androgen suppression, CD14+ cells, dendritic cells, HLA-DR expression, monocytes, myeloid-derived suppressor cells, prostate cancer
Introduction
The lack of an effective cure of androgen-independent prostate cancer (PCa) has stimulated development of immunotherapeutic approaches to this disease. One approach has been the use of patient-derived ex vivo matured dendritic cells (DCs), the antigen-presenting cells unique in their ability to stimulate antigen-naïve T cells in addition to memory T cells [1]. While DC-based treatments have induced clinical responses in one half of 400 PCa patients studied in different clinical trials so far, the responses were only transient (recently reviewed in Ref. [2]). Listed among the reasons for the lack of durable clinical effects have been tumor-associated defects in patients' tumor-specific immunity, including tolerance to prostate-associated antigens, poor expression of HLA molecules by PCa cells, tumor-derived immunosuppression, increased levels of regulatory T cells, etc. (cf. Ref. [3]).
Recently, however, tumor-associated alterations of innate immunity have come into focus as well. For example, patients suffering from metastatic PCa contained fewer circulating myeloid DCs than their age-matched controls [4]. This finding indicates that in PCa patients monocytes do not develop into myeloid DCs as efficiently as they do in healthy subjects, a notion supported by observations that PCa patient serum inhibits monocyte differentiation into DCs and that the degree of inhibition is correlated with higher prostate specific antigen (PSA) levels [5]. Some evidence, though, for aberrant monocytes in PCa has been available for years. For instance, the cells isolated from PCa patient lymph nodes [6] and peripheral blood, later shown to be monocytes [7], could inhibit leukocyte proliferation in vitro [8].
T regulatory (Treg) cells characterized as CD4+ CD25high have recently been documented at an increased level in the blood and tumor tissue of early-stage PCa patients; these cells potently suppressed T cell proliferation in vitro [9]. Interestingly, CD4+CD25+ cells isolated from the blood of healthy subjects suppressed expression of cytokines and HLA class II molecules in monocytes [10]. Treg cells, specified as CD4+CD25+CD127loFoxp3+, directed monocyte differentiation into a phenotype characterized by anti-inflammatory effects and a role in “immune regulation, tissue remodeling, parasite killing, and tumor promotion” [11]. Such monocytes have been designated as “alternatively activated”; while devoid of the ability to express normal levels of proinflammatory molecules such as IL-1β, IL-6, IL-8, MIP-1α, and TNF-α, alternately activated monocytes expressed IL-10, IL-4, and IL-13, the likely mediators of immunosuppression [11]. Taken together, these data imply the possibility of complex mutual interactions of PCa and immunity, but the system has not been characterized within the context of natural history of the disease and the effects of treatment.
In an attempt to validate a standard method of DC maturation from monocytes derived from PCa patients, we found that these cells yielded fewer fully differentiated DCs than monocytes from healthy donors. To investigate the reasons underlying this phenomenon, we characterized phenotypic features of peripheral blood mononuclear cells (PBMC) isolated from newly diagnosed untreated PCa patients (uPCa), from PCa patients treated by standard adjuvant therapy with luteinizing hormone releasing hormone (LHRH)-agonists and an antiandrogen or dexamethasone (tPCa), and from non-cancerous age-matched control subjects (AMC).
Patients, Materials, and Methods
Patients
Patient blood and access to medical records were obtained with the approval of the Mayo Clinic Institutional Review Board. All study subjects received care at or came for second opinion to Mayo Clinic Rochester and participated in the study with informed consent. Subjects were identified for the study by review of medical records at the Mayo Clinic Prostate Cancer SPORE registry (tPCa), Department of Urology (uPCa) and Division of Executive Health (AMC). Most tPCa underwent prostatectomy or radiation as first-line therapy and have since received the standard treatment by luteinizing hormone releasing hormone (LHRH) agonists (leuprolide acetate or goserelin acetate) with or without an antiandrogen (bicalutamide or nilutamide) or dexamethasone; one patient was orchiectomized instead. Subject demographics and pertinent clinical and laboratory data abstracted from patients' charts are shown in Supplementary Table I.
Cell Isolation
On average we drew 45 ml of blood on sodium heparin and an additional 6 ml on ethylenediamine tetraacetate. We isolated PBMCs by buoyant density separation using Lymphoprep separation medium (ICN, Aurora, OH) according to manufacturer's instructions. Cells were counted and assayed for viability by trypan blue exclusion. CD3+ cells and CD14+ cells were isolated from PBMCs by incubation with the pertinent immunomagnetic reagent (Miltenyi Biotec, San Diego, CA) according to manufacturer's instructions. After incubation and washing, we separated the labeled cells on an AutoMACS separator (Miltenyi Biotec) running the POSSEL program.
Cell Characterization by Flow Cytometry
We characterized the cells using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and fluorophore-conjugated monoclonal antibodies with specificity indicated in Table I. By multiple immunostaining we characterized CD3+CD4+ T helper cells, CD3+CD8+ cytotoxic T cells, CD14+HLA-DR+ monocytes, CD19+ B cells, CD56+ NK cells, CD3+CD4+ CD25+CD127low/− T regulatory cells and CD83+ DCs. We fixed the cells in 1.0% paraformaldehyde and then recorded 100,000 cytometry counts per sample. Data were analyzed with CellQuest software (BD Biosciences).
TABLE I. Immunoreagents Used in This Study.
| Antibody specificity | Fluorescent label | Manufacturer |
|---|---|---|
| CD3 | FITC, PE, APC | eBioscience |
| PE-Cy5 | Pharmingen | |
| CD4 | FITC, PE | eBioscience |
| CD8 | FITC, PE, APC | |
| CD14 | eBioscience | |
| CD19 | PE | |
| CD25 | APC | Pharmingen |
| CD56 | FITC | eBioscience |
| CD83 | PE | Beckman Coulter |
| CD127 | PE | Pharmingen |
| HLA-DR | FITC | Biosource |
| PerCP | Pharmingen | |
| IgG | PE | Biosource |
| TGF-βa | PE | R&D Systems |
| IL-10a | PE | R&D Systems |
FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein; APC, allophycocyanin; Cy-5, cyanine 5.
Used for intracellular staining.
Fluorescence Activated Cell Sorting
To isolate CD14+HLA-DRlow/− and CD14+HLA-DRhigh cells, we stained the cells with fluorophore-conjugated monoclonal antibodies specific for CD14 and HLA-DR for 20 min at room temperature in the dark, washed and resuspended at (7−10) × 106/ml in AIM V medium (Invitrogen, Carlsbad, CA) containing 10% human AB serum (HABS; Sigma–Aldrich, St. Louis, MO). Cells were sorted by the use of a FACS Aria fluorescence activated cell sorter (BD Biosciences) under sterile conditions.
Intracellular Staining of TGF-β1 and IL-10
Two hundred microliter of anticoagulated whole blood was distributed per 12 mm × 75 mm polystyrene tube. Cells were stained for CD14 and HLA-DR for 15 min at room temperature in the dark. Erythrocytes were lysed and white cells were fixed with Becton Dickinson (BD) Phosflow Lyse/Fix Buffer (1×) for 10 min at room temperature, centrifuged at 1,500 rpm and supernatant removed. The pellet was resuspended, washed with PBS, centrifuged, permeabilized in 1.0 ml of BD Phosflow Perm Buffer II for 30 min on ice and washed twice with Stain Buffer containing 2.0% fetal bovine serum (SB-FBS; BD Biosciences). Next, the cells were incubated in 100 μl of SB-FBS containing antibodies specific for TGF β1 and IL-10 for 30 min at room temperature in the dark, washed with 2.0 ml of SB-FBS and resuspended in equal volumes of SB-FBS and 4.0% paraformaldehyde and analyzed by flow cytometry for cells stained for CD14/IL-10/HLA-DR and CD14/TGF-β1/HLA-DR.
Preparation of Mature Dendritic Cells
We matured the cells by the modified two-step method we used earlier [12,13]. Briefly, we seeded (2.5−3.0) × 106 CD14+ cells in 1.0 ml of clinical-grade X-VIVO 15 cell culture medium (Cambrex, East Rutherford, NJ) containing 1.0% pooled human AB serum. The first step to immature DCs took place in the presence of granulocyte-macrophage colony stimulating factor (GM-CSF; Berlex, Montville, NJ; 2,800 IU/ml) and interleukin 4 (IL-4; R&D Systems; Minneapolis, MN; 1,000 IU/ml) for 3 days. Final DC maturation was induced by the addition of tumor necrosis factor-α (TNF-α; R&D Systems; 1,100 IU/ml) and prostaglandin E2 (PGE2; Sigma–Aldrich; 1.0 μg/ml) for two additional days. The final product was analyzed for the percent of mature DCs among all viable cells (by expression of CD83, a hallmark of mature DCs) and viable cell yield (the measure of survival calculated by dividing the measured amount of the product by the theoretical (maximal) yield multiplied by 100%; for example, [number of viable cells at the end of incubation/number of viable cells at the beginning of incubation] × 100).
Monocyte Effects on T Cell Proliferation
To assess the effect of autologous monocytes on T cell proliferation, we isolated CD3+ cells from monocyte-depleted PBMCs by CD3-specific immunomagnetic adsorption (Miltenyi Biotec) to 95.8 ± 4.0% purity. Ten million freshly isolated T cells were resuspended in 1.0 ml PBS, combined with one vial of carboxyfluorescein succinimidyl ester (CFSE; Renovar, Madison, WI) and incubated for 10 min at 37°C. Cells were washed twice in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Mediatech) and 1.0% penicillin/streptomycin (Invitrogen) and resuspended in the same medium.
We plated one million CFSE-labeled T cells per well of 24-well plates (Costar, Corning, NY). We incubated the cells alone without or with 20 μl/well of anti-CD3/anti-CD28 bead suspension (Dynabeads, Invitrogen Dynal, Oslo, Norway). To some wells we added autologous CD14+ cells in the ratio 0.1 to 2.0 CD14+ cell per one T cell. The final volume of all wells was 2.0 ml. The cells were incubated for 4 days at 37°C in humidified atmosphere containing 5% CO2. Subsequently, the cells were collected, each sample split in two, and incubated for 10 min at room temperature with allophycocyanin-conjugated anti-CD3 and phycoerythrin (PE)-conjugated anti-CD4 or PE-conjugated anti-CD8 (all from eBioscience; San Diego, CA) according to manufacturer's instructions. The cells were washed, resuspended in PBS containing 1.0% propidium iodide (Renovar), and analyzed by a FACSCalibur flow cytometer and CellQuest software. Viable T cells were gated based on their characteristic position in the forward versus side scatter plot and absence of propidium iodide staining. To analyze the data, we compared the CFSE fluorescence distribution in histograms obtained from T cells incubated alone (negative control), with anti-CD3/anti-CD28 reagent (positive control) or with anti-CD3/anti-CD28 reagent and autologous monocytes.
The effects of isolated CD14+HLA-DRlow/− and CD14+HLA-DRhigh cells on proliferation of autologous T cells were assessed as above with some modifications due to the availability of fewer sorted cells in comparison to unsorted cells. CD14+HLA-DRlow/− or CD14+HLA-DRhigh cells were placed into round-bottom 96-well plates (Costar) in the 1:1 ratio with T cells activated by the anti-CD3/anti-CD28 reagent in the total volume of 200 μl of AIM V medium containing 1.0% penicillin/streptomycin. The cells were incubated for 60 hr when 125 μl of the medium was removed from each well and replaced by the same volume of the fresh medium containing 2.0 μCi [3H]thymidine (Amersham–GE Healthcare, Mt. Pleasant, IL) and incubated for another 12 hr. The cells were harvested by the use of a semiautomatic cell harvester (Skatron Instruments, Sterling, VA) and the incorporated radioactivity was measured by a LS 6000SC scintillation counter (Beckman Coulter, Fullerton, CA).
Statistical Analysis
We report the data as mean ± standard deviation using 5% significance as the limit of statistical significance in all analyses. We assessed the significance of differences in the cells among age-matched noncancerous controls (AMC), newly diagnosed untreated PCa patients (uPCa) and treated PCa patients (tPCa) by Kruskal–Wallis tests. For comparisons of data between any two groups we used the Mann–Whitney tests with Bonferroni correction for multiple comparisons. These nonparametric tests were more robust and thus preferred over their parametric counterparts. We computed the Spearman's correlation coefficients to explore the possible correlations between cell abundance, PSA values, Gleason scores and adjuvant treatment modes and Pearson's correlation coefficients in other cases. In addition, we used Mann–Whitney tests for comparisons of patient treatment effects on cell phenotype. We constructed regression models with an interaction term to investigate treatment effects after controlling for demographic and clinical variables.
We analyzed the T cell proliferation measurements in the following way: The effects of CD3/CD28 stimulation on T cells from tPCa and AMC and the levels of intracellular cytokines were compared by the two-sample t-test and Mann–Whitney test. To acknowledge the correlation between repeated measurements at various monocyte/T cell ratios for the same individual, we compared the effects of the monocyte/T cell ratio on T cell reactivity by repeated-measures ANOVA (separately for CD4+ cells and CD8+ cells). Finally, assuming a linear relationship between measured T cell activation and monocyte/T cell ratios, we used ANCOVA models treating the monocyte/T cell ratio as a continuous predictor. Hence, the interaction term of the experimental group (e.g., CD4+ cells of tPCa) and the respective monocyte/T cell ratio are interpreted as the difference of slopes of the dependence of T cell activation on monocyte/T cell ratio.
Results
More HLA-DRlow/− Monocytes Circulate in PCa Patients Than in Healthy Controls
Based on the published evidence for altered development of myeloid DC in cancer [14] and the presence of altered (HLA Class-II−) monocytes in patients suffering from ovarian carcinoma [15] and melanoma [16], we hypothesized that monocytes in tPCa were also altered. Consequently, by flow cytometry first we determined the percentage of cells in the monocyte region of AMC, uPCa and tPCa; they were 17.6 ± 6.9%, 19.0 ± 14.3%, and 23.9 ± 6.1%, respectively (P = 0.0223 for AMC vs. uPCa and P = 0.0144 for uPCa vs. tPCa). Next, in these cells we analyzed the percent of CD14+ cells; we found that 95.1 ± 4.4%, 92.3 ± 4.5%, and 94.4 ± 3.3% of cells were CD14+ (P > 0.05 for all comparisons). Thus, the three groups did not differ in the amounts of CD14+ cells in the scatter plots. Because of the preponderance of CD14+ cells among monocytes, we use the terms “monocyte” and “CD14+ cell” interchangeably.
To characterize the monocytes in PCa further, we measured the percentage of HLA-DRlow/− cells in freshly isolated CD14+ cells. In this analysis we included the cells from uPCa in addition to those from tPCa. We found that HLA-DRlow/− cells comprised 4.1 ± 6.5% (range: 0.5–23.3%) of CD14+ cells in AMC and 30.7 ± 15.0% (range: 5.1–62.4%) in tPCa, respectively (P < 0.0001; Fig. 1). Newly diagnosed untreated PCa patients exhibited 10.6 ± 14.3% (range: 0.1–50.4%) of HLA-DRlow/− monocytes, that is, more than AMC (P = 0.290), but less than tPCa (P = 0.001).
Fig. 1.
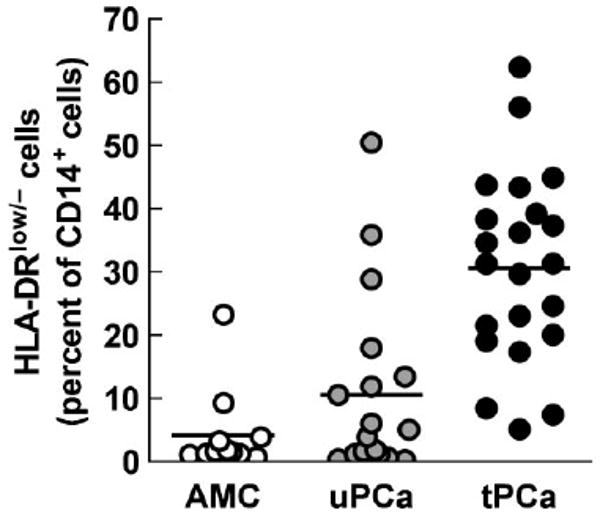
Percentage of HLA-DRlow/− cells among CD14+ cells in peripheral blood of AMC subjects (white circles), newly diagnosed untreated prostate cancer patients (uPCa; gray circles) and tPCa patients (black circles). Horizontal lines indicate mean values. The differences between AMC and tPCa and between uPCa and tPCa are statistically significant (P < 0.0001 and P = 0.001, respectively).
Further we compared the percentage of CD14+HLA-DRlow/− cells in tPCa receiving a LHRH-agonist alone (tPCa/0, 7 patients) with patients additionally receiving an antiandrogen (tPCa/A, 8 patients) or dexamethasone (tPCa/D, 6 patients). The presence of such cells in tPCa/0 (24.3 ± 11.3%, range: 5.1–38.3%) was higher than in AMC and uPCA (P = 0.0018 and P = 0.0131, respectively), but was not statistically significantly different from the levels in tPCa/A (36.7 ± 16.9%, range: 8.4–62.4%) and tPCa/D (31.4 ± 16.1%, range: 7.4–56.0%). More samples in each treatment groups are needed for a definitive resolution whether antiandrogens and dexamethasone contribute to the abundance of CD14+ HLA-DRlow/− cells.
Monocytes From Treated PCa Patients Yield Fewer Mature Dendritic Cells Than Monocytes From Age-Matched Controls
Our standard method for ex vivo DC differentiation includes isolation of CD14+ cells by immunomagnetic adsorption followed by a two-step incubation. The first step requires the presence of GM-CSF, IL-4 and 1.0% human AB serum in X-VIVO 15 medium for 3 days followed by incubation with additional TNF-α and PGE2 for two more days. Starting with CD14+ cells from the blood of healthy blood donors not selected for age, we routinely obtain DCs that are 90.8 ± 7.9% mature (as measured by the expression of CD83) with a yield of 33.9 ± 13.5% (data not shown). To validate this procedure for a clinical trial of DC immunotherapy in androgen-independent tPCa, we prepared DCs from CD14+ cells of patients treated with LHRH agonists and antiandrogens or dexamethasone and one orchiectomized patient (Supplementary Table Ic) and compared the DCs with those prepared from the cells of AMC. Data in Figure 2a show that CD14+ cells from AMC matured into CD83+ cells efficiently and rather uniformly (85.8 ± 6.1%, range: 73.6–92.8%); the cells isolated from tPCa matured less effectively and less uniformly (62.3 ± 30.2%, range: 3.7–98.1%; P = 0.0541). This difference was not related to different final cell yields because the final numbers of total viable cells for AMC and tPCa were indistinguishable, 25.2 ± 16.2% (range: 7.7–63%) for AMC and 25.2 ± 9.3% (range: 6.2–45.3%), respectively (P = 0.543; Fig. 2b).
Fig. 2.
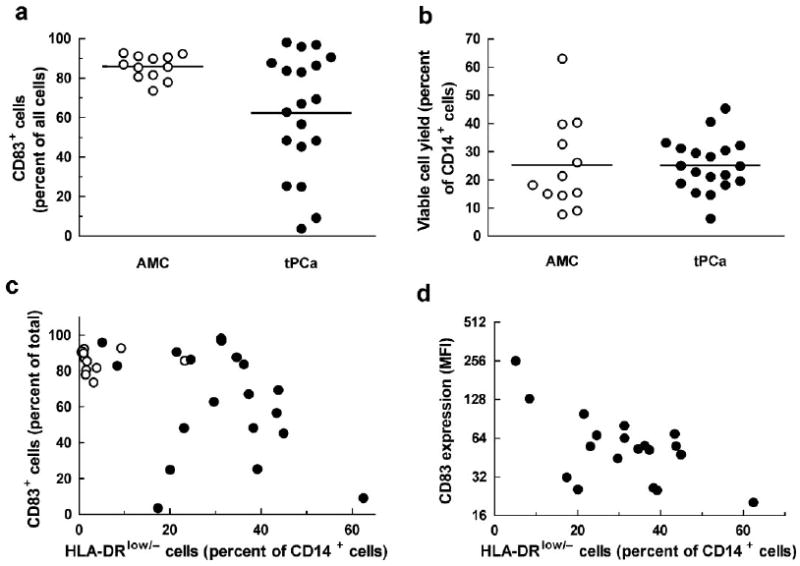
a: Percentage of mature CD83+ dendritic cells in the final preparation and (b) viable cell yield expressed as percentage of the initially plated CD14+ cells for age-matched non-cancerous controls (AMC; white circles) and prostate cancer patients treated by androgen suppression (tPCa; blackcircles). Horizontal lines indicate mean values; the difference between CD83+ cell levels from AMC and tPCa in panel a is statistically significant (P = 0.0541); the difference in yields (b) is not (P = 0.543). c: Percentage of CD83+ cells and (d) CD83 mean fluorescence intensity (MFI) of cells after DC maturation from the CD14+ cells plotted as functions of the percentage of HLA-DRlow/− cells among CD14+ cells.
Levels of HLA-DRlow/− Cells Do Not Predict DC Maturity
To determine the relationship of HLA-DRlow/− cells and CD83+ DCs, we plotted the percentage of CD83+ cells in the final DC product as a function of percentage of HLA-DRlow/− cells among the CD14+ cells. Interestingly, CD14+ cells from AMC matured into CD83+ cells efficiently and independently of the initial content of HLA-DRlow/− cells (R2 = 0.038, P = 0.542); for tPCa, the percentage of CD83+ cells was lower and related to the percentage of HLA-DRlow/− cells among the CD14+ DC precursors (R2 = 0.551, P = 0.0003; Fig. 2c). Interestingly, only in isolates containing more than 17 percent HLA-DRlow/− cells we observed poor DC maturation; this indicates the possibility for the existence of a threshold density of HLA-DRlow/− cells needed to impair differentiation into DCs. An analysis of CD83 expression measured as mean fluorescent intensity (MFI; Fig. 2d) is consistent with the conclusions drawn from Figure 2c: The higher percentage of HLA-DRlow/− cells in the starting monocytes population, the lower CD83 MFI (R2 = 0.3948, P = 0.004). In analogy to the data in Figure 2c, if CD83 MFI measured in AMC cells is included into the analysis, there is no correlation (R2 = 0.0471, P = 0.2411). The percentage of cells surviving in vitro incubation appears independent of the percentage of HLA-DRlow/− cells in the initial CD14+ cell population (P > 0.05 both for AMC and tPCa; data not shown). Thus, solely the level of expression of HLA-DR on CD14+ cells is not sufficient to predict the percentage of CD83+ cells in the final DC preparation.
PCa Monocytes Suppress T-Cell Proliferation
HLA-DRlow/− monocytes found in other pathological states suppress immunity [17–19]. Hence, we compared the effects of tPCa monocytes and AMC monocytes on proliferation of autologous T cells. We isolated monocytes from five additional AMC (containing 3.5 ± 1.0% HLA-DRlow/− cells) and four additional tPCa (containing 8.4 ± 1.8% HLA-DRlow/− cells); these tPCa contained more HLA-DRlow/− cells than AMC (P = 0.041), but contained fewer such cells than tPCa in Figure 1 (P = 0.010). We isolated CD3+ T cells from AMC (97.4 ± 0.4% purity) and tPCa (93.9 ± 2.8% purity, P = 0.211 relative to AMC), stimulated them by anti-CD3/CD28 reagent and analyzed their proliferation (Fig. 3a,b). Both AMC and tPCa CD4+ cells proliferated with high efficiency (87.9 ± 4.2% vs. 85.5 ± 5.7%; P = 0.515), as did CD8+ cells (74.5 ± 7.0% vs. 74.5 ± 2.0%; P = 0.995).
Fig. 3.
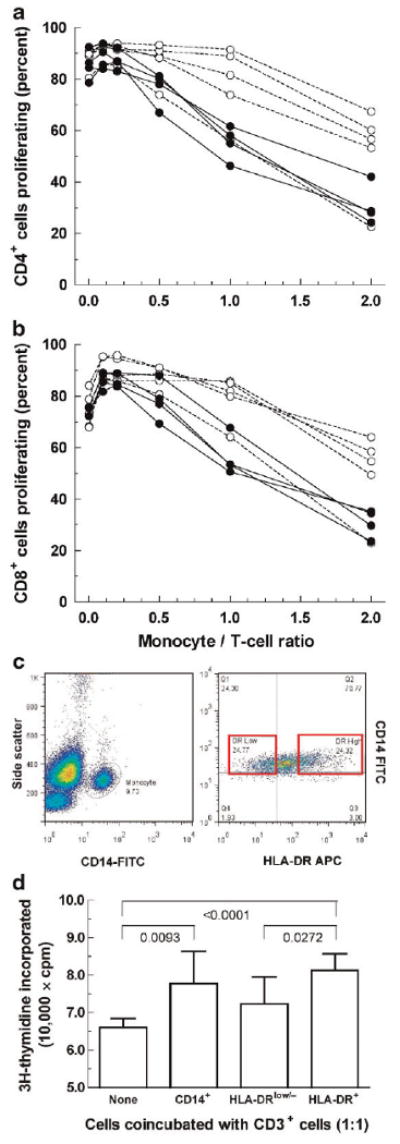
T cell proliferation in the presence of autologous monocytes. a: CD4+ cells; (b) CD8+ cells. White circles, AMC; blackcircles, tPCa. T cells were stimulated by CD3/CD28 ligation in the absence or presence of autologous monocytes. All data were corrected by subtracting the corresponding values measured in unstimulated T cells in the absence of monocytes. There was no difference in proliferation of CD4+ cells (P = 0.515) or CD8+ cells (P = 0.995), but tPCa monocytes inhibited proliferation of autologous CD4+ cells and CD8+ cells more potently than AMC monocytes (P = 0.0036 and P = 0.0044, respectively). c, Left panel: A representative scattergram of mononuclear cells isolated from the blood of a treated PCa patient (P29 in Table II). CD14+ cells in the rightmost peak (monocytes) were sorted into HLA-DRlow/− cells and HLA-DR+ cells (right panel). d: Comparison of the effects of sorted HLA-DRlow/− cells and HLA-DR+ cells (shown in panel c) on proliferation of autologous T cells (measured as DNA synthesis) at the 1:1 ratio. Numbers in the panel designate the P-values for differences between bracketed pairs of groups (two-sided Student's t-test).
To some T cell containing wells we introduced autologous monocytes from AMC (92.1 ± 2.5% purity) or tPCa (89.9 ± 2.2% purity, P = 0.539 relative to AMC) ranging from 0.1 monocyte to 2.0 monocytes per one T cell (the range was limited by the number of cells obtained from a minimally invasive blood draw). The presence of monocytes affected proliferation of T cells (Fig. 3a,b); at ratios of 0.1 and 0.2, monocytes stimulated CD4+ and CD8+ cell proliferation. At higher ratios AMC monocytes had little effect on proliferation of AMC T cells except for the suppressed proliferation at the ratio of two monocytes per one T cells. On the other hand, tPCa monocytes suppressed autologous T cell proliferation at much lower ratios, that is, they were more potent inhibitors than AMC monocytes (P = 0.0036 for CD4+ cells, P = 0.0044 for CD8+ cells). Thus, AMC and tPCa T cells did not differ in the response to CD3/CD28 stimulation, but tPCa monocytes inhibited autologous T cell proliferation more effectively than AMC monocytes.
CD14+HLA-DRlow/− Cells Suppress T Cell Proliferation More Effectively and Express More IL-10 Than CD14+HLA-DR+ Cells
In contrast to CD14+HLA-DRlow/− cells in tPCa patients (Fig. 1), myeloid-derived suppressor cells (MDSCs) in other systems often express little or no CD14 [20–23]. Because CD14+ tPCa monocytes inhibited T cells similarly to such MDSCs [14,20,22,24], we hypothesized that this effect is due to CD14+HLA-DRlow/− cells. Hence, we isolated CD14+HLA-DRlow/− and CD14+HLA-DR+ cells from tPCa patients by fluorescence activated cell sorting (Fig. 3c) and measured the effects of isolated cells on DNA synthesis by activated autologous T cells. (Because of the rather low numbers of cells isolated from the available volume of patient blood, we conducted the experiments only at the 1:1 ratio and measured the effects by radioactive thymidine incorporation.) Data in Figure 3d show that CD14+HLA-DRlow/− cells inhibited T cell proliferation statistically significantly more potently than CD14+ HLA-DR+ cells.
The suppressive effects of MDSCs on T cell proliferation are often mediated by IL-10 and TGF-β, the “M2-type” cytokines [25]. To determine if the suppressive function of CD14+HLA-DRlow/− cells is mediated by such cytokines, we measured and compared the percentages of CD14+HLA-DRlow/− cells and CD14+HLA-DR+ cells that contained intracellular IL-10 and TGF-β1. We found that CD14+HLA-DRlow/− expressed statistically significantly more IL-10 than CD14+HLA-DR+ cells while the difference for TGF-β was not stastistically different (Table II). Interestingly, this effect was characteristic of the cells expressing high and low levels of HLA-DR, respectively, irrespective of whether they were isolated from tPCa or AMC. Although the paucity of available cells isolated by sorting precluded experiments whereby activity of IL-10 and TGF-β would be blocked, the higher level of inhibitory cytokine IL-10 in CD14+HLA-DRlow/− is compatible with their likely role in suppression of T cell proliferation.
TABLE II. Expression of Intracellular IL-10 and TGF-β1 by CD14+HLA-DRlow/− Cells and CD14+ HLA-DR+ Cells From Treated Prostate Cancer Patients (tPCa) and Age-Matched Controls.
| Subject | HLA-DRlow/− cells, percent of CD14+ cells | IL-10 expressing cells | TGF-β1 expressing cells | ||||
|---|---|---|---|---|---|---|---|
| HLA-DRlow/− cells | HLA-DR+ cells | P-valuea | HLA-DRlow/− cells | HLA-DR+ cells | P-valuea | ||
| b P27 | 14.0 | 90.4c | 73.4 | 43.6 | 33.6 | ||
| P28 | 20.8 | 40.6 | 13.5 | 25.9 | 18.6 | ||
| P29 | 24.3 | 89.2 | 69.0 | 0.0244 | 44.3 | 41.2 | 0.5405 |
| A18 | 1.7 | 70.0 | 13.8 | 8.8 | 15.7 | ||
| A19 | 5.0 | 40.4 | 26.4 | 47.2 | 50.2 | ||
Two-sided values calculated by paired Student's t-test for paired samples.
P denotes tPCa patients, A the age-matched controls (both indicated by ** in Supplementary Table I).
Percentage of CD14+HLA-DRlow/− cells and CD14+HLA-DRhigh/+ cells, respectively, expressing IL-10 or TGF-β1.
Percentages of CD4+ Cells, CD8+ Cells and Regulatory T Cells in AMC and PCa Patients Are Largely Indistinguishable
Treg cells have been documented to interfere with therapeutic vaccination [26]. For this reason, we analyzed CD3+ T cells for the frequency of CD4+ cells, CD8+ cells and CD4+CD25+CD127low/− Treg cells [27–29]. Figure 4a shows the ratios of CD4+ cells and CD8+ cells in AMC, uPCa patients and tPCa. There was no difference among the groups in the levels of CD8+ cells, but there were fewer CD4+ T cells as percentage of CD3+ cells in AMC than in uPCa (P < 0.05). Figure 4b shows the percentages of CD25+CD127lo/− cells among CD4+ cells in the three groups. These percentages were low overall with fewer cells in uPCa than in tPCa (P < 0.05).
Fig. 4.
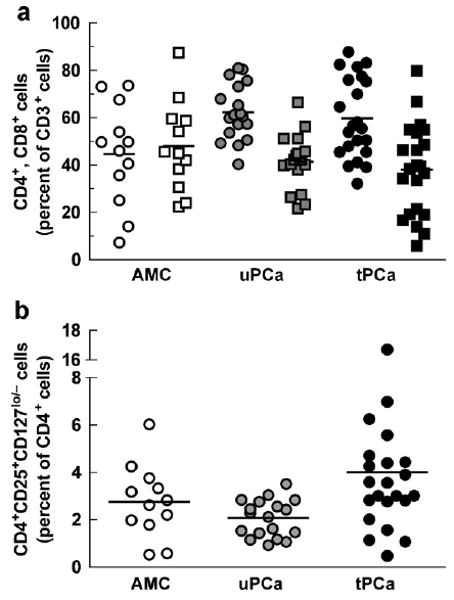
T cells in PBMCs of subjects described in the legend to Figure 1. a: Percent of CD4+ cells (circles) and CD8+ cells (squares) among CD3+ cells. The percentage of CD4+ cells in AMC differed statistically from uPCa(P < 0.05). b: CD4+CD25+CD127low/− T regulatory cells as percent of CD4+ cells. Horizontal lines indicate mean values. The percentage of cells in uPCa differed statistically from tPCa (P < 0.05).
Frequency of HLA-DRlow/− B Cells Is Not Changed in PCa Patients
High levels of HLA-DRlow/− monocytes in tPCa (Fig. 1) prompted the question whether the loss of HLA-DR expression is specific for antigen presenting cells in general or whether it is restricted to monocytes. Thus, we quantified HLA-DR expression in CD19+ B cells. Interestingly, the percentage of HLA-DR+ cells in B cells was statistically indistinguishable among AMC, uPCa and tPCa (Fig. 5). An analysis of HLA-DR+ cells in CD4+ cells and CD8+ cells of the same subjects revealed no difference among the groups either (data not shown). Apparently, diminished expression of HLA-DR in PCa patients appears particular to monocytes.
Fig. 5.
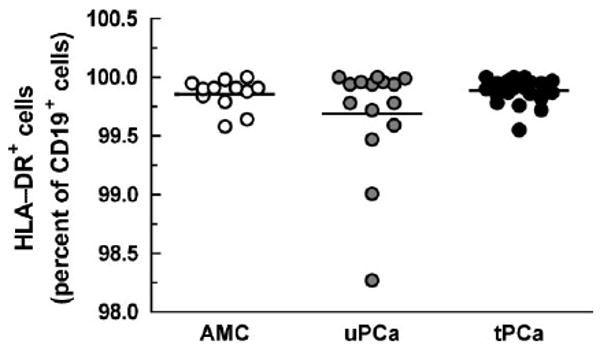
HLA-DR expression on CD19+ B cells from subjects described in the legend to Figure 1. Horizontal lines indicate mean values; these values did not differ statistically.
Androgen Suppression Is Associated With Increased Levels of CD14+HLA-DRlow/− Cells
To detect additional relations among the values measured in AMC, uPCa and tPCa, we undertook a search by the Mann–Whitney test. For example, PSA levels (Fig. 6 and Supplementary Table I) were significantly higher in uPCa and tPCa than in AMC (P < 0.0001 and P < 0.0001, respectively). Similarly, the percent of HLA-DRlow/− cells among CD14+ cells was higher in uPCa and tPCa than in AMC (P = 0.2898 and P < 0.0001, respectively); also, this percentage was higher in tPCa than in uPCa (P = 0.0001).
Fig. 6.
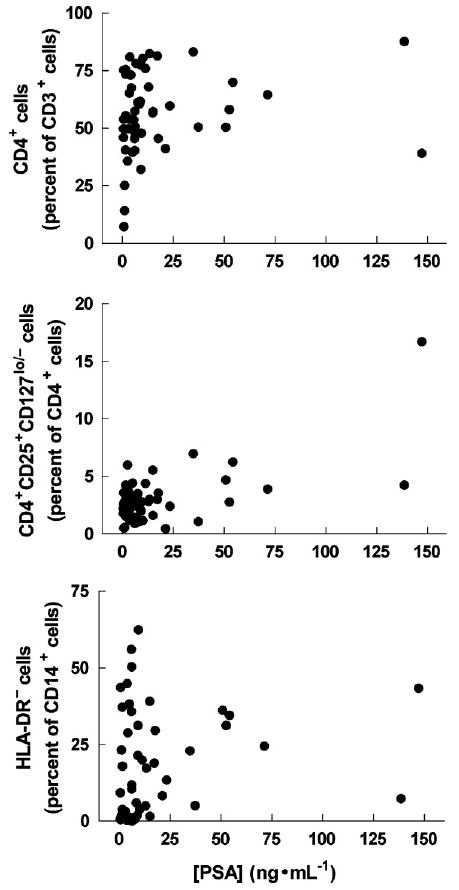
Percent of CD4+ cells among CD3+ cells (upper panel), CD4+CD25+CD127lo/− cells among CD4+ cells (middle panel) and HLA-DRlow/− cells among CD14+ cells (lower panel) plotted as a function of PSA concentrations measured in the blood of all subjects in this study. For statistical details, see the text.
Next we searched by regression analysis for associations between the disease, treatment and percentages of CD14+HLA-DRlow/− cells among all CD14+ cells. We found the levels of CD14+HLA-DRlow/− cells significantly and positively associated with treatment (leuprolide, P = 0.0058; antiandrogen, P = 0.0381), that is, receiving one or both drugs was associated with higher percentages of CD14+HLA-DRlow/− cells. In a regression analysis of the percentages of CD14+HLA-DRlow/− cells versus androgen suppression (all drugs taken into account) and PSA levels, treatment with LHRH-agonist and/or antiandrogen was still significantly and positively associated with percentages of CD14+HLA-DRlow/− cells (P = 0.0027 and 0.0222, respectively); however, in this analysis, PSA levels were not significantly associated with CD14+HLA-DRlow/− cell levels (P = 0.1250). This is different from the significant bivariate Spearman's correlation between CD14+HLA-DRlow/− cells and PSA above, but the nature of this study does not allow us to deduce the underlying reasons for the difference.
Discussion
Attempts to treat cancer by cellular immunotherapy have focused largely on the manipulation of immune effector cells and/or vaccination. These methods have contributed a steady progress in the effectiveness of immunotherapy and evolution of its concepts (cf. Ref. [30]), yet they still do not deliver a predictable and definitive cure [3]. Some have associated this failure with the findings of abnormally high levels of CD4+CD25+ Treg cells [9] and CD8+Foxp3+ Treg cells in tumor tissue [31] and peripheral blood of prostatectomized patients [9]. Others, though, found little difference in the levels of circulating Treg cells between PCa patients and healthy controls, but observed that Treg cells in PCa were more immunosuppressive [32]. We too found similar Treg cell levels in PCa patients and AMC, but the significance of all these findings is uncertain as experiments in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice question whether Treg cells are necessary for induction of T-cell tolerance for PCa [33].
Androgen suppression, the long-standing standard in the treatment of metastatic PCa, boosts thymopoiesis and T cell proliferation [34,35], yet its role in PCa immunotherapy remains unclear. In TRAMP mice, modified by the introduction of influenza hemagglutinin as an artificial prostate-restricted antigen, androgen ablation stimulated immunity by the purported increase of access to antigen [36]. However, it is difficult to extrapolate from the effects of acute androgen deprivation in young mice to elderly cancer patients on chronic androgen suppression. Consequently, the significance of our finding of similar percentage of Treg cells in tPCa patients and AMC needs to be evaluated in terms of possible effects of chronic androgen suppression on T cell function, not just numbers.
Lately there has been an increased interest in the heterogeneous group of myeloid cells that negatively regulate antigen-specific immunity, the myeloid-derived suppressor cells (cf. Ref. [20]). In humans, these cells express CD11b, CD13, CD15, CD33 and CD34, are devoid of lineage markers, CD14, and HLA-DR [20–23] and can suppress CD4+ and CD8+ cell function in vitro [14,24]. In this new area, there are few if any data on these cells in PCa and their role in regulation of human immunity. Similarly, the possible role of androgen suppression in generation and effects of these cells has not been studied.
Prostate Cancer and Androgen Suppression Affect Monocyte Phenotype
To prepare a cellular vaccine for administration to PCa patients, we isolated their CD14+ cells and matured them into DCs by a standard method. In the process, we observed that PCa monocytes differentiated into mature DCs less efficiently than monocytes of age-matched controls. To better understand the reasons for the impairment, we characterized these monocytes and found them significantly different from controls, particularly in patients on androgen suppression therapy. We found that tPCa were higher in the ratio of CD14+HLA-DRlow/−/CD14+HLA-DR+ cells than uPCa who, in turn, exhibited higher ratios than AMC. The increased percentage of HLA-DRlow/− appears characteristic for PCa monocytes, as HLA-DR expression in B cells (and other hematopoietic cells; data not shown) was unchanged indicating that the phenomenon was specific for monocytes. (This, however, does not necessarily mean that B cells in PCa are normal; recently, normal HLA expression was found in CD19 cells associated with systemic plasmocytosis in patients with advanced melanoma, breast cancer, glioma and pancreatic cancer; Ref. [37].) The statistically insignificant increase of HLA-DRlow/− monocytes in uPCa relative to AMC appears related to the disease itself; this increase is statistically significant in patients treated by androgen suppression. Importantly, DC precursors in this study were CD14-positive; hence, they are different from “immature myeloid cells” [24] or “myeloid-derived suppressor cells” [23] that have been defined as CD14-negative.
Despite the statistically significant correlation between HLA-DRlow/− monocytes and androgen suppression, the conclusion that the effects in tPCa are predominantly iatrogenic must await further corroboration. Namely, it is possible that in tPCa the significantly longer time since diagnosis itself contributes to the impairment of monocytes. Additional factors, such as diabetes [38], could affect the phenotype of circulating monocytes as well; however, as only three of the 22 tPCa in this study were diabetic, it is unlikely that diabetes contributed significantly to the present results.
To get a measure of the functional potential of tPCa monocytes in the absence of a technically feasible method in vivo, we compared in vitro the effects of these cells and normal controls on activated autologous T cells. We found that monocytes from tPCa patients inhibited T cell proliferation more effectively than AMC monocytes. The reason for the more pronounced reduction of T cell proliferation by HLA-DRlow/− cells than by HLA-DR+ cells is likely related to higher levels of IL-10 expressed by HLA-DRlow/− cells. IL-10, an M2-type cytokine, suppresses T cell function [25] and, expressed by CD14+ cells, predicts poor outcome in stage IV melanoma [39]. Thus, the lack of HLA-DR expression and IL-10 expression could signify that HLA-DRlow/− cells in PCa patients are similar to M2-type cells observed in other systems.
Malignant Diseases, Sepsis and Trauma Are Associated With Elevated Levels of HLA-DRlow/− Monocytes
The observation that PCa patients exhibit monocytes different from healthy individuals is not new. For example, such monocytes can inhibit the mixed leukocyte reaction [6–8] in analogy to our results. Aberrant monocytes have been observed in ovarian cancer [15], malignant melanoma [16,40], hepatocellular carcinoma [41] and a complex disease such as inflammatory bowel disease [42]. These diseases are associated with increased occurrence of HLA-DRlow/− monocytes that have often been documented for expression of IL-10 and TGF-β, but not IL-12 and tumor necrosis factor-α (TNF-α); the presence of IL-10 and TGF-β and absence of IL-12 and TNF-α can contribute to the ability of these cells to inhibit T cell proliferation [15,25]; our findings of elevated expression of IL-10 in HLA-DRlow/− monocytes and their inhibition of T cell proliferation are fully in line with these observations. In view of these findings it is noteworthy that sera of androgen-independent tPCa patients contained increased levels of IL-4, IL-6 and IL-10 (but not of IL-1β, IL-2, IL-12, and IFN-γ) compared to androgen-dependent patients and noncancerous controls [43]. Similarly, elevated levels of TGF-β have been associated with androgen-deprivation in rats [44] and humans after prostatectomy [45]. Despite these insights, the primary factors responsible for elevated levels of HLA-DRlow/− monocytes and the role of such cells in modulation of immunity and outcome of immunotherapy remain poorly understood.
Although comparison of the natural history of solid tumors with the short-lived sepsis and trauma is tenuous, it is informative to consider the better understood role of HLA Class II− cells in these states [17–19,46,47]. The overall conclusion from sepsis and trauma is that the CD14+HLA-DRlow/− cells are strongly immunosuppressive and predict poor outcome [17,19,47]. A recent study in sepsis established that the early loss of HLA-DR molecule is due to the reduced levels of Class II transactivator and consequential reduction in HLA-DR transcription; the reduction of Class II transactivator correlated with the increased cortisol levels in patients and is also caused by glucocorticoids in vitro [19]. However, a recent study demonstrated that the levels of cortisol in prostatectomized PCa patients are physiological [48] pointing to the likely role of androgen suppression in increased levels of HLA-DRlow/− monocytes.
Androgen Suppression and Prostate Cancer Immunotherapy
The preceding discussion alludes to a rather complex and poorly understood role of androgen suppression in immunotherapy of prostate cancer. On one hand, expanded thymopoiesis and T cell proliferation can stimulate tumor-specific immunity [35,36]. On the other, the immunosuppressive role of HLA Class IIlow/− monocytes has been corroborated by studies of endotoxin-resistant cells [49]. Similar to the HLA-DRlow/− cells isolated from cancer patients [15], endotoxin-resistant HLA-DR− cells express IL-10 and cannot stimulate antigen-specific T cells [49]. In addition, other mechanisms whereby HLA-DRlow/− monocytes suppress immunity are likely involved. For example, this article and others [50] have shown that HLA-DRlow/− monocytes mature less effectively into functional DCs; this can lessen the stimulation of naïve and memory T cells [1,50]. It is also plausible that HLA-DRlow/− monocytes cannot develop into functional macrophages as effectively as normal monocytes; this could reduce the ability of macrophages to re-present the antigens released from tumor cells previously killed by T cells or NK cells [51] and thus maintain the length, amplitude and range of T cell attack on tumor cells. Consequently, the sum total of the effects of androgen suppression on the natural history of disease and development of therapeutic immunity must be evaluated in clinical studies and considered when designing immunotherapeutic strategies aimed at eradication or control of prostate cancer.
Supplementary Material
Acknowledgments
We thank Mayo Clinic patients who generously donated blood for this study and Dr. Brian J. Davis, Leader, Mayo Clinic Prostate Cancer SPORE Clinical Core, Dr. Paul L. Claus and Ms. Dawn Arlander for help with accruing them. Mrs. Adelyn L. Luther, Singer Island, Florida, Mayo Clinic Cancer Center and NIH Grant P50CA91956 supported this work.
Footnotes
The authors have declared no financial conflict of interest in regards to this work.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Thomas-Kaskel AK, Waller CF, Schultze-Seemann W, Veelken H. Immunotherapy with dendritic cells for prostate cancer. Int J Cancer. 2007;121:467–473. doi: 10.1002/ijc.22859. [DOI] [PubMed] [Google Scholar]
- 3.Fox SB, Launchbury R, Bates GJ, Han C, Shaida N, Malone PR, Harris AL, Banham AH, Miller AM, Pisa P. Tumor escape mechanisms in prostate cancer. Cancer Immunol Immunother. 2007;56:81–87. doi: 10.1007/s00262-005-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sciarra A, Lichtner M, Autran Gomez AM, Mastroianni C, Rossi R, Mengoni F, Cristini C, Gentilucci A, Vullo V, Di Silverio F. Characterization of circulating blood dendritic cell subsets DC123+ (lymphoid) and DC11c+ (myeloid) in prostate adenocarcinoma patients. Prostate. 2007;67:1–7. doi: 10.1002/pros.20431. [DOI] [PubMed] [Google Scholar]
- 5.Aalamian-Matheis M, Chatta GS, Shurin MR, Huland E, Huland H, Shurin GV. Inhibition of dendritic cell generation and function by serum from prostate cancer patients: Correlation with serum-free PSA. Adv Exp Med Biol. 2007;601:173–182. doi: 10.1007/978-0-387-72005-0_18. [DOI] [PubMed] [Google Scholar]
- 6.Herr HW. Suppressor cells in the pelvic lymph nodes regional to bladder cancer. J Surg Oncol. 1979;11:289–293. doi: 10.1002/jso.2930110403. [DOI] [PubMed] [Google Scholar]
- 7.Herr HW. Adherent suppressor cells in the blood of patients with bladder cancer. J Urol. 1981;126:457–460. doi: 10.1016/s0022-5347(17)54574-7. [DOI] [PubMed] [Google Scholar]
- 8.Herr HW. Suppressor cells in immunodepressed bladder and prostate cancer patients. J Urol. 1980;123:635–639. [PubMed] [Google Scholar]
- 9.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 10.Taams LS, van Amelsfort JMR, Tiemessen MM, Jacobs KMG, de Jong EC, Akbar AN, Bijlsma JWJ, Lafeber FPJG. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJC, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietz AB, Bulur PA, Erickson MR, Wettstein PJ, Litzow MR, Wyatt WA, Dewald GW, Tefferi A, Pankratz VS, Vuk-Pavlović S. Optimizing preparation of normal dendritic cells and bcr-abl positive mature dendritic cells derived from immunomagnetically purified CD14 positive cells. J Hematotherapy Stem Cell Res. 2000;9:95–101. doi: 10.1089/152581600319676. [DOI] [PubMed] [Google Scholar]
- 13.Dietz AB, Padley DJ, Butler GW, Maas ML, Greiner CW, Gastineau DA, Vuk-Pavlović S. Clinical-grade manufacturing of dendritic cells from CD14+ precursors: Experience from phase 1 clinical trial in chronic myelogenous leukemia and malignant melanoma. Cytotherapy. 2004;6:563–570. doi: 10.1080/14653240410005357-1. [DOI] [PubMed] [Google Scholar]
- 14.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cyotkine protein expression and proliferation of autologous T cells. J Immunol. 1999;163:6251–6260. [PubMed] [Google Scholar]
- 16.Ugurel S, Uhlig D, Pfohler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol Immunother. 2004;53:551–559. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res. 2005;11:311–320. doi: 10.1179/096805105X58733. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons RA, Martinez OM, Lim CR, Horn JK, Garovoy MR. Reduction in HLA-DR, HLA-DQ and HLA-DP expression by Leu-M3+ cells from the peripheral blood of patients with thermal injury. Clin Exp Immunol. 1989;75:371–375. [PMC free article] [PubMed] [Google Scholar]
- 19.Le Tulzo Y, Pangault C, Amiot L, Guilloux V, Tribut O, Arvieux C, Camus C, Fauchet R, Thomas R, Drenou B. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med. 2004;169:1144–1151. doi: 10.1164/rccm.200309-1329OC. [DOI] [PubMed] [Google Scholar]
- 20.Serafini P, Borello I, Bronte V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Ko JS, Bukowski RM, Fincke JH. Myeloid-derived suppressor cells: A novel therapeutic target. Current Oncology Reports. 2009;11:87–93. doi: 10.1007/s11912-009-0014-6. [DOI] [PubMed] [Google Scholar]
- 22.Ostrand-Rosenberg S, Sinha P. Myeloid derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talmadge JE. Pathways mediating the expansion and immunosuppressive acivity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;15:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 24.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DC, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A. Macrophage diversity and polarization: In vivo veritas. Blood. 2006;108:408–409. [Google Scholar]
- 26.van der Vliet HJJ, Koon HB, Atkins MB, Balk SP, Exley MA. Exploiting regulatory T-cell populations for the immunotherapy of cancer. J Immunother. 2007;30:591–595. doi: 10.1097/CJI.0b013e31805ca058. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Putnam AL, Xu-yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med. 2008;233:522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 32.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+ CD25highFoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 33.Degl'Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, Hess-Michelini R, Doglioni C, Bellone M. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 34.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173:6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 35.Aragon-Ching JB, Williams KM, Gulley JM. Impact of androgen-deprivation therapy on the immune system: Implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4963–4977. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 36.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate-prostate cancer/restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpenter EL, Mick R, Rech AJ, Beatty GL, Colligon TA, Rosenfeld MR, Kaplan DE, Chang KM, Domchek SM, Kanetsky PA, Fecher LA, Flaherty KT, Schuchter LM, Vonderheide RH. Collapse of the CD27+ B-cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin Cancer Res. 2009;15:4277–4287. doi: 10.1158/1078-0432.CCR-09-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corrales J, Almeida M, Burgo RM, Hernandez P, Miralles JM, Orfao A. Decreased production of inflammatory cytokines by circulating monocytes and dendritic cells in type 2 diabetic men with atherosclerotic complications. J Diabetes Complications. 2007;21:41–49. doi: 10.1016/j.jdiacomp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Torisu-Itakura H, Lee JH, Huynh Y, Ye X, Essner R, Morton DL. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. J Immunother. 2007;30:831–838. doi: 10.1097/CJI.0b013e318158795b. [DOI] [PubMed] [Google Scholar]
- 40.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Marani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 41.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Haile LA, von Wasielewski R, Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: A new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Wise GJ, Marella VK, Talluri G, Shirazian D. Cytokine variations in patients with hormone treated prostate cancer. J Urol. 2000;164:722–725. doi: 10.1097/00005392-200009010-00024. [DOI] [PubMed] [Google Scholar]
- 44.Kyprianou N, Isaacs JT. Expression of transforming growth factor-β in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989;3:1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- 45.Sinnreich O, Kratzsch J, Reichenbach A, Glaeser C, Huse K, Birkenmeier G. Plasma levels of transforming growth factor-1β and α2-macroglobulin before and after radical prostatectomy: Association to clinicopathological parameters. Prostate. 2004;61:201–208. doi: 10.1002/pros.20062. [DOI] [PubMed] [Google Scholar]
- 46.Fumeaux T, Pugin J. Is the measurement of monocytes HLA-DR expression useful in patients with sepsis? Intensive Care Med. 2006;32:1106–1108. doi: 10.1007/s00134-006-0205-7. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman JA, Weinberg KI, Azen CG, Horn MV, Dukes L, Starnes VA, Woo MS. Human leukocyte antigen-DR expression on peripheral blood monocytes and the risk of pneumonia in pediatric lung transplant recipients. Transpl Infect Dis. 2004;6:147–155. doi: 10.1111/j.1399-3062.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- 48.Heracek J, Urban M, Sachova J, Kuncova J, Eis V, Mandys V, Hampl R, Starka L. The endocrine profiles in men with localized and locally advanced prostate cancer treated with radical prostatectomy. Neuroendocrinol Lett. 2007;28:45–51. [PubMed] [Google Scholar]
- 49.Wolk K, Kunz S, Crompton NEA, Volk HD, Sabat R. Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. J Biol Chem. 2003;278:18030–18036. doi: 10.1074/jbc.M207714200. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Hong S, Yang JP, Qian J, Shpall E, Kwak LW, Yi Q. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients' monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006;108:4071–4077. doi: 10.1182/blood-2006-04-016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corthay A. CD4+ T cells cooperate with macrophages for specific elimination of MHC class II-negative cancer cells. Adv Exp Med Biol. 2007;590:195–208. doi: 10.1007/978-0-387-34814-8_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


