Abstract
Medulloblastoma is the most frequent malignant pediatric brain tumor with a dismal prognosis in 30% of cases. We examined the activity of AEE788, a dual inhibitor of human epidermal receptor (HER) 1/2 and vascular endothelial growth factor receptor (VEGFR) 1/2, in medulloblastoma preclinical models. Established lines (Daoy and D283), chemoresistant (DaoyPt), and ectopically HER2-overexpressing (DaoyHER2) cells expressed diverse levels of total and activated AEE788 target receptors. In vitro, AEE788 inhibited cell proliferation (IC50 from 1.7 to 3.8 µM) and prevented epidermal growth factor- and neuregulin-induced HER1, HER2, and HER3 activation. Inhibition of Akt paralleled that of HER receptors. In vivo, AEE788 growth inhibited Daoy, DaoyPt, and DaoyHER2 xenografts by 51%, 45%, and 72%, respectively. Immunohistochemical analysis of mock- and HER2-transfected xenografts revealed that the latter showed, along with high HER2 expression, high VEGFR2 staining in tumor and endothelial cells and increased expression of the endothelial marker CD31. AEE788 reduced the activation of target receptors and angiogenesis. In 21 primary medulloblastoma, HER2 expression significantly correlated (P < .01) with VEGFR2 (r = 0.56) and VEGF (r = 0.61). In conclusion, AEE788 shows similar growth-suppressive activities in chemosensitive and chemoresistant medulloblastoma cells in vitro and in vivo. Ectopic HER2 overexpression sensitizes cells to AEE788 in vivo, but not in vitro, possibly through host-mediated processes. Together with the experimental data, the finding that HER2 positively correlates with VEGFR2 and VEGF in human medulloblastoma specimens indicates HER2-overexpressing medulloblastoma as the subset that most likely might benefit from AEE788 treatment.
Introduction
Medulloblastoma is the most common malignant brain tumor of childhood [1]. Despite intensive treatment, approximately 30% of children with medulloblastoma die of their disease, whereas survivors often experience long-term iatrogenic sequelae [2]. New agents that are more effective and less toxic should be developed.
As long as the molecular bases of tumorigenesis increase, new avenues for treating cancer are being explored. Because of the interplay between signaling pathways in tumor and tumor-associated cells, the single-targeted molecular agents are giving the way to agents able to concurrently inhibit multiple targets and biologic processes [3]. Aberrant signals through receptor tyrosine kinases, including those of the human epidermal receptor (HER) family, activate proliferation and prosurvival pathways that confer selective growth advantage to tumor cells [4]. In addition, tumor cells require the formation of new vessels for nutrient and oxygen supplies [5]. Numerous ligands are involved in the coordinated processes that lead to angiogenesis, but vascular endothelial growth factor (VEGF) seems to play a pivotal role in controlling mitogenesis and survival of endothelial cells [6]. VEGF binds to both VEGF receptor 1 (VEGFR1, Flt1) and VEGFR2 (KDR, Flk1) on the surface of endothelial cells, VEGFR2 being, however, the main mediator of VEGF signaling.
A functional link between HER family members and VEGF has been established. Tumor cells can be stimulated by activation of HER1 (EGFR, ErbB1) to secrete VEGF, which, in turn, induces angiogenesis through paracrine mechanisms [7]. HER2 (ErbB2/neu) has also been associated with increased angiogenic potential in experimental and clinical models. In breast cancer, HER2 signaling induced by ectopic overexpression of HER2 or ligand stimulation increases VEGF expression in vitro [8], and in biopsy specimens, HER2 expression positively correlates with VEGF [9].
These data lend the experimental support for combined targeting of HER- and VEGFR-dependent pathways in clinical settings [10]. One of the agents under development is AEE788, a member of the 7H-pyrrolo[2,3] class of pyrimidines, which inhibits tyrosine kinase activity of HER1/2 and VEGFR1/2 with similar affinity, thus potentially blocking both HER-driven proliferation of tumor cells and vasculature neoformation mediated by VEGFRs [11]. AEE788 has demonstrated antiproliferative activity against cell lines and xenografts from different tumors, such as carcinomas of lung, prostate, thyroid, and colon [11–13]. Clinical trials with AEE788 in tumors, including those of the brain, are ongoing, and results are awaited (www.clinicaltrials.gov).
Medulloblastoma might be a candidate for AEE788 treatment because of the expression of AEE788-sensitive targets in this tumor. Particularly, HER2 increases angiogenic potential in medulloblastoma preclinical models [14], and HER2 is overexpressed in a sizable subpopulation of patients, being associated with more aggressive disease, poor survival, and chemoresistance [15]. VEGF receptors and ligands are coexpressed inmedulloblastoma cells and patient samples, suggesting an autocrine role for this loop in medulloblastoma tumorigenesis [16].
In the present study, we investigated the therapeutic potential of AEE788 in medulloblastoma by using commercially available medulloblastoma lines, cells with acquired drug resistance, and cells with ectopic expression of HER2. We found that AEE788 inhibits the proliferation of medulloblastoma lines and that chemoresistance is not associated with resistance to AEE788 in vitro and in vivo. In xenografts, ectopic HER2 overexpression increases VEGFR2 expression in tumor cells and angiogenesis and results in a greater response to AEE788 antitumor activity. In primary human medulloblastoma, HER2 expression significantly correlates (P < .01) with the expression of VEGF and VEGFR2. Together, these data suggest that AEE788 might have a therapeutic potential in medulloblastoma, identifying HER2 as a possible predictive marker of responsiveness to the agent.
Materials and Methods
Reagents
AEE788 (kindly provided by Novartis Pharmaceuticals, Basel, Switzerland) was dissolved in dimethyl sulfoxide to a 10-mM stock solution. For oral administration, AEE788 was dissolved immediately before use in N-methylpyrrolidone and polyethylene glycol 300 (1:9; Sigma, Dorset, UK).
The following primary antibodies were used: HER1, p-HER1 (Tyr1173), and actin from Santa Cruz Biotechnology (Santa Cruz, CA); p-HER2 (Tyr1248) from Upstate Biotechnology (Lake Placid, NY); and HER2, p-HER3 (Tyr1289), Akt, p-Akt (Ser473), extracellular signal-regulated kinase (ERK) 1/2, p-ERK1/2 (Thr202/Tyr204), VEGFR2 from Cell Signaling Technology (Beverly, MA) for Western blot analysis. For immunohistochemistry, the following primary antibodies were used: HER1, p-HER1 (Tyr1173) (Santa Cruz Biotechnology), HER2, p-HER2 (Tyr1221/1222), VEGFR2, p-VEGFR2 (Tyr1175, Cell Signaling Technology), and antimouse CD31 (BDPharMingen, San Diego, CA). Horseradish peroxidase-conjugated secondary antibodies were from Vector (Burlingame, CA).
Cell Lines and Culture Conditions
The human medulloblastoma cell lines D283 and Daoy were obtained from American Type Culture Collection (Rockville, MD). D283 cells were grown according to the American Type Culture Collection's recommendations. Cisplatinum-resistant DaoyPt cells were established by continuous exposure to stepwise-increasing concentrations of cisplatinum up to 1.5 µM. For HER2 overexpression, Daoy cells were transfected with either pcDNA3.1 empty vector or pcDNA3.1-HER2 expression vector and selected under 100 µg/ml of G418 (Sigma) [17]. Daoy cells and derivatives were maintained in 10% fetal bovine serum (FBS)/RPMI supplemented with l-glutamine, a penicillin/streptomycin mixture (Sigma), and the appropriate selecting agent that was removed at least 1 week before any experiment was performed. Different clones of HER2-overexpressing Daoy cells were used giving similar results. Therefore, only data from clone no. 20, called DaoyHER2, have been reported. Daoy transfected with empty vector (DaoyV) behaved as untransfected Daoy cells.
Cell Viability Assay
Cell viability assays with AEE788 were performed in 2% FBS-containing medium, as previously reported [11,12]. Cells were seeded into six-well tissue culture plates at the appropriate density to prevent confluence throughout the experiment. After 24 hours, cells were exposed to vehicle or increasing concentrations of AEE788 for 72 hours. Viable cells as judged by trypan dye exclusion were counted and expressed as a percent of the control. IC50 values were then determined by mathematical curve fitting by using CalcuSyn for Windows software package (Biosoft, Cambridge, UK).
RNA Preparation and Reverse Transcription-Quantitative Polymerase Chain Reaction
Total RNA was extracted from cells using TRIzol (Invitrogen Corp, Paisley, UK) according to the manufacturer's protocol. One microgram of total RNA was reverse-transcribed with Moloney murine leukemia virus reverse transcriptase and random primers (Invitrogen Corp). Gene expression was quantified by real-time quantitative polymerase chain reaction (qPCR) performed on a 7500 Real-time PCR Systems (Applied Biosystems, Foster City, CA). PCRs were done using Applied Biosystems Master Mix reagents as per manufacturer's instructions. TaqMan gene expression assays for HER1, HER2, VEGFR2, and VEGF and the reference normalization gene hypoxanthine guanine phosphorybosyltransferase (HPRT) were obtained from Applied Biosystems. Each amplification reaction was performed in triplicate, and the average of the three threshold cycles (Ct) of each gene was normalized to that of HPRT for each sample to get ΔCt (ΔCt = Cttarget gene - CtHPRT). The ΔCt was then converted to the relative amount of tissue target messenger RNA (mRNA) by the formula 2-ΔCt.
Ligand Stimulation and Western Blot Analysis
For ligand stimulation, 80%confluent cells were starved overnight in 0.5% FBS medium. Cells were then exposed to AEE788 (0.1–5 µM) for 2 hours and subsequently stimulated for 10 minutes with 25 ng/ml epidermal growth factor (EGF; Sigma) or 50 ng/ml neuregulin (NRG; Sigma). At the end of treatment, cells were scraped in lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1 µg/ml pepstatin, and 1 mM phenylmethylsulfonyl fluoride). Total lysates were subjected to electrophoresis on 8% to 10% polyacrylamide gels, transferred to a Hybond nitrocellulose membrane (Amersham Pharmacia, Buckinghamshire, UK), and probed with appropriate dilutions of primary antibodies. After incubating with horseradish peroxidase-conjugated secondary antibodies, immunoblots were visualized using the ECL detection system (Amersham Pharmacia).
Animals and Tumor Growth Inhibition Studies
All animal investigations complied with the guidelines of the Istituto Superiore di Sanità (Rome, Italy) on experimental neoplasia in animals. Medulloblastoma cells (20 x 106) were injected subcutaneously together with an equal volume of Matrigel (Becton Dickinson, Franklin Lakes, NJ) in both flanks of athymic nude mice (Charles River, Calco, Italy). Mice were randomly divided into two groups of 10 animals per group, and either vehicle or 50 mg/kg AEE788 was administered orally thrice a week for 4 weeks. Tumor volume (TV) and total weight were monitored every 3 days. TVs were calculated by the formula: TV = d2 x D/2, where d and D are the shortest and longest diameters, respectively. The efficacy of drug treatment was assessed as percentage tumor volume inhibition (TVI) in treated (T) versus control (C) mice according to the formula: TVI = 100 - (T/C x 100). Two-tailed Student's t tests were applied to compare tumor growth between treated and control groups; the differences were considered statistically significant at P < .05.
Immunohistochemistry
Xenograft specimens were fixed with 4% paraformaldehyde, paraffin-embedded, and cut into 3-µm sections. Sections were deparaffinized, and endogenous peroxidase was blocked with 3% hydrogen peroxide in phosphate buffer. After microwaving sections in the appropriate buffer for antigen retrieval, nonspecific protein binding was blocked with 20% normal goat/rabbit serum. Sections were incubated overnight at 4°C with primary antibodies at the following working dilutions: 1:100 for p-HER1, HER1, p-VEGFR2, and CD31; 1:50 for p-HER2; 1:150 for VEGFR2; and 1:500 for HER2. Sections were then incubated with the secondary anti-mouse/rabbit/goat EnVision System-HRP (DakoCytomation, Carpinteria, CA) for 30 minutes at room temperature. After washing, the signal was detected by EnVisionr+ System-horseradish peroxidase (DakoCytomation) as per the manufacturer's instructions. Slides were counterstained with Mayer hematoxylin and were finally mounted. Immunohistochemical staining for CD31 was by the streptavidin-biotin peroxidase method using the Vectastatin ABC system (Vector).
Reverse Transcription-qPCR Analysis of Human Medulloblastoma
Surgical specimens of primary medulloblastoma (6 desmoplastic, 12 classic, and 3 anaplastic/large cells, according to the World Health Organization's criteria) were collected from 13 male and 8 female patients with institutional review board approval. Tumor samples were snap-frozen in liquid nitrogen in the operating room and then stored in liquid nitrogen until further analysis. Total RNA was extracted using RNeasy Kit (Qiagen, Venlo, NE) and retrotranscribed with random primers. Complementary DNA quality was confirmed by PCR analysis of β-actin internal control expression. Real-time qPCR analysis was performed as described to determine the expression level of HER2, VEGFR2, VEGFR1, VEGF, basic fibroblast growth factor (bFGF), transforming growth factor α (TGFα), and HPRT.
Statistical Analysis
Spearman correlation coefficients were calculated to assess associations between the mRNA expression levels of the different genes. The statistical significance level was set at P < .05. Calculations were performed with SPSS software package, version 12.0 (SPSS, Inc, Chicago, IL).
Results
AEE788 Inhibits the Proliferation of Medulloblastoma Cell Lines
In preliminary experiments, we characterized the response of Daoy cells and derivatives to therapeutics commonly used in the treatment of medulloblastoma. Cisplatinum-selected DaoyPt cells resulted 18-fold resistant to cisplatinum and cross-resistant to carboplatinum and etoposide (10- and 4-fold, respectively; Figure 1A) compared with Daoy cells, whereas HER2-overexpressing DaoyHER2 cells were significantly resistant to cisplatinum and carboplatinum (4.9- and 2.1-fold, respectively) but not to etoposide. DaoyV cells transfected with the empty vector showed IC50 values similar to those of untransfected parental cells.
Figure 1.
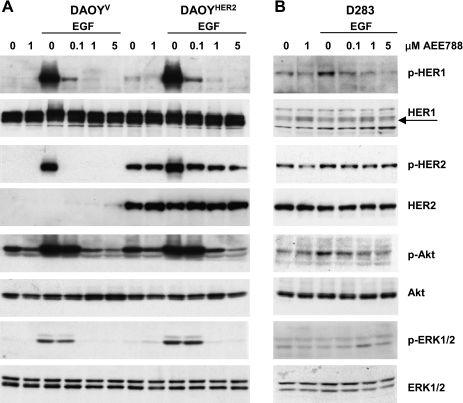
Antiproliferative effects of AEE788 and expression of AEE788-sensitive targets in medulloblastoma cell lines. (A) Sensitivity to antineoplastic agents or AEE788 expressed as IC50 in Daoy, cisplatinum-selected DaoyPt, Daoy transfected with HER2 (DaoyHER2) or empty vector (DaoyV), and D283 cells. Viability assays were performed after 72 hours of treatment. *P < .01, **P < .001 compared with Daoy cells (two-tailed Student's t test). (B, D) RT-qPCR analysis of expression of HER1 and HER2 (B) and VEGF and VEGFR2 (D) inmedulloblastoma lines and in the glioma lines U87 and A172. Bars show means ± SD of three determinations of each target gene normalized to the endogenous control HPRT in each sample. (C) Expression levels of total and activated HER1, HER2, and VEGFR2 in medulloblastoma cell lines. Cells were serum-starved overnight in 0.5% FBS medium, and Western blot analysis was performed to either the total or the phosphorylated levels of each protein. Equal loading was verified by actin immunoblot analysis. HUVEC cells were used as a positive control for VEGFR2 expression. No phosphorylated VEGFR2 was detected. HUVEC indicates human umbilical vein endothelial cells.
We next evaluated the growth-inhibitory effects of AEE788 in these lines and in D283 cells. The proliferation of both D283 and Daoy cells was inhibited by AEE788 in a dose-dependent manner, with IC50 values of 1.7 ± 0.1 and 3.8 ± 0.2 µM, respectively (P < .001; Figure 1B, and data not shown). DaoyPt, DaoyHER2, and DaoyV cells did not show a significantly different response to AEE788 compared with Daoy.
AEE788-Sensitive Targets Are Expressed and Activated at Variable Levels in Medulloblastoma Cell Lines
We determined the expression of AEE788-sensitive targets in all lines. D283 cells expressed high levels of HER2 mRNA and protein, whereas Daoy cells showed high levels of HER1 (Figure 1, B and C). Phosphotyrosine content paralleled the expression of the corresponding receptor in each line. Phosphorylated (activated) and total HER1 was undetectable with 1-minute exposure in D283 cells but showed a clear signal with longer exposures (Figure 2B). DaoyPt cells displayed a receptor profile similar to that of Daoy cells, whereas DaoyHER2 cells expressed 20-fold more HER2 mRNA and consistently higher levels of total and activated protein.
Figure 2.
Inhibition of EGF-triggered signaling pathways in medulloblastoma lines by AEE788. DaoyV and DaoyHER2 (A) and D283 (B) cells were serum-starved overnight and incubated with increasing concentrations of AEE788 2 hours before a 10-minute exposure to EGF (25 ng/ml). Cell lysates were subjected to immunoblot analysis with antibodies to the phosphorylated and total HER1, HER2, Akt, and ERK1/2.
Accordingly to previous data [16], our lines coexpressed VEGF and VEGFR2 at mRNA levels (Figure 1D), with no substantial difference in parental and derived Daoy cells. Total VEGFR2 protein was barely detectable, whereas the activated form was not (Figure 1C, and data not shown). To assess the relevance of VEGF/VEGFR2 as an autocrine/paracrine loop in medulloblastoma, we compared the expression of both genes in our lines to that in lines from malignant glioma, a typically angiogenic tumor that expresses in culture both VEGF and VEGFR2 [18]. Reverse transcription (RT)-qPCR analysis demonstrated that both genes were expressed at similar or higher amounts in medulloblastoma compared with glioma lines (Figure 1D).
AEE788 Inhibits EGF-Induced Signaling in Medulloblastoma Cell Lines
On binding to cognate ligands, receptor tyrosine kinases, including HERs and VEGFRs, activate downstream Akt and ERK pathways that promote cell proliferation and survival [4,6]. To determine the effects of AEE788 on HER-mediated signaling, serum-starved medulloblastoma lines were treated with increasing concentrations of AEE788 for 2 hours and then stimulated with the HER1 ligand EGF for 10 minutes. In Daoy lines, HER1 and HER2 were activated by EGF and dose-dependently inhibited by AEE788 (Figure 2A, and data not shown for Daoy and DaoyPt cells). EGF increased also the phosphorylation of Akt and ERK1/2, whose inhibition by AEE788 paralleled that of HER1. Of note, Akt was still phosphorylated in baseline conditions and was downregulated by 1 µM AEE788, suggesting the presence of a constitutive, AEE788-sensitive activation of Akt in Daoy cells.
Overall, EGF induced a modest activation of HER1-mediated signaling in D283 cells (Figure 2B). A slightly increased phosphorylation was obvious only in HER1 and Akt proteins and was inhibited dose-dependently by AEE788, whereas no modulation of p-ERK1/2 was observed. HER2 was only minimally stimulated by EGF and reported to the unstimulated levels by AEE788. However, AEE788 was not or was scarcely effective in inhibiting constitutively active HER2 in both DaoyHER2 and D283 lines. Expression of total HER1, HER2, Akt and ERK1/2 proteins did not change throughout all of the experiments.
Because of the low level of VEGFR2 expression in our cell lines, we were unable to induce its phosphorylation on stimulation with either EGF or VEGF (data not shown). In contrast, DaoyHER2 growing in vivo express high levels of VEGFR2 (see below).
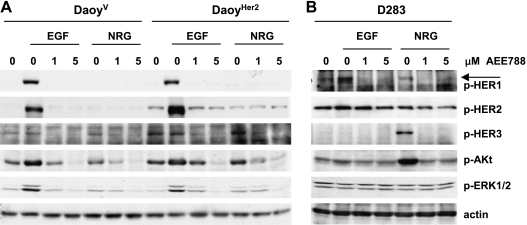
AEE788 Inhibits NRG-Induced Signaling
We next investigated whether AEE788 could inhibit signaling triggered by ligands other than EGF because D283 cells had little EGF induced activation despite their lowest IC50 for cellular growth. Among ligands of the HER family, NRG has been reported to play a role in medulloblastoma tumorigenesis [19]. NRG binds to the “kinase dead” HER3 that preferentially signals as a complex with HER2, suggesting that HER2 overexpression might sensitize cells to stimulation by NRG [4]. DaoyV, DaoyHER2, and D283 cells were serum-starved, treated with AEE788, and then stimulated with NRG and, for comparison, with EGF. Unlike EGF, NRG did not activate either HER1 or HER2 over their basal level in DaoyV and DaoyHER2 cells, nor did it increase the activity of Akt or ERK1/2 (Figure 3A). With respect to DaoyV, DaoyHER2 cells displayed higher levels of ligand-independent p-HER3, which were not further induced by either EGF or NRG. In both lines, as little as 1 µM AEE788 reduced the level of HER3 phosphorylation below the baseline. By contrast, treatment with NRG, but not EGF, caused a striking increase in HER3 activity in D283 cells, with a concomitant marked activation of Akt, that was effectively prevented by AEE788 (Figure 3B). Again, no phosphorylation of ERK1/2 was observed.
Figure 3.
Inhibition of NRG-induced signaling in medulloblastoma cells. DaoyV and DaoyHER2 (A) and D283 (B) cells were serum-starved overnight and treated with the indicated concentrations of AEE788 2 hours before a 10-minute exposure to EGF (25 ng/ml) or NRG (50 ng/ml). Cell lysates were subjected to immunoblot analysis with antibodies to the phosphorylated HER1, HER2, HER3, Akt, and ERK1/2. Actin was used as a loading control.
AEE788 Inhibits the Growth of Medulloblastoma Tumors In Vivo
We compared the antitumor activity of AEE788 against Daoy, DaoyPt, DaoyHER2, and DaoyV xenografts. AEE788 caused a statistically significant reduction in tumor volume of Daoy and DaoyPt xenografts, with a TVI of 51% and 45%, respectively (Figure 4, A and B). DaoyV xenografts behaved as Daoy (data not shown). On the DaoyHER2 xenografts, AEE788 induced a more pronounced tumor inhibition (TVI = 72%; Figure 4C). All the mice survived until the end of the 4-week treatment period, with a less than 15% body weight loss at worst, which was partially recovered by the end of the experiment (Figure 4, D–F).
Figure 4.
Antitumor activity of AEE788 on human medulloblastoma xenografts. Mice bearing Daoy (A), DaoyPt (B), and DaoyHER2 (C) xenografts received orally either vehicle only or AEE788 at a dosage of 50 mg/kg thrice a week for 4 weeks. Points shown are mean values for groups of 8 to 10 mice. Bars, SE. Drug activity was defined by percentage TVI. (D–F) Effects of AEE788 treatment on the body weight changes of animals bearing Daoy (D), DaoyPt (E), and DaoyHER2 (F) xenografts.
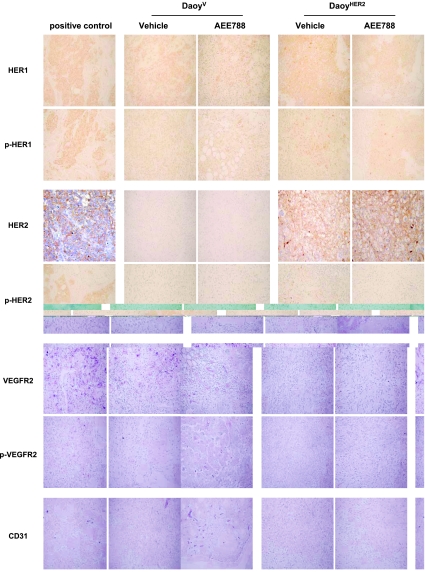
Because of the higher antitumor activity in DaoyHER2 xenografts, we investigated the biologic effects of AEE788 in formalin-fixed specimens from DaoyV and DaoyHER2 tumors at the end of treatment (Figure 5). We evaluated the levels of expression and the phosphorylation status of HER1, HER2, and VEGFR2. In both models, phosphorylated HER1 moderately decreased after treatment, consistently with a decrease in the number of HER1-positive cells. Treated sections showed lymphocytic infiltration and microcystic areas, as a consequence of treatment, as already observed in tumors from other tissues [20]. Expectedly, DaoyHER2 xenografts exhibited a strong and diffuse immunopositivity for HER2, whose phosphorylation levels were decreased by AEE788. In these xenografts, an increase in immunoreactivity against VEGFR2 was also evident, which localized to both endothelial cells of neoformed vessels and tumor cells. In treated tumors, the level of staining was lower because of both a reduction in the number of positive cells and staining intensity. Overall, phosphorylated VEGFR2 was less intense than that of total protein and was reduced by treatment. To confirm neoangiogenesis in DaoyHER2 xenografts, sections from tumors were stained with an antibody against the endothelial marker CD31. Consistent with the VEGFR2 data, a slight increase in CD31 immunoreactivity was only detected in HER2-transfected cells and was reversed by treatment.
Figure 5.
Effects of AEE788 on the expression and phosphorylation/activation status of AEE788-sensitive targets in DaoyV and DaoyHER2 xenografts. Tumors were harvested and processed for immunohistochemical analysis at the end of treatment. Sections were stained for expression of HER1, p-HER1, HER2, p-HER2, VEGFR2, p-VEGFR2 and CD31 (an endothelial cell marker), and counterstained with hematoxylin. Positive controls were as follows: breast cancer for HER1, p-HER1, and p-HER2; ovarian cancer for VEGFR2 and p-VEGFR2; endometrial cancer for HER2; and rat uterus for CD31. Compared with DaoyV xenografts, DaoyHER2 xenografts showed increased expression of total and phosphorylated HER2 and VEGFR2 and of CD31. Treatment of mice with AEE788 decreased CD31 expression and the phosphorylation of HER2 and VEGFR2. Original magnification, x20.
HER2 Expression Positively Correlates with VEGF and VEGFR2 Expression in Human Medulloblastoma Specimens
To establish whether HER2 could be associated with angiogenesis in clinical medulloblastoma, we evaluated the transcriptional expression of angiogenesis-related genes in 21 fresh-frozen surgical samples by RT-qPCR analysis. Therefore, we examined the expression of VEGF, VEGFR2, VEGFR1, bFGF, and TGFα [5]. HER2 expression positively correlated with VEGF (r = 0.61, P < .01), VEGFR2 (r = 0.56, P < .01), and bFGF (r = 0.60, P < .01) but not with VEGFR1 or TGFα (Figure 6).
Figure 6.
HER2 expression correlates positively with some angiogenesis- related genes in human medulloblastoma samples. The transcript expression levels of the indicated genes were measured by RT-qPCR analysis in 21 medulloblastoma samples as previously described. Spearman rank correlation coefficients with P values are shown.
Discussion
In this study we show that AEE788 inhibits the proliferation of different medulloblastoma cell lines, including chemoresistant and HER2-overexpressing cells, in vitro and in vivo, by interfering with EGF- and NRG-mediated signaling pathways. Off-target inhibition of HER3 contributes to AEE788 effects beyond its canonical targets, potentially expanding AEE788's therapeutic applications. In vivo, the antitumor activity of AEE788 is increased in xenografts, with ectopic overexpression of HER2, possibly because AEE788 inhibits both HER2-induced angiogenesis and autocrine signaling mediated by HER2 and de novo expression of VEGFR2 in tumor cells. These data, together with the significantly positive correlation of HER2 with VEGF and VEGFR2 in human medulloblastoma samples, indicate HER2-overexpressing medulloblastoma as the subset that might benefit most from AEE788 treatment.
The sensitivity of medulloblastoma lines to AEE788 as expressed as IC50 values ranged from ∼2 to 4 µM, values below the intratumoral concentrations achievable in vivo [11], indicating that AEE788 could be effective in medulloblastoma at clinically relevant doses. Our in vitro results are in close agreement with those reported in cell lines from other tumors [12,21,22]. However, this is the first report on AEE788 activity on cells with acquired resistance or ectopic overexpression of HER2. Of note, HER2 signaling in our medulloblastoma cells resulted in resistance to platinum compounds, and HER2 overexpression is associated with chemoresistance in medulloblastoma patients [15]. Pleomorphic drug resistance has previously been observed after transfection of HER2 in tumor cells [23], and we found increased expression and activation of endogenous HER2 in chemoresistant cells from glioma and ovarian carcinoma [24]. Our in vivo experiments demonstrated a similar (in Daoy and DaoyPt xenografts) or increased (in DaoyHER2 xenografts) antitumor activity of AEE788. Therefore, AEE788 proves to be able to circumvent chemoresistance resulting from either continuous exposure to drugs or HER2-mediated oncogenic signals, suggesting that AEE788 could be as effective in chemo-naive as in pretreated medulloblastoma patients.
AEE788 effectively prevented EGF-induced phosphorylation of HER1 and transphosphorylation of HER2, concurrently blocking the downstream signaling molecules Akt and ERK1/2. However, although AEE788 is targeted to both HER1 and HER2 with similar affinity in in vitro kinase assays [11], it was scarcely or not effective on constitutively activated HER2, which spontaneously forms by ligand-independent homodimerization in HER2-overexpressing cells [25]. Together, these data indicate that AEE788's inhibitory effects on HER2 phosphorylation in cell-based assays could be mainly due to the blockade of transphosphorylating HER1 rather than to a direct effect on HER2 kinase activity.
Another novel finding of our work is AEE788's capability of blocking NRG-dependent HER3 activation. On binding to NRG, the “kinase dead” HER3 dimerizes with other HER receptors, preferably HER2 [4], functioning as a scaffold to activate the PI3K/Akt pathway because of having multiple p85/p110a docking sites [26]. We found that NRG strongly activated the HER3/PI3K/Akt route in D283 cells that display high levels of endogenous HER2, but it did not do so in DaoyHER2 cells. These data indicate that HER2 in the presence of high HER1 does not redirect cells to NRG signaling and that the inhibitory effects of AEE788 in these cells are mainly due to the blockade of EGF-dependent HER1 activation. By contrast, HER2 overexpression in the presence of low HER1 might switch cells to an NRG-triggered HER3 pathway, which is highly sensitive to inhibition by AEE788.
Although HER3 is not an easily drugable kinase because intrinsically inactive, growing evidence shows that HER3 modulates the response to inhibitors of the HER pathways in cell lines from different tumors [26–28]. Given the importance of HER family trans-signaling in cancer cell biology, research is directing toward agents able to simultaneously inhibit HER1-, HER2-, and HER3-mediated pathways [29]. In this light, AEE788's therapeutic potential could be explored in new and ampler clinical settings.
In vivo, but not in vitro, isogenic HER2 overexpression significantly sensitized cells to AEE788 effects. This dissociation of in vitro and in vivo efficacy is consistent with HER2 inducing host-mediated processes that are sensitive to AEE788 inhibition. A greater tumor growth inhibition in medulloblastoma xenografts with ectopic overexpression of HER2 has previously been reported after treatment with HER inhibitors or antiangiogenesis agents [14,17] and almost exclusively ascribed to the blockade of the increased vascularization induced by HER2 [14]. In keeping with these data, we found neoangiogenesis in DaoyHER2 xenografts as detected by the expression of endothelial-associated VEGFR2 and CD31 that were both reduced by treatment. However, direct effects of AEE788 on tumor cells cannot be excluded. Indeed, AEE788 caused a 50% TVI in Daoy xenografts, in which activation of HER1 signaling only was observed in vivo and was inhibited by the drug. In DaoyHER2 xenografts, in addition to HER1 and HER2 activation, de novo expression of VEGFR2 in tumor cells might contribute to a prosurvival/proliferation signaling in vivo because activated and total VEGFR2 were easily detectable in xenografts but scarcely in vitro. Consistent with our observation, colon carcinoma cells growing in culture did not express VEGFRs, whereas they did in vivo [13]. Therefore, new and/or enhanced oncogenetic signaling, which DaoyHER2 xenografts rely on, could sensitize them to AEE788's inhibitory effects. Factors in the tumor microenvironment, such as cytokines or hypoxia, might upregulate VEGFR2 expression, with molecular mechanisms similar to those described for VEGF [30].
The nonendothelial VEGFR2 expression that has been observed in cell lines and biopsy specimens of different cancers, including medulloblastoma, implies a role for VEGFR2 beyond neovascularization [16,31,32]. In vitro, the VEGF/VEGFR2 system mediates proliferation of medulloblastoma cells [16]. Also, in human medulloblastoma, the concomitant expression of VEGF and receptors in tumor cells suggests that VEGFR2 mediates amitogenic stimulus in response to VEGF[16].
Neoangiogenesis has been correlated with HER2 expression in surgical samples of breast cancer [9]. The correlation that we found between the expression of HER2 and that of the angiogenesis-related genes VEGF, VEGFR2, and bFGF is a novel finding in clinical medulloblastoma and hints at HER2 eliciting an angiogenic signal also in this tumor. However, the lack of correlation between HER2 and VEGFR1 suggests that the HER2-associated VEGFR2 pathway could be related not only to newly formed vessels but also to tumor cells. Indeed, VEGFR2 mediates mitogenesis and survival signaling, whereas VEGFR1 plays a “decoy” function by sequestering VEGF and preventing its interaction with VEGFR2 [6]. Of interest, kinome profiling in pediatric brain tumors revealed a consistent activation of VEGFR2 only in the medulloblastoma samples, which suggests a relevant role for this signaling specifically in this tumor [33].
In summary, we have provided experimental evidence that blockade of HER and VEGFR signaling pathways by AEE788 might have a therapeutic potential in medulloblastoma, mostly in those overexpressing HER2. However, identification of other molecular correlates of AEE788 responsiveness is warranted to prospectively identify tumors that are more likely to benefit from AEE788 treatment.
Acknowledgments
The authors thank Iris Meco for statistical advice, Gabriella Cusano for technical assistance, and Novartis Pharmaceuticals for supplies of AEE788.
Footnotes
This work was supported by Fondazione per l'Oncologia Pediatrica and the Italian Association for the Fight against Neuroblastoma (Pensiero Project). The authors disclose any commercial affiliations or financial interests that may be considered conflicts of interest regarding this article.
References
- 1.Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004;5:209–218. doi: 10.1016/S1470-2045(04)01424-X. [DOI] [PubMed] [Google Scholar]
- 3.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33:407–420. doi: 10.1053/j.seminoncol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Goldman CK, Kim J, Wong WL, King V, Brock T, Gillepsie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klos KS, Wyszomierski SL, Sun M, Tan M, Zhou X, Li P, Yang W, Yin G, Hittelman WN, Yu D. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;66:2028–2037. doi: 10.1158/0008-5472.CAN-04-4559. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D. ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer. 2002;94:2855–2861. doi: 10.1002/cncr.10553. [DOI] [PubMed] [Google Scholar]
- 10.Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5:521–530. doi: 10.1038/ncponc1161. [DOI] [PubMed] [Google Scholar]
- 11.Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J, et al. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- 12.Younes MN, Yazici YD, Kim S, Jasser SA, El-Naggar AK, Myers JN. Dual epidermal growth factor receptor and vascular endothelial growth factor receptor inhibition with NVP-AEE788 for the treatment of aggressive follicular thyroid cancer. Clin Cancer Res. 2006;12:3425–3434. doi: 10.1158/1078-0432.CCR-06-0793. [DOI] [PubMed] [Google Scholar]
- 13.Yokoi K, ThaKer PH, Yazici S, Rebhun RR, Nam DH, He J, Kim SJ, Abbruzzese JL, Hamilton SR, Fidler IJ. Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res. 2005;65:3716–3725. doi: 10.1158/0008-5472.CAN-04-3700. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Gajjar A, Hernan R, Kocak M, Fuller C, Lee Y, McKinnon PJ, Wallace D, Lau C, Chintagumpala M, Ashley DM, et al. Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol. 2004;22:984–993. doi: 10.1200/JCO.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Slongo ML, Molena B, Brunati AM, Frasson M, Gardiman M, Carli M, Perilongo G, Rosolen A, Onisto M. Functional VEGF and VEGF receptors are expressed in human medulloblastomas. Neuro Oncol. 2007;9:384–392. doi: 10.1215/15228517-2007-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meco D, Servidei T, Riccardi A, Ferlini C, Cusano G, Zannoni GF, Giangaspero F, Riccardi R. Antitumor effect in medulloblastoma cells by gefitinib: ectopic HER2 overexpression enhances gefitinib effects in vivo. Neuro Oncol. 2009;11:250–259. doi: 10.1215/15228517-2008-095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knizetova P, Ehrmann J, Hlobilkova A, Vancova I, Kalita O, Kolar Z, Bartek J. Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 2008;7:2553–2561. doi: 10.4161/cc.7.16.6442. [DOI] [PubMed] [Google Scholar]
- 19.Gilbertson RJ, Clifford SC, MacMeekin W, Meekin W, Wright C, Perry RH, Kelly P, Pearson AD, Lunec J. Expression of the ErbB-neuregulin signaling network during human cerebellar development: implications for the biology of medulloblastoma. Cancer Res. 1998;58:3932–3941. [PubMed] [Google Scholar]
- 20.Zannoni GF, Vellone VG, Carbone A. Morphological effects of radiochemotherapy on cervical carcinoma: a morphological study of 50 cases of hysterectomy specimens after neoadjuvant treatment. Int J Gynecol Pathol. 2008;27:274–281. doi: 10.1097/PGP.0b013e31815b1263. [DOI] [PubMed] [Google Scholar]
- 21.Younes MN, Park YW, Yazici YD, Gu M, Santillan AA, Nong X, Kim S, Jasser SA, El-Naggar AK, Myers JN. Concomitant inhibition of epidermal growth factor and vascular endothelial growth factor receptor tyrosine kinases reduces growth and metastasis of human salivary adenoid cystic carcinoma in an orthotopic nude mouse model. Mol Cancer Ther. 2006;5:2696–2705. doi: 10.1158/1535-7163.MCT-05-0228. [DOI] [PubMed] [Google Scholar]
- 22.Goudar RK, Shi Q, Hjelmeland MD, Keir ST, McLendon RE, Wikstrand CJ, Reese ED, Conrad CA, Traxler P, Lane HA, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–112. [PubMed] [Google Scholar]
- 23.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, Schmidt M, Mills GB, Mendelsohn J, Fan Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 24.Servidei T, Riccardi A, Ferlini C, Ferlini C, Riccardi R. Chemoresistant tumor cell lines display altered epidermal growth factor receptor and HER3 signaling and enhanced sensitivity to gefitinib. Int J Cancer. 2008;123:2939–2949. doi: 10.1002/ijc.23902. [DOI] [PubMed] [Google Scholar]
- 25.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res. 2010;16:1373–1383. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2009;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 28.Junttila TT, Akita RW, Parson K, Fields C, Lewis Phillips PC, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, Beck S, Marshall G, Davenport S, Callis R, et al. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB receptor blockade in cancer. Clin Cancer Res. 2010;16:1159–1169. doi: 10.1158/1078-0432.CCR-09-2353. [DOI] [PubMed] [Google Scholar]
- 30.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 31.Keefe SM, Cohen MA, Brose MS. Targeting vascular endothelial growth factor receptor in thyroid cancer: the intracellular and extracellular implications. Clin Cancer Res. 2010;16:778–783. doi: 10.1158/1078-0432.CCR-08-2743. [DOI] [PubMed] [Google Scholar]
- 32.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 33.Sikkema AH, Dicks SH, den Dunnen WF, ter Elst A, Scherpen FJ, Hoving EW, Ruijtenbeek R, Boender PJ, de Wijn R, Kamps WA, et al. Kinome profiling in pediatric brain tumors as a new approach for target discovery. Cancer Res. 2009;69:5987–5995. doi: 10.1158/0008-5472.CAN-08-3660. [DOI] [PubMed] [Google Scholar]