Abstract
This study examined the preparation of high-capacity silica supports containing immobilized protein G. A maximum content of 39 mg protein G/g silica was obtained when using 100 Å pore size silica, followed by 33 mg/g for 50 Å silica and 9.3–24 mg/g for 300–4000 Å silica. The surface coverage of protein G increased with pore size, with a maximum level of 0.037 μmol/m2 being obtained for 4000 Å silica. These supports gave comparable apparent activities (i.e., 30–47% binding to rabbit immunoglobulin G, or IgG), with the highest binding capacities (71–77 mg IgG/g silica) being obtained for 50–100 Å silica.
Keywords: Protein G, Immobilization, Immunoglobulins, Antibodies, Silica, High-capacity supports, Immunoaffinity chromatography
Protein G is a bacterial cell surface protein that is commonly used as a binding agent in affinity chromatography and other methods for the selective adsorption of immunoglobulins and antibodies [1–4]. This protein is produced by groups C and G streptococci and binds to the Fc regions of many types of immunoglobulins, including all subclasses of mouse immunoglobulin G (IgG), rabbit IgG, goat IgG, and human IgG (see Ref. 2 for a most extensive list). Protein G has strong binding to these targets at a neutral or slightly acidic pH (e.g., pH 5–7.5) and yet can be made to release any retained immunoglobulins at pH 2.5–3.0 [2]. These properties have made protein G a popular binding agent for the purification of immunoglobulins and as a secondary binding agent that can be used to adsorb antibodies for use in applications such as immunoaffinity chromatography or immunoassays [1–4].
Over the last decade there have been several examples in which immobilized protein G has been used in small affinity columns or in affinity capture systems for the adsorption of immunoglobulins [5–8]. The small size of many of these columns and affinity sorbents ideally requires that a relatively large amount of protein G be present in a small volume for the effective capture of the desired target [8]. However, no previous studies have determined the maximum amount of protein G that can be placed on common porous supports (e.g., HPLC-grade silica) for such work. The purpose of this report was to estimate the maximum amount of protein G that could be immobilized to silica with various pore sizes and to examine the binding of these supports to immunoglobulins, using rabbit IgG as a model.
All of the supports used in this study were HPLC-grade porous silica with an average particle size of 7 μm and nominal pore sizes of 50, 100, 300, 500, 1000 or 4000 Å (see Supplementary Material for details on the experimental methods, reagents and equipment used in this study). According to the manufacturer, these supports had surface areas of 420, 350, 100, 35, 25 and 10 m2/g, respectively. Recombinant protein G with the albumin binding domain removed was used as the immobilized binding agent. The protein G was immobilized using the Schiff base method [2], which has been shown in previous work to give higher activities for protein G and protein A (i.e., a related bacterial cell wall protein) than other amine-based coupling methods [2,3,9]. At least a two times excess of protein G was present versus the maximum amount that was later found to be immobilized to any of the materials that were examined in this study. After immobilization, part of each support was washed and dried under vacuum, with the dried support then being measured in triplicate by using a bicinchoninic acid protein assay [10], with protein G being utilized as the standard and diol silica as the blank.
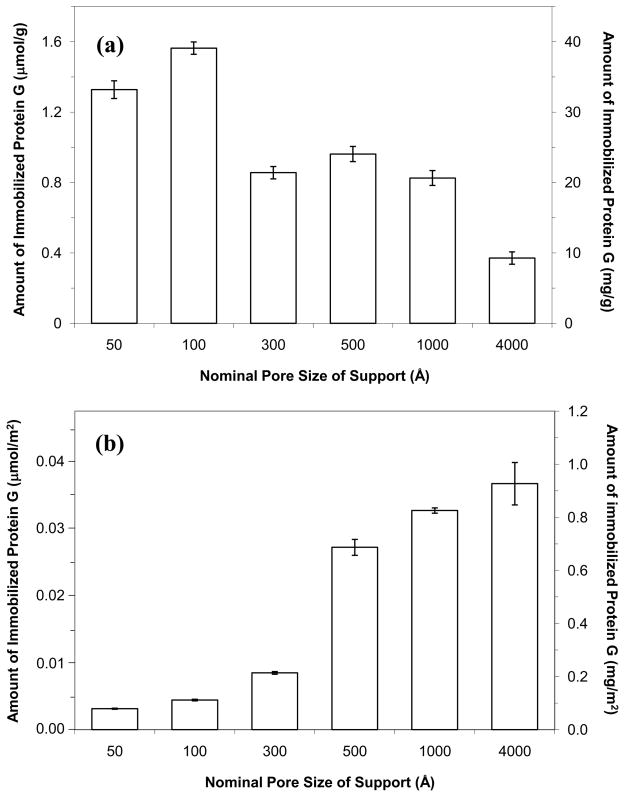
Figure 1(a) shows the maximum amount of protein G that was immobilized onto silica with various pore sizes and when using the Schiff base method. The highest protein content was obtained for the 100 A support, which contained 1.56 (± 0.04) μmol protein G/g silica or 39 (± 1) mg protein G/g silica (note: the numbers in parentheses represent ± 1 S.D. of the mean). The second highest amount was obtained for the 50 Å support, which gave 33 (± 1) mg protein G/g silica. A decrease in the amount of immobilized protein G per gram of support was seen as the pore size increased to 300 through 4000 Å, with roughly 21–24 mg protein G/g silica being obtained for the 300–1000 Å pore size supports and 9.3 mg/g being measured for 4000 Å pore size silica. It has previously been suggested in work with the immobilization of other proteins and in theoretical calculations of effective diffusivity that the optimum pore size for protein immobilization is roughly 3–5 times the diameter of the protein [11,12]. Based on a previous crystal structure and hydrodynamic studies [13,14], the estimated diameter of recombinant protein G with the albumin binding removed was less than 30–35 Å. This size range agreed with the data shown in Figure 1(a), which confirmed that a maximum protein content was obtained when using a support with pores that were roughly three times the diameter or protein G (i.e., as noted when using 100 Å pore size silica).
Figure 1.
Amount of protein G that was immobilized by the Schiff base method to silica with various pore sizes. The results are shown in terms of (a) the moles or mass of protein G that was immobilized per gram of support and (b) the moles or mass that was immobilized per unit surface area of the support. The error bars represent a range of ± 1 S.D. of the mean (n = 3). The pore sizes listed here and throughout this paper are nominal, average values provided by the manufacturer of silica supports. A distribution in these pore sizes is actually present and this distribution may vary slightly from one batch to the next of the same support.
The amount of immobilized protein G was also examined in terms of the quantity that was coupled per unit area for each type of silica. The results are shown in Figure 1(b), which indicate that there was an increase in amount of immobilized protein G per unit area as the pore size of the support was increased. This effect has been observed in previous studies for other proteins (e.g., IgG and Fab fragments) [12] and is a result of the protein being able to access more surface area on the support as the pores increase in size. The use of a support with a pore size of 300 Å or smaller resulted in a large fraction of the total surface area being inaccessible to protein G, thus resulting in a relatively low surface coverage. Silica with a pore size of 500 Å or larger gave a dramatic increase in surface coverage, with a maximum value of 0.037 (± 0.003) μmol/m2 or 0.93 (± 0.08) mg/m2 being measured for 4000 Å pore size silica. This trend fits with previous observations that have been made with other proteins on porous silica [12].
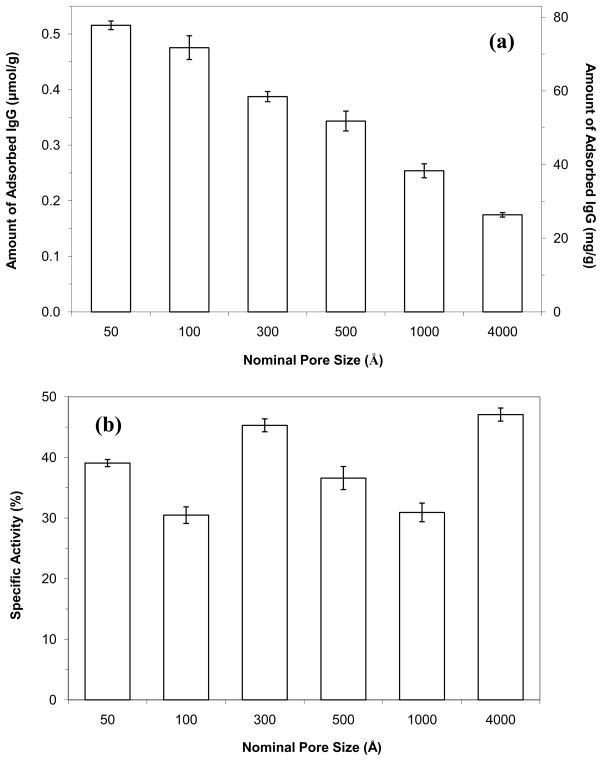
The binding capacity and activity for each protein G support were examined by placing these materials into affinity columns and conducting frontal analysis. Rabbit IgG was used as the model analyte for this work. The results are summarized in Figure 2. In Figure 2(a), the 50 and 100 Å pore size supports gave the largest overall binding capacities for rabbit IgG, with values in the range of 71–77 mg protein per g support (or approximately 32–35 mg/mL for a column packed with these materials). As the pore size of the support was increased to larger values, there was a decrease in binding capacity that followed the general trend noted for the total amount of immobilized protein G in Figure 1(a). The maximum binding capacities obtained in this study were approximately 1.6 to 1.7-fold higher than values listed for commercial supports that contain protein G immobilized to comparable materials.
Figure 2.
Amount of rabbit IgG that was adsorbed to protein G supports prepared by using silica with various pore sizes. The protein G supports that were used in this figure were the same supports examined in Figure 1. The specific activities were calculated by assuming a maximum of one IgG could bind to each protein G molecule. The error bars represent a range of ± 1 S.D. of the mean (n = 3).
The apparent activity of the immobilized protein G in each type of column was found by dividing the measured binding capacity by the total amount of immobilized protein G. These results are given in Figure 2(b). In this case, all of the supports had similar apparent activities in the range of 30–47%. These values indicated that about one out of every three protein G molecules could bind to rabbit IgG when the support was saturated with this target. Some of this protein G may have lost its activity during immobilization (e.g., see similar work with protein A in Ref. [9]). Steric hindrance between neighboring protein G molecules in their binding to IgG would also be expected to account for part of the drop in apparent activity below 100%. The similarity of the apparent activities in Figure 2(a) indicates that steric hindrance arising from pore size effects was not an issue for the adsorption of IgG to protein G that had been immobilized to the 50 to 4000 Å porous silica.
Previous work has examined the amount of rabbit IgG that can be covalently immobilized to the same types of supports that were used in this present study [12]. Both general amine-based coupling methods (e.g., the Schiff base and 1,1′-carbonyldiimidazole techniques) and a site-specific immobilization technique (e.g., the coupling of IgG through oxidized carbohydrate chains in its Fc region to a dihydrazide-activated support) were studied in this previous report. The results for these various methods were fairly comparable, with contents for rabbit IgG of 212 (± 20) mg/g being obtained for 100 Å pore size silica and 255 (± 12) mg/g being reported for 300 Å pore size silica when using site-selective coupling by the dihydrazide method [12]. A comparison of these values with those in Figure 2(a) indicates that the covalent coupling of immunoglobulins/antibodies to these supports can allow for about a three-fold higher maximum coverage of these agents than when using secondary adsorption of the same agents to immobilized protein G.
In summary, this report investigated the immobilization of protein G to silica supports with regards to the maximum protein content that could be obtained for these materials. These data agreed with previous observations that maximum content for an immobilized protein is generally obtained when using a pore size that is 3–5 times the protein’s diameter. The surface coverage of protein G was found to increase with pore size and all supports gave apparent activities in the range of 30–47% for rabbit IgG. Although these binding capacities are lower than those that can be obtained for the covalent immobilization of antibodies, they should still be appropriate for the use of protein G in affinity microcolumns or miniaturized analytical systems [5–8]. In addition, using protein G as a secondary binding agent can allow adsorbed immunoglobulins/antibodies to be released at a mildly acidic pH for column regeneration, and this binding agent can allow a variety of immunoglobulins/antibodies to be adsorbed to the same support [2–8]. The ability of protein G to attach to the Fc region of immunoglobulins is another attractive feature that can aid in obtaining site-selective attachment and high activity for adsorbed antibodies. These properties should continue to make protein G supports, like those developed in this report, valuable tools in immunoaffinity separations, immunoassays and related bioanalytical applications.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health under grant R01 GM044931 and was conducted in facilities that were renovated under NIH grant RR015468-01.
Abbreviations
- IgG
immunoglobulin G
Appendix A. Supplementary Material
Supplementary material associated with this article can be found in the online version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bjorck L, Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984;133:969–974. [PubMed] [Google Scholar]
- 2.Hermanson GT, Mallia AK, Smith PK. Immobilized Affinity Ligand Techniques. Academic Press; Boca Raton: 1992. [Google Scholar]
- 3.Hage DS, Bian M, Burks R, Karle E, Ohnmacht C, Wa C. Bioaffinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. CRC Press; Boca Raton: 2006. pp. 101–126. [Google Scholar]
- 4.Hage DS, Phillips TM. Immunoaffinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. CRC Press; Boca Raton: 2006. pp. 127–172. [Google Scholar]
- 5.Phillips TM. Microanalytical methods based on affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. CRC Press; Boca Raton: 2006. pp. 763–787. [Google Scholar]
- 6.Cole LJ, Kennedy RT. Selective preconcentration for capillary-zone electrophoresis using protein G immunoaffinity capillary chromatography. Electrophoresis. 1995;16:549–556. doi: 10.1002/elps.1150160190. [DOI] [PubMed] [Google Scholar]
- 7.Shen H, Aspinwall CA, Kennedy RT. Dual microcolumn immunoassay applied to determination of insulin secretion from single islets of Langerhans and insulin in serum. J Chromatogr B. 1997;689:295–303. doi: 10.1016/s0378-4347(96)00336-2. [DOI] [PubMed] [Google Scholar]
- 8.Clarke W, Roy Choudhuri A, Hage DS. Analysis of free drug fractions by ultra-fast immunoaffinity chromatography. Anal Chem. 2001;73:2157–2164. doi: 10.1021/ac0009752. [DOI] [PubMed] [Google Scholar]
- 9.Hage DS, Walters RR, Hethcote HW. Split-peak affinity chromatographic studies of the immobilization-dependent adsorption kinetics of protein A. Anal Chem. 1986;58:274–279. doi: 10.1021/ac00293a003. [DOI] [PubMed] [Google Scholar]
- 10.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 11.Gustavsson PE, Larsson PO. Support materials for affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. CRC Press; Boca Raton: 2006. pp. 15–33. [Google Scholar]
- 12.Clarke W, Beckwith JD, Jackson A, Reynolds B, Karle EM, Hage DS. Antibody immobilization to high-performance liquid chromatography supports: characterization of maximum loading capacity for intact immunoglobulin G and Fab fragments. J Chromatogr A. 2000;888:13–22. doi: 10.1016/s0021-9673(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 13.Derrick JP, Wigley DB. Crystal structure of a streptococcal protein G domain bound to an Fab fragment. Nature. 1992;359:752–754. doi: 10.1038/359752a0. [DOI] [PubMed] [Google Scholar]
- 14.Akerstrom B, Bjorck L. A physiological study of protein G, a molecule with unique immunoglobulin G-binding properties. J Biol Chem. 1986;261:10240–10247. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.