INTRODUCTION
Attention deficit/hyperactivity disorder (ADHD) is one of the most common childhood diagnoses. Symptoms, which can continue through adolescence and adulthood, include difficulties in staying focused, paying attention, controlling behavior, and managing hyperactivity or overactivity.1 Children with ADHD may struggle with low self-esteem, troubled relationships, and poor performance in school.1,2 In 2006 in the U.S., ADHD was diagnosed in about 4.5 million children 5 to 17 years of age.3 From 3% to 7% of school-aged children have ADHD. In some studies, estimated rates are higher in community samples.1
Stimulants constitute the first-line treatment for ADHD in children. The response rate is approximately 70%, based on reduced hyperactivity or increased attention, as rated by parents, teachers, or researchers.4,5 At least 80% of children respond to one stimulant medication. For those who do not respond to a particular stimulant because of intolerable side effects, another recommended stimulant medication should be tried.6
Nonstimulants are also used in children or adolescents who show a fair response to stimulants or who experience adverse effects from them. Currently, atomoxetine (Strattera, Lilly) is indicated for the treatment of ADHD in children six years of age and older, teenagers, and adults. Extended-release (long-acting) guanfacine HCl (Intuniv, Shire), approved by the FDA in 2009, is used as part of a treatment program to control symptoms of ADHD in children and adolescents 6 to 17 years of age, but it is not approved for adults with ADHD. Combination therapy comprising both atomoxetine and guanfacine, along with behavioral and psychological interventions, is often beneficial in ADHD.7
CHEMISTRY AND PHARMACOLOGY8
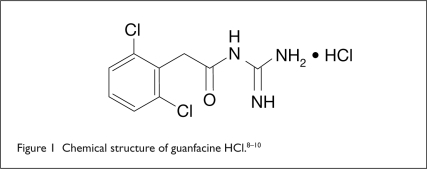
Intuniv is a once-daily, extended-release (ER) matrix tablet formulation of Tenex (Reddy, A. H. Robins).9,10 The chemical designation is N-amidino-2-(2,6-dichlorophenyl) acetamide monohydrochloride. The molecular formula is C9H9Cl2N3O • HCl, and the molecular weight is 282.55. The drug’s chemical structure is shown in Figure 1.
Figure 1.
This selective alpha2A-adrenergic receptor agonist is not a central nervous system (CNS) stimulant. The mechanism of action in ADHD is not known, but the drug appears to work on certain receptors in the prefrontal cortex, a part of the brain where behaviors related to ADHD, such as inattention and impulsiveness, are thought to be controlled.
PHARMACOKINETICS AND PHARMACODYNAMICS8
Guanfacine HCl is absorbed orally, with a bioavailability of 80%. It is taken once daily, usually in the morning, but it should not be taken with high-fat meals. The drug is approximately 70% bound to plasma proteins, and it is widely distributed throughout the body, with a volume of distribution (Vd) of 6.3 L/kg (276–347 L). Fifty percent of the drug is metabolized in the liver, primarily to the glucuronide and sulfate of 3-hydroxyguanfacine, oxidized mercapturic acid derivatives, and other minor metabolites.
Guanfacine HCl is metabolized primarily by cytochrome P-450 (CYP) 3A4. It is a substrate of CYP 3A4 and CYP 3A5, and exposure is affected by inducers and inhibitors of these enzymes.
Renal excretion is 50%, and the elimination half-life is 17 hours (range, 10–30 hours). Patients on dialysis can be given their customary doses of guanfacine HCl because the drug is poorly dialyzed.
This product has an affinity that is 15 to 20 times higher for the alpha2A receptor subtype than for the alpha2B or alpha2C subtypes. As an antihypertensive agent, guanfacine stimulates alpha2A-adrenergic receptors, thereby reducing sympathetic nerve impulses from the vasomotor center to the heart and blood vessels. The result is a decrease in peripheral vascular resistance and a reduced heart rate.
CLINICAL TRIALS
Biederman et al.11
An eight-week, multicenter, randomized, double-blind, placebo-controlled safety and efficacy study of guanfacine ER was conducted in 345 patients from 6 to 17 years of age. The study showed improved total scores on the Attention-Deficit/Hyperactivity Disorder Rating Scale (ADHD–RS) IV from baseline compared with placebo.
After a washout period lasting approximately one week (or as long as five times the half-life of the current ADHD medication that the patient was taking), the following agents were administered:
guanfacine 2 mg/day (n = 87); baseline ADHD–RS IV total score, 36.57 points
guanfacine 3 mg/day (n = 86); baseline ADHD–RS IV total score, 37.15 points
guanfacine 4 mg/day (n = 86); baseline ADHD–RS IV total score, 38.49 points
matched placebo (n = 86)
All patients started taking 1 mg/day. The dose was increased weekly by 1 mg/day until the final assigned dose was reached. The dose was decreased weekly by 1 mg/day after five weeks until a dosage of 2 mg/day was reached in patients who chose to enroll in the open-label extension study or 1 mg/day for one week and then discontinued in those patients who did not enroll in the extension study.
ADHD–RS IV total scores from baseline to the last treatment week of the titration/maintenance period (the primary endpoint) were significantly decreased in the guanfacine ER group (mean reduction, −6.7 points) compared with placebo (mean reduction, −8.9 points; P < 0.0001). In addition, the placebo-adjusted least squares mean endpoint change from baseline was significant as follows:
guanfacine 2 mg/day: −7.7 points; 95% confidence interval (CI), −12.25 to −3.15 points (P = 0.0002)
guanfacine 3 mg/day: −7.95 points; 95% CI, −12.5 to −3.4 points (P = 0.0001)
guanfacine 4 mg/day: −10.39 points; 95% CI, −14.97 to −5.82 points (P < 0.0001)
During the dosage titration/maintenance phase, the highest mean systolic and diastolic blood pressure (BP) changes from baseline, respectively, were as follows:
guanfacine 2 mg/day: −7 and −3.8 mm Hg
guanfacine 3 mg/day: −7 and −4.7 mm Hg
guanfacine 4 mg/day: −10.1 and −7.1 mm Hg
The highest mean pulse rate changes from baseline were −5.7, −8.1, and −8 beats/minute with guanfacine 2 mg/day, 3 mg/day, and 4 mg/day, respectively. Common adverse effects reported in the study were somnolence, fatigue, upper abdominal pain, and sedation. There were no significant changes in mean height or weight in patients receiving guanfacine ER.
Sallee et al.12
The efficacy of guanfacine ER in ADHD was demonstrated in a nine-week, multicenter, randomized, double-blind, placebo-controlled study of 324 patients 6 to 17 years of age. ADHD–RS IV total scores were significantly improved from baseline compared with placebo. Only patients who weighed less than 50 kg (110 pounds) were enrolled.
After a washout period lasting approximately one week (or as long as five times the half-life of the current ADHD medication), 62 patients received guanfacine ER 1 mg/day; 65 patients received 2 mg/day, 65 patients received 3 mg/day, 66 patients received 4 mg/day, and 66 patients received matched placebo.
In the first three study weeks, dosage titration was performed, followed by a three-week maintenance period and a three-week dose-tapering phase. ADHD–RS IV total scores from baseline to the last treatment week of the titration/maintenance period (the primary endpoint) were decreased by 19.6 ± 13.9 points:
1 mg/day, −20.4 points (P = 0.004)
2 mg/day, −18 points (P = 0.018)
3 mg/day, −19.4 points (P = 0.0016)
4 mg/day, −20.9 points (P = 0.0006)
placebo, −12.2 ± 13 points
Placebo-adjusted least squares mean endpoint changes from baseline were significant as follows:
guanfacine 1 mg/day: −6.75 points (P = 0.0041)
guanfacine 2 mg/day: −5.41 points (P = 0.0176)
guanfacine 3 mg/day: −7.34 points (P = 0.0016)
guanfacine 4 mg/day: −7.88 points (P = 0.0006)
Common side effects reported more often in patients who received guanfacine ER, compared with placebo, included somnolence, headache, and fatigue. No significant changes in height or weight were reported for the study drug.
DRUG–DRUG INTERACTIONS8
There was a three-fold increase in the area-under-the-curve (AUC) concentration of guanfacine when it was given with ketoconazole (Nizoral, Janssen). Caution should be used when guanfacine ER is given with other CYP 3A4 and 3A5 inhibitors such as ketoconazole. Patients should be monitored for hypotension, bradycardia, and sedation. Dose adjustments may be needed if guanfacine ER is taken with a CYP 3A4 inducer because the AUC concentration of guanfacine ER is decreased by 70%.
Patients should be monitored for potential CNS side effects when guanfacine ER is given with valproic acid because of increased concentrations of valproic acid with coadministration. Potential additive pharmacodynamic effects (e.g., hypotension and syncope) must be monitored when guanfacine ER is taken with other antihypertensive agents. Sedation and somnolence may be experienced if guanfacine ER is taken with alcohol, sedatives, hypnotics, benzodiazepines, barbiturates, or antipsychotic agents.
MONITORING REQUIREMENTS
In most cases, the American Academy of Pediatrics (AAP) does not recommend the routine use of electrocardiograms or routine subspecialty cardiology evaluations before initiating stimulant therapy to treat ADHD, even though the American Heart Association (AHA) had previously recommended this step to detect cardiac conditions that might put children at risk for sudden cardiac death. Based on AAP and AHA consensus statements, the following cardiac monitoring recommendations have been established to aid clinicians in evaluating children receiving stimulants (including guanfacine) for ADHD:8,13,14
A thorough examination should be conducted before patients begin taking guanfacine for ADHD. Special attention should be given to symptoms that suggest a cardiac condition (e.g., palpitations, near syncope, or syncope).
A complete family and patient history should be obtained to check for conditions associated with sudden cardiac death.
Clinicians should determine whether patients are currently using other prescribed or over-the-counter agents.
Patients should be evaluated for cardiac murmurs, hypertension, physical findings associated with Marfan syndrome, and signs of arrhythmias.
Further evaluation is indicated if the family history, patient history or physical findings suggest cardiac disease during the initial visit or at follow-up visits. If indicated, a pediatric cardiologist should be consulted.
Patients should continue to be assessed for cardiac symptoms and any changes in family history at follow-up visits.
BP and heart rate should be evaluated at the baseline assessment, during routine follow-up visits within one to three months, and at follow-up appointments every six to 12 months.
DOSAGE AND ADMINISTRATION
For children 6 to 17 years of age with ADHD, the starting dose of guanfacine ER is 1 mg once daily; this amount may be increased in increments of 1 mg/week, up to 4 mg, the recommended maximum daily dose. A maintenance dose of 0.05 to 0.08 mg/kg once daily, up to 0.12 mg/kg, may be considered. For patients discontinuing guanfacine ER, the dose should be tapered in decrements of no more than 1 mg every three to seven days. Patients switching from guanfacine immediate-release (IR) must discontinue the IR formulation and should receive a titrated dose of guanfacine ER initially at 1 mg once daily; this may be increased in increments of 1 mg/week up to 4 mg/day.8
The effect of renal impairment on the pharmacokinetics of guanfacine in children has not been studied. In adults with impaired renal function, the cumulative urinary excretion of guanfacine and the renal clearance decreased as renal function decreased. In patients on hemodialysis, dialysis clearance was about 15% of the total clearance. The low dialysis clearance suggests that hepatic metabolism increases as renal function decreases. It may be necessary to adjust the dose in patients with significant renal impairment.8 Few studies show any accumulation of guanfacine in patients with renal failure, and dosing adjustments do not appear warranted.15–17
The effect of hepatic impairment on guanfacine levels in children has not been assessed. In adults, guanfacine is cleared both by the liver and the kidney, and approximately 50% of guanfacine clearance is hepatic. Dose adjustments might be necessary in patients with significant hepatic failure.18
CONTRAINDICATIONS, WARNINGS, AND PRECAUTIONS8
Guanfacine ER is contraindicated in patients with a known hypersensitivity to guanfacine or to any component of the product. Precautions are advised for those with a history of bradycardia, cardiovascular disease, heart block, hypotension, and syncope.
To avoid the risk of rebound hypertension, patients should not stop therapy abruptly. The concomitant use of alcohol and other known depressants, as well as other guanfacine-containing drugs (e.g., Tenex) should be avoided.
Patients should not drive or operate heavy equipment until they know how they respond to guanfacine ER. The safety and efficacy of the drug have not been established in children younger than six years of age. It is unknown whether the drug is effective if used for longer than nine weeks.
ADVERSE REACTIONS8
Most common and dose-related adverse reactions include somnolence, sedation, abdominal pain, dizziness, hypotension or decreased BP, dry mouth, irritability, nausea, decreased appetite, and constipation.
P&T COMMITTEE CONSIDERATIONS
When considering whether to add guanfacine ER (Intuniv) to the hospital formulary, P&T committee members should note that the drug’s efficacy and safety might be comparable to drugs (particularly stimulants) that are already available, without most of the adverse effects. Guanfacine ER will most likely be used on an outpatient basis or for in-patients who have been clinically stabilized with no chest or abdominal pain or other acute medical condition. The drug may be considered a possible alternative for treating ADHD, but it is not expected to replace any agents currently indicated for this condition.
COST
Guanfacine ER (Intuniv) might not offer any economic advantage over other drugs already on the formulary. The approximate price of one tablet (in strengths of 1, 2, 3, and 4 mg) is $5.50 to $5.62.19 Each package contains 100 tablets.
CONCLUSION
Although there is no simple cure for ADHD, some treatments do relieve symptoms effectively. Standard therapies include educational approaches as well as psychological or behavioral modifications with or without medication. A diagnosis of ADHD can provoke anxiety, and symptoms can be a challenge for parents and children alike. However, treatment can make a difference, and most children with ADHD grow up to be vibrant, active, and successful adults. In general, guanfacine ER should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
Footnotes
Disclosure: The author reports no financial or commercial relationships in regard to this article.
REFERENCES
- 1.Attention-deficit and disruptive behavior disorders Diagnostic and Statistical Manual of Mental Disorders 4th rev ed (DSM-IV-TR)Washington, D.C: American Psychiatric Association; 2000 [Google Scholar]
- 2.American Academy of Pediatrics clinical practice guideline: Diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105(5):1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 3.National Health Interview Survey, 2006 (machine-readable data file and documentation) Hyattsville, Md: National Center for Health Statistics; 2007. Available at: www.cdc.gov/nchs/nhis.htm [Google Scholar]
- 4.Miller A, Lee SK, Raina P, et al. A review of therapies for attention deficit hyperactivity disorder Ottawa: Canadian Coordinating Office for Health Technology Assessment; 1998; RFP #ADHD-1196. Available at: www.ccohta.ca/main-e.html Accessed June 29, 2010. [Google Scholar]
- 5.Schachter HM, Pham B, King J, et al. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. Can Med Assoc J. 2001;165(11):1475–1488. [PMC free article] [PubMed] [Google Scholar]
- 6.A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 7.Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 8.Wayne, Pa.: Shire; 2010. Intuniv (guanfacine extended-release oral tablets), product information. [Google Scholar]
- 9.Bridgewater, N.J.: Reddy; 2006. Tenex (guanfacine HCl tablets), product information. [Google Scholar]
- 10.Richmond, Va.: A.H. Robins Co.; 1996. Tenex (guanfacine), product information. [Google Scholar]
- 11.Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(1):e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 12.Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215–226. doi: 10.1089/cap.2008.0080. [DOI] [PubMed] [Google Scholar]
- 13.Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451–453. doi: 10.1542/peds.2008-1573. [DOI] [PubMed] [Google Scholar]
- 14.Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving stimulant drugs: A scientific statement from the American Heart Association Congenital Cardiac Defects Committee of the Council on Cardiovascular Disease in the Young and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407–2423. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- 15.Bennett WM, Aronoff GR, Golper TA, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults. 3rd ed. Philadelphia: American College of Physicians; 1994. [DOI] [PubMed] [Google Scholar]
- 16.Kirch W, Kohler H, Braun W, et al. The influence of renal function on plasma concentration, urinary excretion, and antihypertensive effect of guanfacine. Clin Pharmacokinet. 1980;5(5):476–483. doi: 10.2165/00003088-198005050-00005. [DOI] [PubMed] [Google Scholar]
- 17.Kiechel JR. Pharmacokinetics of guanfacine in patients with impaired renal function and in some elderly patients. Am J Cardiol. 1986;57(9):18E–21E. doi: 10.1016/0002-9149(86)90718-6. [DOI] [PubMed] [Google Scholar]
- 18.Kiechel JR. Pharmacokinetics and metabolism of guanfacine in man: A review. Br J Clin Pharmacol. 1980;10(Suppl 1):25S–32S. doi: 10.1111/j.1365-2125.1980.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guanfacine extended-release tablets. Available at: www.shirepricing.com/Documents/INTUNIV-long-form.pdf Accessed July 8, 2010; and McKesson.