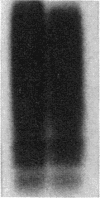
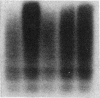
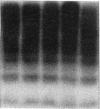
Abstract
Synthetic oligodeoxynucleotides complementary to the break-point junction of bcr-abl transcripts selectively inhibit the proliferation of Philadelphia-positive leukemic cells, but residual leukemic cells persist in antisense oligodeoxynucleotides-treated cultures. Cyclophosphamide derivatives such as mafosfamide and 4-hydroperoxycyclophosphamide are used at high doses for purging of Philadelphia leukemic cells from marrows but such treatment can be associated with delayed engraftment and prolonged cytopenias. To develop a more effective procedure that might optimize the killing of leukemia cells and the sparing of normal hematopoietic progenitor cells, a 1:1 mixture of Philadelphia leukemic cells and normal bone marrow cells was exposed to a combination of a low dose of mafosfamide and bcr-abl antisense oligodeoxynucleotides and assayed for growth ability in clonogenic assays and in immunodeficient mice. Bcr-abl transcripts were not detected in residual colonies, and cytogenetic analysis of individual colonies revealed a normal karyotype. Normal but not leukemic hematopoietic colonies of human origin were also detected in marrows of immunodeficient mice 1 mo after injection of the treated cells. Our results indicate that a combination of a conventional chemotherapeutic agent and a tumor-specific antisense oligodeoxynucleotide is highly effective in killing leukemic cells and in sparing a much higher number of normal progenitor cells as compared with high-dose mafosfamide treatment. This offers the prospect of a novel and more selective ex vivo treatment of chronic myelogenous leukemia.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apperley J. F., Mauro F. R., Goldman J. M., Gregory W., Arthur C. K., Hows J., Arcese W., Papa G., Mandelli F., Wardle D. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol. 1988 Jun;69(2):239–245. doi: 10.1111/j.1365-2141.1988.tb07628.x. [DOI] [PubMed] [Google Scholar]
- Bortin M. M., Horowitz M. M., Gale R. P. Current status of bone marrow transplantation in humans: report from the International Bone Marrow Transplant Registry. Nat Immun Cell Growth Regul. 1988;7(5-6):334–350. [PubMed] [Google Scholar]
- Bron D., Stryckmans P. Removal of tumour cells from bone marrow: overview. Eur J Cancer Clin Oncol. 1989 Feb;25(2):163–166. doi: 10.1016/0277-5379(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Butturini A., Gale R. P. How can we cure leukaemia? Br J Haematol. 1989 Aug;72(4):479–485. doi: 10.1111/j.1365-2141.1989.tb04309.x. [DOI] [PubMed] [Google Scholar]
- Carlo-Stella C., Mangoni L., Piovani G., Almici C., Garau D., Caramatti C., Rizzoli V. In vitro marrow purging in chronic myelogenous leukemia: effect of mafosfamide and recombinant granulocyte--macrophage colony-stimulating factor. Bone Marrow Transplant. 1991 Oct;8(4):265–273. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claxton D., Suh S. P., Filaccio M., Ellerson D., Gaozza E., Andersson B., Brenner M., Reading C., Feinberg A., Moen R. Molecular analysis of retroviral transduction in chronic myelogenous leukemia. Hum Gene Ther. 1991 Winter;2(4):317–321. doi: 10.1089/hum.1991.2.4-317. [DOI] [PubMed] [Google Scholar]
- Coulombel L., Kalousek D. K., Eaves C. J., Gupta C. M., Eaves A. C. Long-term marrow culture reveals chromosomally normal hematopoietic progenitor cells in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. N Engl J Med. 1983 Jun 23;308(25):1493–1498. doi: 10.1056/NEJM198306233082502. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Degliantoni G., Mangoni L., Rizzoli V. In vitro restoration of polyclonal hematopoiesis in a chronic myelogenous leukemia after in vitro treatment with 4-hydroperoxycyclophosphamide. Blood. 1985 Mar;65(3):753–757. [PubMed] [Google Scholar]
- Dubé I. D., Arlin Z. A., Kalousek D. K., Eaves C. J., Eaves A. C. Nonclonal hemopoietic progenitor cells detected in long-term marrow cultures from a Turner syndrome mosaic with chronic myeloid leukemia. Blood. 1984 Dec;64(6):1284–1287. [PubMed] [Google Scholar]
- Fouillard L., Van Den Akker J., Laporte J. P., Najman A., Perot C., Lopez M., Douay L., Isnard F., Taillemite J. L., Gorin N. C. Autogreffe de moelle osseuse traitée par mafosfamide en phase d'accélération de leucémie myéloïde chronique. Inversion de l'évolution clinique. Presse Med. 1989 Nov 4;18(36):1785–1788. [PubMed] [Google Scholar]
- Goldman J. M., Lu D. P. New approaches in chronic granulocytic leukemia--origin, prognosis, and treatment. Semin Hematol. 1982 Oct;19(4):241–256. [PubMed] [Google Scholar]
- Gorin N. C., Aegerter P., Auvert B., Meloni G., Goldstone A. H., Burnett A., Carella A., Korbling M., Herve P., Maraninchi D. Autologous bone marrow transplantation for acute myelocytic leukemia in first remission: a European survey of the role of marrow purging. Blood. 1990 Apr 15;75(8):1606–1614. [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Kamel-Reid S., Dick J. E. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988 Dec 23;242(4886):1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- Lapidot T., Pflumio F., Doedens M., Murdoch B., Williams D. E., Dick J. E. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992 Feb 28;255(5048):1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- Mortensen B. T., Ernst P., Philip P. Mafosfamide (ASTA-Z-7654) in vitro treatment of bone marrow does not eradicate Philadelphia-positive cells in chronic myeloid leukaemia. Eur J Haematol. 1988 Sep;41(3):218–222. doi: 10.1111/j.1600-0609.1988.tb01184.x. [DOI] [PubMed] [Google Scholar]
- Negrin R. S., Kiem H. P., Schmidt-Wolf I. G., Blume K. G., Cleary M. L. Use of the polymerase chain reaction to monitor the effectiveness of ex vivo tumor cell purging. Blood. 1991 Feb 1;77(3):654–660. [PubMed] [Google Scholar]
- Nicolaides N. C., Gualdi R., Casadevall C., Manzella L., Calabretta B. Positive autoregulation of c-myb expression via Myb binding sites in the 5' flanking region of the human c-myb gene. Mol Cell Biol. 1991 Dec;11(12):6166–6176. doi: 10.1128/mcb.11.12.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro L., Matera L., Ritz J., Levis A., Palumbo A., Biagini G. Establishment of a Ph1-positive human cell line (BV173). J Natl Cancer Inst. 1983 Mar;70(3):447–453. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schiffer C. A., Aisner J., Dutcher J. P., Wiernik P. H. Sustained post-transfusion granulocyte count increments following transfusion of leukocytes obtained from donors with chronic myelogenous leukemia. Am J Hematol. 1983 Aug;15(1):65–74. doi: 10.1002/ajh.2830150108. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Singer J. W., Arlin Z. A., Najfeld V., Adamson J. W., Kempin S. J., Clarkson B. D., Fialkow P. J. Restoration of nonclonal hematopoiesis in chronic myelogenous leukemia (CML) following a chemotherapy-induced loss of the Ph1 chromosome. Blood. 1980 Sep;56(3):356–360. [PubMed] [Google Scholar]
- Skorski T., Szczylik C., Ratajczak M. Z., Malaguarnera L., Gewirtz A. M., Calabretta B. Growth factor-dependent inhibition of normal hematopoiesis by N-ras antisense oligodeoxynucleotides. J Exp Med. 1992 Mar 1;175(3):743–750. doi: 10.1084/jem.175.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczylik C., Skorski T., Nicolaides N. C., Manzella L., Malaguarnera L., Venturelli D., Gewirtz A. M., Calabretta B. Selective inhibition of leukemia cell proliferation by BCR-ABL antisense oligodeoxynucleotides. Science. 1991 Aug 2;253(5019):562–565. doi: 10.1126/science.1857987. [DOI] [PubMed] [Google Scholar]
- Takahira H., Nishimura J., Shibata K., Hirata J., Umemura T., Nawata H. Lineage non-specific down regulation of P210bcr/abl in the CML cell line, KU-812-F, during differentiation. Leuk Res. 1990;14(9):801–808. doi: 10.1016/0145-2126(90)90074-j. [DOI] [PubMed] [Google Scholar]
- Talpaz M., McCredie K. B., Mavligit G. M., Gutterman J. U. Leukocyte interferon-induced myeloid cytoreduction in chronic myelogenous leukemia. Blood. 1983 Sep;62(3):689–692. [PubMed] [Google Scholar]
- Thomas E. D., Clift R. A., Fefer A., Appelbaum F. R., Beatty P., Bensinger W. I., Buckner C. D., Cheever M. A., Deeg H. J., Doney K. Marrow transplantation for the treatment of chronic myelogenous leukemia. Ann Intern Med. 1986 Feb;104(2):155–163. doi: 10.7326/0003-4819-104-2-155. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhan A. G., Humphries R. K., Eaves C. J., Barnett M. J., Phillips G. L., Kalousek D. K., Klingemann H. G., Lansdorp P. L., Reece D. E., Shepherd J. D. Detection of breakpoint cluster region- negative and nonclonal hematopoiesis in vitro and in vivo after transplantation of cells selected in cultures of chronic myeloid leukemia marrow. Blood. 1990 Dec 1;76(11):2404–2410. [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Nucleotide sequence heterogeneity of alpha satellite repetitive DNA: a survey of alphoid sequences from different human chromosomes. Nucleic Acids Res. 1987 Sep 25;15(18):7549–7569. doi: 10.1093/nar/15.18.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. M., Yeager A. M. Predictive value of colony-forming unit assays for engraftment and leukemia-free survival after transplantation of chemopurged syngeneic bone marrow in rats. Exp Hematol. 1991 Mar;19(3):179–184. [PubMed] [Google Scholar]
- van Denderen J., van der Plas D., Meeuwsen T., Zegers N., Boersma W., Grosveld G., van Ewijk W. Immunologic characterization of the tumor-specific bcr-abl junction in Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1990 Jul 1;76(1):136–141. [PubMed] [Google Scholar]