Abstract
Breast cancer is the second leading cause of malignant effusions in cancer patients. Pleural effusion indicates incurable disease with limited palliative treatment options and poor outcome. Here, we demonstrate the therapeutic efficacy of measles virus (MV) vaccine strain derivative against malignant pleural effusion in an MDA-MB-231 xenograft model of advanced breast cancer. Both systemic intravenous (i.v.) and intrapleural (t.t.) administered virus caused massive infection and syncytia formation in the pleural tumor deposits. Intrapleural administration of 1.5 × 106 plaque-forming units (PFU) total dose of MV significantly improved median survival by approximately 80% compared to the control animal group. Furthermore, we tested human dendritic cells as carriers for delivery of oncolytic MV infection to breast cancer pleural metastases. Carrier-delivered MV infection prevented accumulation of the pleural exudate and also significantly improved the survival of the treated mice. This is the first demonstration of the therapeutic potential of oncolytic virotherapy against malignant pleural effusions in a pre-clinical model of advanced breast cancer.
Keywords: Measles virus, Pleural effusion, Breast cancer, Oncolytic virotherapy
Introduction
Malignant pleural effusion is a frequent complication of advanced cancer with more than 200,000 cases diagnosed annually in the USA [1]. Exudates accumulate as a result of increased production and obstructed resorption following malignant cell infiltration and growth in the pleural cavity [2]. Lung and breast cancer are the leading cause of malignant pleural effusion; other causes include hematologic malignancies, ovarian cancer, gastrointestinal tract neoplasms, and primary pleural mesothelioma. Development of pleural effusions due to the metastatic involvement of the pleura is associated with incurable disease and poor outcome. Advanced breast carcinoma is the second most common cause (>20%) of malignant effusions [3]. Although a variety of surgical methods and chemotherapeutic agents have been tested, the treatment is largely palliative, including symptomatic control and chemically induced pleurodesis to prevent exudate accumulation [4]. Development of more aggressive alternative therapeutic approaches to manage malignant effusions and to improve the quality of life of breast cancer patients with advanced disease is needed.
Oncolytic virotherapy has been recently approved for the treatment of cancer patients in Asia and multiple phase I and II clinical trials are ongoing in the USA. Measles virus (MV), a member of the Paramyxoviridae family, is a virus with a lipoprotein envelope and negative-strand, non-segmented RNA genome [5]. Replication competent derivatives of MV Edmonston vaccine strain have shown considerable anti-tumor effect in preclinical models [6–11]. These viruses enter through the natural measles receptors CD46 and CD150 [12] and spread by cell–cell fusion causing giant syncytia formation and killing of the tumor cells. Attenuated MV strains demonstrated potent oncolytic activity against human cancer xenografts in immunocompromized mice, including subcutaneous breast cancer tumors [13]. An important mechanism which explains the selectivity of the measles virus vaccine strains against tumor cells, including breast cancer cells, pertains to overexpression of the measles virus receptor CD46 in tumor cells [13, 14], with low expression levels in normal cells [7, 9, 14]. Clinical activity of MV has been demonstrated in patients with cutaneous T cell lymphoma treated intratumorally with live Edmonston-Zagreb vaccine strain [15]. Currently, phase I clinical trials utilizing oncolytic MV are ongoing for patients with multiple myeloma, glioblastoma, and advanced ovarian cancer [11].
Here, we examined a novel approach for the treatment of malignant pleural effusion in cancer patients utilizing replication competent MV as an anticancer agent. We developed a reproducible pleural effusion model of advanced breast cancer in mice by implantation of human tumor cells in the pleural cavity. MV administered either intrapleurally (t.t) or systemically caused massive infection of pleural tumor deposits. T.t. therapeutic injections of oncolytic MV or MV-infected primary human dendritic cell (DC) carriers significantly improved the survival of animals bearing pleural tumor xenografts.
Materials and methods
Cell lines, viruses, and vectors
Vero cells, human monocytic U-937, and human breast cancer cell lines MCF-7, MDA-MB-231, and ZR-75-1 were derived from ATCC, Rockville, MD. The cells were maintained in DMEM culture medium, supplemented with 10% fetal bovine serum and antibiotics (Invitrogen, Carlsbad, CA). The U-937 line was grown in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) with 10% fetal bovine serum. Red fluorescent protein expressing clones of MDA-MB-231 cells (MDA-231-RFP) were generated using a lentiviral vector as described previously [16]. MDA-MB-231 cells expressing firefly luciferase (F-lu) were produced following transduction with the pSIN-CSGWdlNotl lentivector encoding F-lu reporter (pSIN-Luc). The vector was generated by deleting one of the two NotI sites in the GFP-expressing HIV vector construct, pSIN-SEW [17], which allowed one-step cloning of genes of interest between the BamHI and NotI restriction sites. The firefly luciferase gene was amplified by PCR primers, and cloned into the BamHI and NotI sites of pSIN-CSGWdlNotI to generate pSIN-Luc. The firefly luciferase-expressing lentiviral vector was produced by standard 3 plasmid transfection in 293T cells as described previously [18].
The MDA-231-lu cells were used for animal engraftment and in vitro experiments. Green fluorescent protein (GFP) expressing MV (MV-GFP) was propagated and titrated on Vero cells as described [19, 20]. Rescue of the MV encoding firefly luciferase reporter (MV-Luc) has been reported recently [11]. MV titer was calculated in both PFU and tissue culture infectious dose 50% (TCID50) per ml.
Human dendritic cell isolation and serum collection
Collection of blood samples from healthy volunteers was approved by Institutional Review Board. Peripheral blood mononuclear cells were obtained with a post-collection leucoreduction system as previously described [21]. The monocytic population was isolated by CD14 positive selection using CD14 microbeads (Miltenyi Biotec, Auburn, CA). The cells were cultured with human recombinant GM-CSF (R & D Systems, Minneapolis, MN). Mature DCs were produced by the stimulation of CD14+ cells with growth factors as described previously [21, 22]. Serum samples were collected from 9 AB, Rh+ healthy individuals and were heat-inactivated at 56°C for 30 min. Neutralizing antibody titer against MV-GFP was determined by virus neutralization (VN) test on Vero cells as described previously [23].
All sera had protective antibody titers (Supplementary Table 1). Serum HS-935 with more than 1:2,500 titer in plaque reduction neutralization test 50% (PNT50) was used for passive immunization of mice in experiments in vivo.
Assessment of the MV antitumor effect in vitro
MDA-MB-231 and MDA231-lu-P4 cells isolated from lung metastases in mice were transferred to 96-well plate at density of 104 per well in 0.1 ml culture medium. The cells were inoculated at a multiplicity of infection of 1.0 using MV-GFP diluted in Opti-MEM (Invitrogen). Cell viability was determined at 24, 48, 72 h and 7 days post infection using an MTT proliferation assay (ATCC, Rockville, MD). Uninfected or inoculated with inactivated virus Vero cells were used as control.
Assessment of cell-mediated transfer of MV infection in vitro
U-937 monocytic cells, immature (IDC) or mature (MDC) dendritic cell carriers were inoculated with MV-GFP (at a multiplicity of infection 0.5) of for 18 h. The efficiency of infection of cell carriers was determined 48–72 h later by fluorescent microscopy. Infected MDCs were treated with 1:10 diluted human serum to neutralize all non-internalized virions on the cell surface. After washing ×3 in Opti-MEM, the carriers were transferred to monolayers of MCF-7 breast cancer cells in the presence of different dilutions of AB group-pooled human serum (Sigma-Aldrich). Human serum HS-935 isolated from a AB+ blood group donor with a high MV neutralizing antibody titer was also used in the experiment. MV-GFP alone was used as a control. The number of GFP positive syncytia per well were counted after 48-h incubation and compared to those of control wells incubated without antibodies. Efficiency of carrier-based delivery of MV-GFP infection in the presence of neutralizing antibodies was compared to that of free MV-GFP as described previously [16].
Animals and in vivo experiments
Female athymic nude mice were purchased from Harlan, Indianapolis IN. All in vivo experiments were approved by the Mayo Foundation Institutional Animal Care and Use Committee.
Development of a pleural effusion xenograft model
Third or fourth in vivo passage MDA231-lu cells (MDA231-lu-P3 and MDA231-lu-P4) were isolated from lung metastatic nodules in i.v. engrafted in mice. In order to develop a metastatic pleural effusion model, 106 exponentially grown cells in 10 μl phosphate buffered saline (PBS) were implanted by fine needle t.t. injection in the left pleural cavity of 4–5 week-old female nude mice. Successful engraftment was confirmed by live body imaging. Three days after tumor cell implantation, mice were anesthetized and injected intraperitoneally (i.p.) with the F-lu substrate D-luciferin (Gold Biotechnology, St. Louis, MO). Images were taken using Xenogen Ivis 200 System (Caliper Life Sciences, Hopkinton, MA). F-lu expressing breast cancer cells MCF-7 and ZR-75-1 were also implanted in the pleural space of nude mice and tumor growth was monitored in the same way as for the MDA-231-lu xenografts.
Assessment of MV infection, and in vivo efficacy of measles virotherapy in pleural effusion xenograft models
Four–five-week-old female nude mice (five per group) were implanted by t.t. injection of MDA-231-lu-P3 and engraftment was verified by bioluminescence imaging as described above. Advanced pleural tumors were treated on day 25 by a single systemic i.v. or local t.t. injection of 106 PFU of MV-GFP. Mice were sacrificed on day 4, 5, 7, and 10 after treatment and pleural fluid was collected. Pleural effusion cells and tumor deposits in the pleural cavity were examined by fluorescent microscopy for MV-GFP infection. Tumor cells and pleural fluid were overlaid on Vero cells for virus isolation.
In a separate experiment, groups of five female nude mice were engrafted t.t. with RFP expressing MDA-231-RFP cells. MV-Luc was injected i.v. or t.t. on day 20 in a single dose of 106 PFU. Pleural tumor infection was monitored by in vivo imaging using the Xenogen machine.
To assess the antitumor activity of this approach in vivo, 4–5-week-old female nude mice were engrafted as described above. On day 3, mice were imaged and assigned to groups in order to ensure comparable bioluminescence intensity of pleural xenografts among the treated animals. Mice were treated either with three repeated t.t. injections of 5 × 105 PFU (in 50 μl volume) on days 5, 13, and 19 or five i.v. injections of 106 PFU MV-GFP (in 100 μl) on days 5, 9, 13, 16, and 19. Control groups were injected with PBS. Criteria to sacrifice the animals were weight loss >20% of the body weight, difficult breathing, and cyanosis.
MV and MV-infected cell carrier therapy in passively immunized animals
Mice were engrafted by t.t. implantation of 106 MDA231-lu-P4 cells and groups of equal bioluminescence intensity were arranged after bioluminescence imaging on day 3. Strongly neutralizing AB blood group human serum (HS-935) was used for passive immunization. On day 3, mice were injected i.p. with 250 μl heat-inactivated serum. Passively immunized animals were bled before the first therapeutic injection and measles protective titer was determined by VN test. Immunization with 100 μl human serum was repeated before the second and third MV-GFP injections. Survival of the treated mice was compared to the passively immunized untreated group or control animals injected with inactivated virus. In a pilot experiment, MDC carrier cells were inoculated with MV-GFP (multiplicity of infection 2.0) for 18–24 h and washed 3–4 times in PBS before injection. Mice were treated with three repeated t.t. injections of 106 PFU of MV-GFP or 106 MV-GFP-infected carriers in 50 μl PBS on day 5, 13, and 19. Therapeutic efficacy was compared to control groups injected with PBS or uninfected MDCs.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA). Survival curves were plotted according to the Kaplan–Meyer method, and survival times of the treated groups were compared using the logrank test. A P value of <0.05 was considered significant.
Results
MV has significant anti-tumor effect against human breast cancer cells in vitro
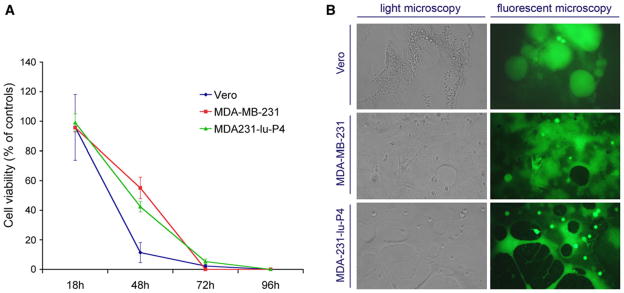
Two days post inoculation at a multiplicity of infection 1.0, MV-GFP caused 100% infection of MDA-MB-231 monolayers with formation of giant multinucleated syncytia. Cell viability was reduced by approximately 50% at 48 h and all tumor cells were killed 72–96 h after MV infection (Fig. 1). No difference in the killing curves was observed between the MDA-MB-231 line and lung metastatic derivative MDA231-lu-P4 cells.
Fig. 1.
MV-GFP in vitro killing effect in breast cancer cells MDA-MB-231 and MCF-7 and control Vero cells. Results from MTT cell viability assay are shown in (a). 48 h post inoculation, MV-GFP-induced giant syncytia formation in breast cancer cell monolayers (b). Light microscopy pictures are shown with corresponding fluorescent microscopy images
Dendritic cells can transfer MV infection in the presence of neutralizing antibodies
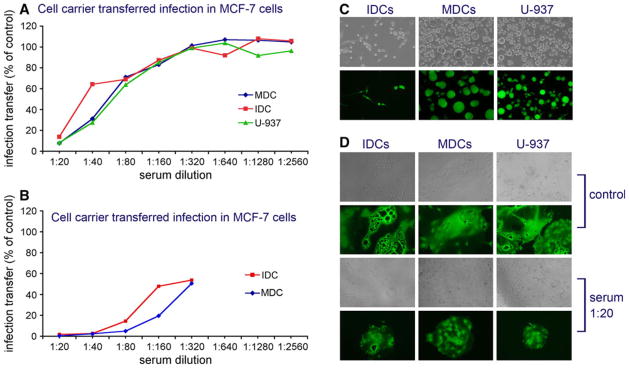
MV-infected MDCs were more efficient than cell-free virions in infecting MCF-7 and MDA-MB-231 breast cancer monolayers in the presence of human neutralizing serum. Results using IDC and MDC carriers were similar to those of the control U-937 monocytic line (Fig. 2a). Neutralization titer for MV infection in breast cancer cells was higher than the VN titer for Vero cells. Heterofusion inhibition 50% (HFI50) by the pooled AB+ serum was similar (1:42–1:64) for the different carriers (Table 1). When the highly neutralizing single donor serum HS-935 was employed, cell-mediated delivery by dendritic cells was inhibited to a somewhat greater extend (1:196 and 1:264 for IDCs and MDCs, respectively, Fig. 2b). Nevertheless, all carriers were able to transfer MV infection by heterofusion in the presence of more than 20-fold higher serum concentration compared to infection by free virions (Table 1). The size as of MV-induced syncytia in the target MCF-7 cells was not affected significantly by the presence of neutralizing antibodies in 1:20 dilution (Fig. 2d).
Fig. 2.
Cell carrier-based in vitro transfer of MV-GFP infection to MCF-7 breast cancer cells in the presence of neutralizing antibodies. MDC, IDC, or control U-937 monotypic cell carriers were overlaid on the MCF-7 monolayers and incubated for 2 days in the presence of different dilutions of pooled human AB+ serum (a) or highly neutralizing AB+ serum HS-935 (b). Results are presented as percent carrier transferred infection in the presence of serial serum dilutions, as compared to controls (no antibody). Infection and syncytia formation in infected carrier cells are shown in (c). Although the heterofusion efficiency was reduced more than 80% by the 1:20 diluted pooled serum (see a), the size of syncytia in MCF-7 cells after transfer of MV-GFP infection by the cell carriers was not significantly affected (d). Virus neutralization of the free MV-GFP virions is shown in Table 1
Table 1.
MV neutralization and heterofusion inhibition antibody titer of the pooled AB+ blood group human serum for different cell lines and dendritic cell carriers
| Test | VN100 or HFI100 | PNT50 or HFI50 |
|---|---|---|
| MV-GFP | ||
| VN on Vero cells | 1:80 | 1:522 |
| VN on MDA-MB-231 | 1:320 | 1:2250 |
| VN on MCF-7 | 1:160 | 1:1557 |
| MV-GFP-infected cell carriers | ||
| IDC/MCF-7 | < 1:20 | 1:42 |
| MDC/MCF7 | < 1:20 | 1:59 |
| U-937/MCF-7 | < 1:20 | 1:64 |
First three rows show VN and PNT50 in Vero cells and two breast cancer lines. The next three rows show heterofusion inhibitory titer in dendritic cells (IDCs or MDCs) and the control U-937 monocytic line VN100 complete (100%) virus neutralization, HFI100 complete heterofusion inhibition, VN virus neutralization, IDC immature dendritic cells, MDC mature dendritic cells, PNT50 plaque reduction neutralization titer 50%, HFI50 serum dilution that reduced with 50% cell-mediated transfer of infection
Metastatic MDA-MB-231 cells grow aggressively causing pleural effusions in t.t.-engrafted animals
Three human breast cancer lines were tested for their ability to grow in the pleural cavity of immunocompromized mice. The cells were transduced with self-inactivating lentiviral vectors for stable expression of F-lu. Reporter gene expression allowed in vivo monitoring of tumor burden using quantitative bioluminescence imaging. The MCF-7 and ZR-75-1 lines were successfully implanted with tumor implants persisting for more than 3 months in the pleural space of the animals. Both lines failed to produce symptomatic disease and pleural exudates, despite the fact that the MCF-7 line has been originally isolated from a patient with pleural effusion. Estrogen-independent MDA-MB-231 breast cancer cells are known to cause metastatic xenograft tumors. We were able to isolate a highly aggressive sub-population of cells from a lung metastatic nodule by serial i.v. passages in mouse lungs. Stable reporter gene expression by MDA231-lu cells was verified after cloning from a single cell per well in 96-well plates and subsequent F-lu assay (data not shown). MDA231-lu-P3 pleural tumors implanted via t.t. injection induced massive accumulation of exudate, associated with respiratory distress and severe weight loss. Mice succumbed or required euthanasia within 5–7 weeks after engraftment (Supplementary Fig. 1). Organ examination revealed small tumor nodules growing on the parietal or visceral pleura and massive pleural effusion with abundant tumor cells. Some animals developed hemorrhagic exudates.
MV caused massive infection in the pleural tumor deposits following both systemic and t.t. administration
Athymic nude mice with advanced MDA231-lu-P3 pleural tumors were treated by a single injection of 106 PFU of MV-GFP via i.v. or t.t. route on day 25 post-implantation. Direct examination of malignant effusions revealed large number of GFP positive tumor cells and giant MV-induced syncytia. Both routes of administration efficiently delivered oncolytic MV to thoracic or visceral pleura tumor deposits. Infection persisted for more than 7 days and replicating virus was recovered by Vero cell overlay (Fig. 3a, c). Massive infection of pericardial metastases was observed in i.v.-treated mice (Fig. 3b, d) indicating that distant tumor lesions were accessible for systemic MV delivery.
Fig. 3.
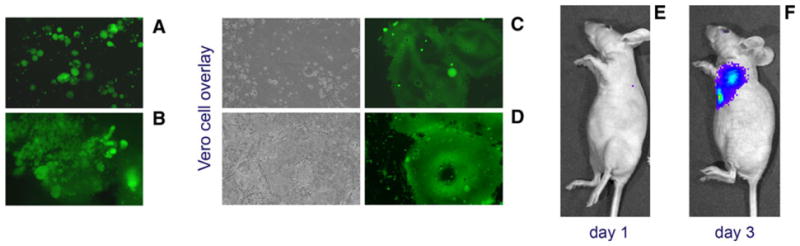
In vivo MV-GFP infection of pleural MDA231-lu-P3 tumor deposits and breast cancer cells in the pleural effusion. Massive infection and large syncytia in cancer cells floating in the pleural fluid was observed on day 7 after t.t. injection of the virus (a). Systemic i.v. injection of MV-GFP caused diffuse infection in pericardial tumor deposits (b). Pleural fluid and tumor samples were collected and examined directly by fluorescent microscopy (a and b). Infected cancer cells from pleural fluid and tumor deposits were isolated and MV was recovered after overlaying on Vero cells (c and d). T.t. treatment with MV construct expressing F-lu caused progressive infection in the MDA-231-RFP pleural tumors as demonstrated by bioluminescence imaging on day 1 (e) and day 3 (f)
The F-lu reporter allowed in vivo monitoring of the bio-distribution of viral infection in mice using bioluminescence imaging. Rapid spread of infection in the pleural MDA-231-RFP tumor deposits was observed on days 1–3 after a single t.t. injection of 106 PFU of MV-Luc (Fig. 3e, f).
MV therapy delayed malignant effusion accumulation and prolonged survival of mice bearing pleural breast cancer xenografts
MDA231-lu-P4 cells were implanted by t.t. injection and tumor growth in the pleural cavity was verified using bioluminescence imaging (Fig. 4a). Local t.t. therapy with a total dose of 1.5 × 106 PFU of MV-GFP significantly improved survival by approximately 80% (Fig. 4b). More specifically, median survival for the treated group was 54.5 days versus 30.5 days for control animals (P = 0.001). While not statistically significant, a strong protective trend against malignant effusion was also observed after systemic virus administration (Fig. 4c). Five repeated i.v. injections of MV-GFP prolonged the survival by 45.5% (from 22 to 32 days). Persistent MV infection at the tumor lesions was detected in all animals tested, and the virus was isolated on Vero cell monolayers (Fig. 4d).
Fig. 4.
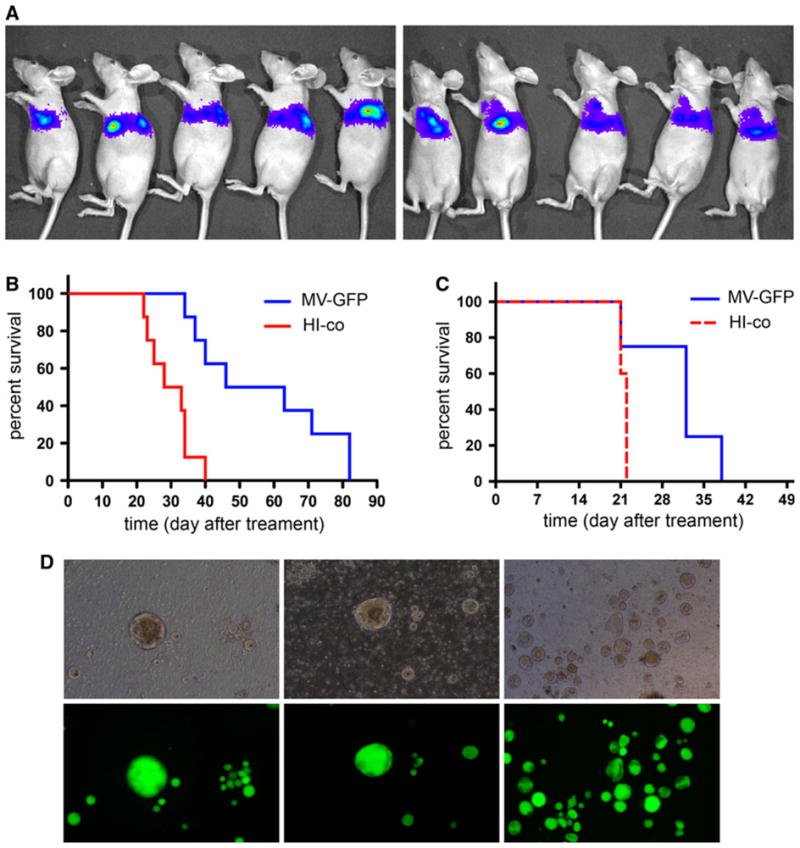
In vivo therapeutic efficacy of oncolytic MV against malignant pleural effusion in an advanced breast cancer model. Nude mice were engrafted t.t. in the left pleural space with MDA231-lu-P4 cells and tumor growth was confirmed by bioluminescence imaging (a). Local t.t. therapy with three repeated injection of MV-GFP (1.5 × 106 PFU total dose) significantly (P = 0.001) improved median survival of the animals from 30.5 to 54.5 days compared to the control (b). Strong trend (P = 0.0675) of survival prolongation was observed also for i.v. treated mice (c). b and c: MV-GFP-treated mice (MV-GFP, blue line), heat-inactivated virus-treated controls (HI-co, red line). Large multinucleated syncytia of infected tumor cells were detected by fluorescent microscopy of the pleural effusion samples from treated mice (d)
MV therapy in the presence of protective anti-measles immunity
A pilot study with six mice per group was used to test the therapeutic efficacy of cell-mediated MV delivery in the presence of protective humoral anti-measles immunity. Carrier-based therapy was compared to the data from a parallel experiment in pre-immune animals treated with free MV virions. The groups were arranged according to the bioluminescent intensity of t.t.-implanted MDA231-lu-P4 xenografts on day 3 (Supplementary Fig. 2A). Infected-dendritic cell carriers (MDCs) or MV-GFP-free virions were injected t.t. on day 5 post-engraftment either in non-immune animals or animals passively immunized with human AB+ group serum (the treatment protocol is summarized in Supplementary Fig. 2B). Mice were bled prior to the therapeutic injection and the serum VN titer was determined. All sera from passively immunized animals had protective [24, 25] anti-measles titers (Table 2). Mice were re-immunized with 150 μl human serum before each therapeutic MV injection to maintain the protective antibody level in the plasma. Infection efficiency of the carriers was monitored 48 h after inoculation with MV-GFP (or 24 h after injection in mice, respectively). MV infection efficiency for different donor MDCs was 17.3% (first therapeutic injection), 48.6% (second injection), and 47.4% (third injection). Both MV alone and cell carriers significantly improved survival of the treated non-immune mice (Fig. 5a, b). Passive immunization with human serum significantly decreased the therapeutic effect of free MV virions (Fig. 5c). In contrast, two of the six animals were long-term survivors in the pre-immunized group that was treated with MV-infected MDC carriers (Fig. 5d).
Table 2.
VN serum titer in mice passively immunized with human AB+ serum (ABS) before initiation of the therapy with either MV or infected MDC carriers
| ABS-co | MV-ABS | MDC-co- ABS | MDC- MV-ABS | |
|---|---|---|---|---|
| PNT50 for the individual animals | 240 | 139 | 168 | 132 |
| 204 | 230 | 195 | 174 | |
| 226 | 154 | 195 | 188 | |
| 200 | 177 | 153 | 195 | |
| 161 | 181 | 159 | 171 | |
| 265 | 282 | 379 | 159 | |
| 161 | 240 | |||
| Mean ± SD | 208 ± 39 | 200 ± 52 | 208 ± 86 | 170 ± 22 |
Mice were immunized i.p. with 250 μl of the highly protective HS-935 serum. All animals had an anti-measles antibody titer that is considered protective (above 1:120) for humans. The data are presented as PNT50 titer. VN titer in non-immune mice was <1:16. ABS-co = PBS treated immunized mice, MV-ABS = MV-GFP treated immunized mice, MDC-co-ABS = immunized mice treated with MDC inoculated with heat inactivated MV-GFP, MDC-MV-ABS = immunized mice treated with MV-GFP infected MDC
Fig. 5.
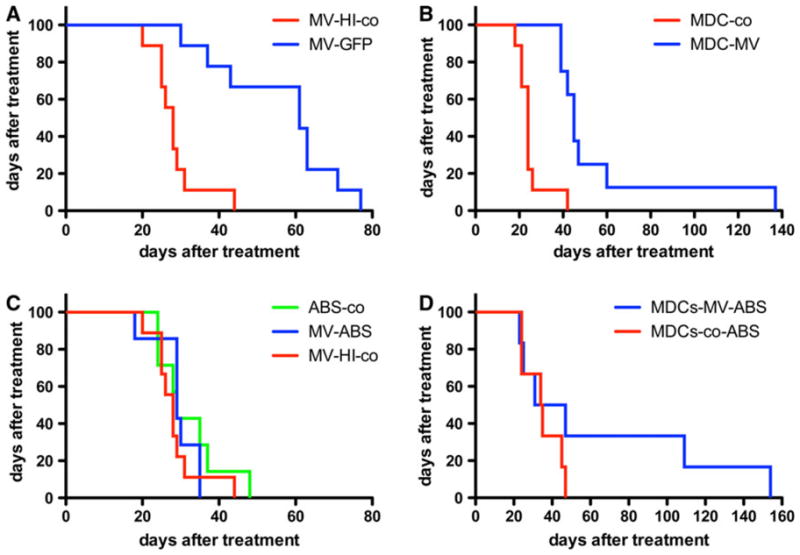
Survival experiment after t.t. treatment of passively immunized and non-immune mice bearing pleural breast cancer MDA231-lu-P4 xenografts. Both MV-GFP (blue line) (a) and MV-GFP-infected MDC (MDC-MV, blue line) carriers (b) protected against early malignant effusion accumulation and significantly improved survival of the animals compared to the control groups (P <0.001). Heat-inactivated virus (MV-HI-co) or MDCs inoculated with inactivated MV-GFP (MDC-co) were used as controls (red line in a and b). In contrast, passive immunization with human serum HS-935 (ABS in the figure) inhibited the therapeutic effect of free virions (c). MV-GFP treatment (MV-ABS, blue line) was compared to those of heat-inactivated virus (MV-HI-co, red line) or PBS-treated immunized mice (ABS-co, green line). In contrast, two of six immunized and treated with MV-GFP-infected carriers mice (MDCs-MV-ABS, blue line) were long-term survivors with pleural effusion formation delayed by months (d)
Discussion
The present study examines the antitumor potential of a novel strategy, i.e., use of oncolytic viruses for local or systemic treatment of pleural tumor deposits and prevention of malignant exudate formation. In order to explore the viral oncolysis approach, we established a reproducible model of breast cancer pleural xenografts in mice. Following serial passages in lungs, the MDA-MB-231 line evolved into a highly aggressive cell variant causing development of massive exudates in the pleural space within 4–5 weeks post t.t. implantation. Initial microarray analysis of MDA231-lu-P4 cells revealed elevated gene expression of cyclooxygenase-2, matrix metalloproteinase 1, and epiregulin compared to the original cell line confirming the clinical relevance of the model (Iankov and Galanis, unpublished data), since these genes play a key role controlling breast cancer cell spread and formation of lung metastases in patients [26]. Our previous studies have shown that MV Edmonston vaccine strain derivatives are able to infect breast cancer lines through the CD46 receptor, causing syncytia formation and apoptotic cell death [13].
In this study, we explored both intrapleural and systemic delivery of MV for the treatment of malignant pleural effusions in breast cancer xenograft models. Both treatment approaches were well tolerated, prevented early exudate accumulation, and prolonged survival of experimental animals. These results represent the first evidence supporting the clinical use of oncolytic viruses for the management of malignant effusions in breast cancer patients. MV or other vectors could be instilled via a thoracic catheter or video-assisted thoracoscopic surgery in the pleural space. Tumor cell infection and response to treatment can be easily monitored; furthermore, the likelihood of virus neutralization due to circulating antibodies is decreased. In our xenograft models, intrapleural administration of MV caused rapid spread of infection in the pleural tumor deposits.
Nevertheless, systemic delivery of MV has the advantage of better distribution of oncolytic infection to other metastatic sites. This was confirmed by the massive viral replication observed in pericardial metastatic tumor deposits following i.v. injection of MV-GFP. However, pre-existing antiviral immunity in this context can represent a potential impediment. Cancer patients can have MV neutralizing antibodies and cellular-mediated immunity as a result of immunization or natural infection in the past [5]. Cell carrier-based delivery, therefore, has recently been proposed as a strategy to evade the pre-existing humoral immunity in the plasma [27]. Our in vitro data showed that MV-infected dendritic cells can transfer infection to breast cancer cells via heterofusion in the presence of high titer of neutralizing antibodies. Our previous observations indicated that MV-infected carriers, mimicking the natural spread of infection, could be a useful delivery system in the presence of low virus-neutralizing antibody titers of in vivo [16]. In this study, we tested the effect of protective humoral response on the local and systemic MV therapy of metastatic pleural tumors. All passively immunized mice possessed protective anti-measles serum titers above 1:120 according to the criteria for immunization in humans. Our data showed that protective antibody levels against wild-type MV infection significantly decreased the effect of free-virion therapy. In contrast to free virions, however, 30% of pre-immunized mice treated with MV-infected MDC carriers were long-term survivors, indicating the potential application of this approach as a means of circumventing systemic immune response.
Collection of additional clinical information, including status of systemic MV immunity and neutralizing antibody titers in the pleural fluid of patients with advanced breast cancer would be important prior to clinical translation of this approach. It is also possible that local inflammatory response is equally important to the oncolytic effect in order to prevent the re-accumulation of exudate. Thus, MV could be engineered to express cytokines or inflammatory factors that could induce local inflammation and pleurodesis. The significant role of inflammation has been confirmed in clinical trials using immunomodulatory agents, including staphylococcal superantigens [28]. Studies on pathogenetic mechanisms have also revealed the important role of cancer cell secreted factors in pleural fluid accumulation [29]. Other factors, such as neutrophil attraction, fibrinolysis inhibition, and pH of the exudates, could also affect the results of locally administered virotherapy [30–32]. We are currently testing MV strains engineered to encode genes with pro-inflammatory function. Expression of such factors even by a limited number of infected tumor cells or MV cell carriers, by creating a local inflammatory reaction, could further increase the therapeutic benefit of MV virotherapy, and prevent fluid re-accumulation.
In conclusion, in this study, we have for the first time demonstrated improved survival and prevention of pleural fluid accumulation in aggressive breast cancer xenograft models using a measles-based virotherapy approach; these proof of principle experiments can serve as the basis for the development of an oncolytic virotherapy strategy in the treatment of patients with malignant pleural effusions.
Supplementary Material
Acknowledgments
This work was supported by the Atwater grant and P50CA108961.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-009-0602-z) contains supplementary material, which is available to authorized users.
References
- 1.Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346(25):1971–1977. doi: 10.1056/NEJMcp010731. [DOI] [PubMed] [Google Scholar]
- 2.DeCamp MM, Jr, Mentzer SJ, Swanson SJ, Sugarbaker DJ. Malignant effusive disease of the pleura and pericardium. Chest. 1997;112(Suppl 4):291S–295S. doi: 10.1378/chest.112.4_supplement.291s. [DOI] [PubMed] [Google Scholar]
- 3.Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer. 2006;54(1):1–9. doi: 10.1016/j.lungcan.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83(2):235–250. doi: 10.4065/83.2.235. [DOI] [PubMed] [Google Scholar]
- 5.Griffin D. Measles virus. In: Knipe DM, Howley PM, editors. Fields virology. 4. Lippincott, Williams & Wilkins; Philadelphia, PA: 2001. pp. 1401–1441. [Google Scholar]
- 6.Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA, Fielding AK. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97(12):3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 7.Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R, Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98(7):2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 8.Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8(5):527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 9.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63(10):2462–2469. [PubMed] [Google Scholar]
- 10.Iankov ID, Hillestad ML, Dietz AB, Russell SJ, Galanis E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol Ther. 2009;17(8):1395–1403. doi: 10.1038/mt.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Msaouel P, Iankov ID, Allen C, et al. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate. 2009;69(1):82–91. doi: 10.1002/pros.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- 13.McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, Harvey ME, Zollman PJ, Russell SJ, Galanis E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99(2):177–184. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 15.Heinzerling L, Kunzi V, Oberholzer PA, Kundig T, Naim H, Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106(7):2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- 16.Iankov ID, Blechacz B, Liu C, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15(1):114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 17.Demaison C, Parsley K, Brouns G, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of immunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13(7):803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 18.Noser JA, Towers GJ, Sakuma R, et al. Cyclosporine increases human immunodeficiency virus type 1 vector transduction of primary mouse cells. J Virol. 2006;80(15):7769–7774. doi: 10.1128/JVI.02427-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73(11):9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprex WP, McQuaid S, Roscic-Mrkic B, Cattaneo R, McCallister C, Rima BK. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J Virol. 2000;74(17):7972–7979. doi: 10.1128/jvi.74.17.7972-7979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietz AB, Bulur PA, Emery RL, et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46(12):2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 22.Dietz AB, Bulur PA, Knutson GJ, Matasic R, Vuk-Pavlovic S. Maturation of human monocyte-derived dendritic cells studied by microarray hybridization. Biochem Biophys Res Commun. 2000;275(3):731–738. doi: 10.1006/bbrc.2000.3372. [DOI] [PubMed] [Google Scholar]
- 23.Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. Development of a novel efficient fluorescence-based plaque reduction microneutralization assay for measles virus immunity. Clin Vaccine Immunol. 2008;15(7):1054–1059. doi: 10.1128/CVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 25.Ward BJ, Aouchiche S, Martel N, et al. Measurement of measles virus-specific neutralizing antibodies: evaluation of the syncytium inhibition assay in comparison with the plaque reduction neutralization test. Diagn Microbiol Infect Dis. 1999;33(3):147–152. doi: 10.1016/s0732-8893(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 26.Eltarhouny SA, Elsawy WH, Radpour R, Hahn S, Holzgreve W, Zhong XY. Genes controlling spread of breast cancer to lung “gang of 4”. Exp Oncol. 2008;30(2):91–95. [PubMed] [Google Scholar]
- 27.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren S, Terman DS, Bohach G, et al. Intrapleural staphylococcal superantigen induces resolution of malignant pleural effusions and a survival benefit in non-small cell lung cancer. Chest. 2004;126(5):1529–1539. doi: 10.1378/chest.126.5.1529. [DOI] [PubMed] [Google Scholar]
- 29.Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax. 1999;54(8):707–710. doi: 10.1136/thx.54.8.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bielsa S, Salud A, Martinez M, et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med. 2008;19(5):334–339. doi: 10.1016/j.ejim.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Crnjac A, Sok M, Kamenik M. Impact of pleural effusion pH on the efficacy of thoracoscopic mechanical pleurodesis in patients with breast carcinoma. Eur J Cardiothorac Surg. 2004;26(2):432–436. doi: 10.1016/j.ejcts.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Psathakis K, Calderon-Osuna E, Romero-Romero B, et al. The neutrophilic and fibrinolytic response to talc can predict the outcome of pleurodesis. Eur Respir J. 2006;27(4):817–821. doi: 10.1183/09031936.06.00097505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.