Abstract
Objective
To evaluate the efficacy of an integrated multiple risk intervention delivered mainly during pregnancy, in reducing such risks (smoking, environmental tobacco smoke exposure, depression and intimate partner violence) postpartum.
Design
Data from this randomized controlled trial were collected prenatally and on average 10 weeks postpartum in six prenatal care sites in the District of Columbia. African Americans were screened, recruited and randomly assigned to the behavioral intervention or usual care. Clinic-based, individually tailored counseling was delivered to intervention women. The outcome measures were number of reisks reported postpartum and reduction of these risks between baseline and postpartum.
Results
The intervention was effective in significantly reducing the number of risks reported in the postpartum period. In Bivariate analyses, the intervention group was more successful in resolving all risks (47% compared with 35%, p=0.007), number needed to treat=9, 95% confidence interval [CI] 5-31) and in resolving some risks (63% compared with 54%, p=0.009), number needed to treat=11, 95% CI 7-43) as compared with the usual care group. In logistical regression analyses, women in the intervention group were more likely to resolve all risks (OR=1.86, 95% CI: 1.25-2.75) and in resolving at least one risk (OR=1.6, 95% CI: 1.15-2.22).
Conclusions
An integrated multiple risk factor intervention addressing psychosocial and behavioral risks delivered mainly during pregnancy can have beneficial effects in risk reduction postpartum.
Introduction and Background
Medical, behavioral, and psychosocial risks may influence the health of women and their infants starting at pre-conception through pregnancy, delivery, and postpartum period. Psychosocial and behavioral risk factors such as smoking, environmental tobacco smoke exposure (ETSE), depression and intimate partner violence (IPV) have been associated with adverse pregnancy outcomes and poor long term health.”).1-4 Interventions addressing such factors in pregnancy have been variably successful.5,6 Those with modest success addressed risk factors in isolation despite their frequent co-occurrence. More recent studies have adopted an integrated approach addressing multiple risks simultaneously.7 Such interventions, when tested in women in the childbearing age, may hold greater promise in reducing reproductive morbidities.6
The intensity of psychosocial and behavioral risk factors may vary during pregnancy and after delivery. For example, women who stop smoking early in pregnancy may be unable to maintain cessation following delivery.8 Depression may become intensified postpartum.9,10 IPV, a factor associated with poor pregnancy outcomes,11,12 may vary in frequency and intensity in pregnancy2,13 and after delivery.14 Similarly, attitudes toward avoidance of ETSE in pregnancy and postpartum may be influenced by parents' evaluation of risk, and perceived difficulty in maintaining a smoke free home environment.15 Interventions designed to reduce psychosocial and behavioral risk during pregnancy may have an impact beyond delivery.16 Evidence of such postpartum effects is demonstrated in some studies.17,18
The purpose of this study is to evaluate the efficacy of a cognitive behavioral intervention delivered during pregnancy in reducing behavioral risks in the postpartum period. The risks addressed included depression, IPV, smoking and ETSE in a population of African American pregnant women.
Participants and Methods
Recruitment occurred at six prenatal care (PNC) clinics between July 2001 and October 2003. Primary and secondary hypotheses were specified in advance. To ensure statistical power for testing the hypotheses that the integrated intervention would achieve statistically significant reductions in the targeted risk factors, sample size determination was essential. Assuming a 5% level of significance, 80% power, 20% drop-out rate, and 20% loss to follow-up, a sample size of 1,750 pregnant women was required in order to retain 1,050 at the end of the study (525 in each care group). This number allowed detection of 10-20% reductions in risk-specific factors in the intervention group (IG) as compared to the usual care (UCG). Details are published in El-Khorazaty et al.19
During 28 months of recruitment, 4,213 women were approached for Audio-Computer Assisted Survey Interview screening, and 2,913 women consented. Eligibility criteria included minority status, age ≥ 18 years, ≤ 28 weeks of pregnancy, DC resident, English speaking, and reporting any of the four designated risks (active smoking, ETSE, depression, and IPV). Smoking was defined as smoking in the six months prior to pregnancy or since learning they were pregnant. ETSE was defined as exposure to smokers at home, in the same room, or in a car. Depression was evaluated using the Beck Depression Inventory II.20,21 For IPV, women were asked if a current or previous partner, boyfriend, husband, or baby's father had pushed, shoved, slapped, kicked, or physically hurt them or forced them to have sexual intercourse in the last year, or if they were afraid of their current partner. Eligible women were invited to participate and those interested consented to study participation.
A baseline interview gathered sociodemographic data and information on the designated risks. Survey data items assessing smoking and ETSE were taken or adapted from the Smoke-Free Families screening and baseline questionnaires,22 the 1990 and 1998 National Health Interview Survey supplements,23-26 and ETSE intervention studies.27 Additional items assessed stage of change for smoking cessation28,29 and processes of change.30 Depression was identified using the Hopkins Symptom Checklist, a validated, widely used depression score based on a set of twenty items.31,32 Respondents rated each item on a 5-point scale from 0=Not at all to 4=Extremely distressed by various symptoms within the past month (e.g., poor appetite, feeling lonely or blue, restless sleep, thoughts of death and dying). Responses were summed, divided by 20, and resulting scores classified into one of two groups; ≤.75 = remission or no depression and > .75 depression. Survey items for IPV included the Conflict Tactics Scale33-35, a validated scale to measure risk of intimate partner violence and safety behaviors items.36-38 The conflict tactics scale measured annual frequency of physical assault and sexual coercion (partner to self). A single item assessed whether any IPV on the Conflict Tactics Scale occurred during pregnancy (either partner to self or self to partner). Active smoking was defined as smoking a puff of a cigarette in the previous week, ETSE as having been in the same room or area where someone was smoking in the past week; depression as having a score >0.75 on Hopkins Symptom Checklist; and IPV was confirmed if a woman reported being subjected to any of the actions on the revised Conflict Tactics Scale at least once by her partner in the past year. After telephone interview, women were randomized to IG or UCG.
Interventions for active and passive smoking were based on the Social Cognitive Theory39,40 and the Transtheoretical Model.41 The smoking interventions used a Cognitive-Behavioral Therapy orientation42-44 tailored to individuals' “stage” of readiness for change. Active smoking intervention included the “Smoking Cessation or Reduction in Pregnancy Treatment” materials,45-47 and incorporated some content from the “Pathways to Change” manual. 48 ETSE intervention paralleled the active smoking intervention. The primary objectives were to promote smoking cessation, ETSE avoidance and/or reduction depending on the participants' readiness.
The cognitive behavioral therapy intervention for depression was adapted from a group intervention by Miranda and Munoz.49 The IPV intervention was adapted from the Parker-McFarlane structured intervention,13 to individualized counseling sessions. Behavioral counseling for IPV was integrated from a brochure-based approach using Dutton's Empowerment theory50 found to be effective in increasing safety behaviors and reducing IPV at 6 and 12 months postpartum.13 To be considered adequate, the intervention was intended to be delivered prenatally in a minimum of four sessions, with eight sessions considered complete. Up to two postpartum booster sessions were offered, reinforcing skills and goals and adapted to specific postpartum stressors.
The intervention delivered by Masters' degree trained counselors, lasted 36±15 minutes per session prenatally and 38±13 minutes postpartum. On average, participants attended 3.9 prenatal sessions and 0.8 postpartum sessions. Fifty-four percent of the IG attended at least 4 prenatal sessions, while 51% attended at least one postpartum. (For details on Project DC-HOPE, see El-Khorazaty et al.19). Eight women (6 IG and 2 UCG) were identified as suicidal (during intervention or data collection) and were referred immediately to mental health care and excluded from further participation. The anticipated additional cost to providing the intervention would be the hiring of a Master's level social or mental health professional and the space needed to deliver the service privately. Additional infrastructure costs are limited.
Women randomized to IG received behavioral counseling addressing the risk factors they reported. Two follow-up interviews were administered during the second trimester (22-26 weeks gestation) and third trimester (34-38 weeks gestation). A final interview was conducted at 10.3 weeks postpartum on average. During these interviews women were evaluated for smoking, depression, and ETSE using same the definitions as at baseline. IPV was defined as victimization during the time interval between two consecutive interviews. Interviewers were blinded to group assignment, while participants and the intervention team were not.
To preserve the randomization, participant data were analyzed according to their randomized group assignment, regardless of receipt of intervention, using an intent-to-treat approach. Of the 913 African American women with one or more risks at baseline, 723 participated in the postpartum telephone interview. In order to make an intent-to-treat analysis possible, multiple imputation (MI) methodology was implemented to estimate missing data for the remaining 190 (49 drop-outs, 111 lost to follow-up, and 30 deemed ineligible) using the sequential regression imputation method described by Raghunathan, et al.51 A linear, logistic, or polytomous regression model was used to impute continuous, binary, or categorical missing values. MI was performed to create five complete datasets, using IVEware imputation and variance estimation software.52
Analyses were conducted for each of the five imputed datasets and the results were combined results using the MIANALYZE procedure in SAS53 to obtain the multiple imputation inference. The final parameter estimates and the associated standard errors account for both within- and between-imputation variance. Basic descriptive statistics provided information regarding characteristics of participating women stratified by randomization assignment. Comparisons were conducted using General Linear Models for continuous variables and Generalized Linear Models for categorical variables. The GLM and GENMOD procedures in SAS were used. Frequencies presented in the tables are rounded to the nearest integer.
To quantify postpartum behavioral changes and to assess effects of the intervention, the distributions of risks in the two randomized groups at baseline and postpartum were compared. Within-person changes in number of risks over time was also evaluated. Each woman was characterized with respect to number of risks (1-4) at baseline. Each of the 913 participants was categorized postpartum in one of three ways: (1) Resolving all risks (RA); (2) Resolving some, but not all, risks (RS); and (3) Resolving no risks or increasing the number of risks (RN).
Intervention effectiveness was measured contrasting the proportion of women RA versus the proportion RN in the two groups. The proportions of participants resolving all or some risk were also compared between groups. To evaluate the impact of the intervention on risk status at postpartum, we used logistic regression analyses. Analyses were conducted using the LOGISTIC procedure in SAS version 9.1.53 Control variables included age; education; marital status; employment status; enrollment in Medicaid and WIC; illicit drug and alcohol use at baseline; previous premature delivery, pregnancy loss, or live birth; time of PNC initiation; gestational age at baseline; smoking, ETSE, depression, and IPV at baseline.
All study related activities were approved by the Howard University IRB, the designated IRB of record for the clinical study sites, the RTI International IRB and the National Institute of Child Health and Human Development IRB.
Results
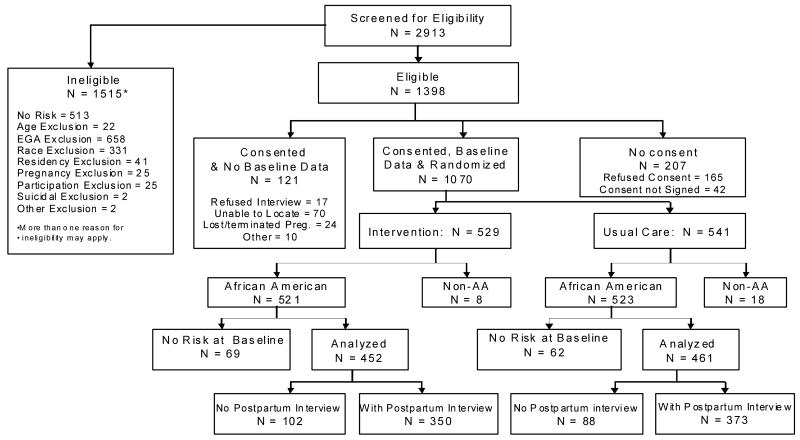
A total of 1,398 women met eligibility requirements (See Figure 1), of whom 85% (1,191) consented to participate in a baseline telephone interview before randomization; 1,070 (89.8%) completed the baseline interview. Of those, 157 were excluded from further analysis due to completion of pregnancy (n=4) before baseline interview or other than African American minority status (n=22). In addition, because of using different instruments (Audio-Computer Assisted Survey interview and baseline interview) were used to assess risks, 131 women who were screened-in to the study showed no risk at baseline interview and were excluded. Site- and risk-specific block randomization was conducted following the baseline interview. Of those African American pregnant women with risk reported at baseline, 452 were randomized to the IG and 461 to the UCG. (See Figure 1). 723 (79.2%) women were contacted postpartum, 350 were in the IG and 373 in the UCG (See Figure 1).
Figure 1. Screening, Eligibility, Recruitment, Randomization & Follow-up: Project DC-HOPE.
* 157 women were excluded from analysis either because they were non-AA (22), pregnancy loss (4), or because they had no risk at baseline (131).
Characteristics of study participants are described in Tables 1 and 2. No significant differences were detected when comparing IG to UCG or all participants to those retained in the study in the postpartum period. (For more information on retention, see El-Khorazaty et al.19). At baseline, ETSE was most commonly reported risk (82.7%), followed by depression (50.7%) and IPV (36.8%) among those with at least one risk. The least common risk at baseline was active smoking, reported by 21.7%. Approximately 72.5% of participants reported one or two risks, while 27.5% of participants had three to four risks. Women exposed only to ETSE represented the highest percentage (28.2% of the 913), followed by women exposed to ETSE and depression (14.5%), those exposed to ETSE, depression and IPV (13.8%), women who were exposed to ETSE and IPV (7.7%), and those with depression only (7.0%) (data not shown). Women reporting all four risks represented 4.1% of the sample.
Table 1. Participant characteristics self-reported at baseline.
| Characteristics | Category | Intervention (N=452) |
Usual Care (N=461) |
P-value |
|---|---|---|---|---|
| Maternal age | Mean ± SE | 24.4 ± 0.3 | 24.8 ± 0.3 | 0.217 |
| Education level | < High school | 141 (31.3%) | 143 (31.0%) | 0.649 |
| HS graduate/GED | 214 (47.3%) | 210 (45.6%) | ||
| At least some college | 97 (21.4%) | 108 (23.4%) | ||
| Currently working | Yes | 157 (34.8%) | 167 (36.2%) | 0.648 |
| Worked prior to pregnancy ˆ | Yes | 164 (55.8%) | 174 (59.7%) | 0.339 |
| Relational status | Single/separated/ widowed/divorced | 341 (75.5%) | 353 (76.6%) | 0.686 |
| Married or living with partner | 111 (24.5%) | 108 (23.4%) | ||
| Household size | Mean ± SE | 3.9 ± 0.1 | 4.0 ± 0.1 | 0.161 |
| Medicaid | Yes | 366 (80.9%) | 355 (77.0%) | 0.153 |
| WIC | Yes | 203 (44.9%) | 195 (42.2%) | 0.422 |
| Alcohol use during pregnancy | Yes | 103 (22.7%) | 105 (22.8%) | 0.969 |
| Illicit drug use during pregnancy | Yes | 66 (14.6%) | 54 (11.7%) | 0.201 |
| Previous pregnancy | Yes | 377 (83.4%) | 397 (86.1%) | 0.252 |
| Gravidity | Mean ± SE | 3.7 ± 0.1 | 3.8 ± 0.1 | 0.576 |
| Previous live birth | Yes | 306 (67.7%) | 327 (70.9%) | 0.290 |
| Number of live births † | Mean ± SE | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.980 |
| Previous miscarriage * | Yes | 147 (38.9%) | 155 (39.1%) | 0.967 |
| Previous stillbirth * | Yes | 16 (4.2%) | 23 (5.8%) | 0.325 |
among women not currently working
among women with a previous pregnancy.
among women with a previous live birth.
Table 2. Comparison of Enrolled and Retained Participants in the Postpartum Period.
| Characteristics | Category | All participants (N=913) |
Women with Postpartum interview (N=723) |
P-value |
|---|---|---|---|---|
| Sociodemographic Characteristics | ||||
| Maternal age | Mean (years) ± SE | 24.6 ± 0.2 | 24.7 ± 0.2 | 0.752 |
| Education level | < High school | 284 (31.1%) | 219 (30.3%) | 0.715 |
| HS graduate/GED | 424 (46.5%) | 338 (46.8%) | ||
| At least some college | 205 (22.4%) | 166 (22.9%) | ||
| Currently working | Yes | 324 (35.5%) | 262 (36.3%) | 0.750 |
| Worked prior to pregnancy ˆ | Yes | 338 (57.7%) | 274 (59.8%) | 0.508 |
| Relational status | Single/separated/widowed/divorced | 694 (76.0%) | 539 (74.6%) | 0.498 |
| Married or living with partner | 219 (24.0%) | 184 (25.4%) | ||
| Household size | Mean ± SE | 3.9 ± 0.1 | 3.9 ± 0.1 | 0.828 |
| Medicaid (Baseline interview) | Yes | 720 (78.9%) | 570 (78.8%) | 0.972 |
| WIC (Baseline interview) | Yes | 398 (43.5%) | 314 (43.4%) | 0.943 |
| Other Exposures During Pregnancy | ||||
| Alcohol use during pregnancy (Baseline interview) | Yes | 208 (22.7%) | 167 (23.0%) | 0.885 |
| Illicit drug use during pregnancy (Baseline interview) | Yes | 120 (13.1%) | 96 (13.3%) | 0.937 |
| Reproductive History | ||||
| Gravidity (Baseline interview) | Mean ± SE | 3.8 ± 0.1 | 3.7 ± 0.1 | 0.773 |
| Number of live births † | Mean ± SE | 2.2 ± 0.1 | 2.1 ± 0.1 | 0.548 |
| Previous miscarriage (Baseline interview)* | Yes | 302 (39.0%) | 243 (39.1%) | 0.968 |
| Previous stillbirth (Baseline interview) * | Yes | 39 (5.0%) | 32 (5.2%) | 0.923 |
| Previous premature delivery (Baseline abstraction) † | Yes | 114 (18.1%) | 91 (18.2%) | 0.956 |
| Pregnancy ‘intended’ | Yes | 304 (33.3%) | 233 (32.2%) | 0.655 |
| Pregnancy ‘wanted’ | Yes | 693 (75.9%) | 547 (75.6%) | 0.878 |
| Trimester of PNC initiation | 1st Trimester | 551 (60.3%) | 441 (60.9%) | 0.817 |
| 2nd Trimester | 344 (37.7%) | 268 (37.1%) | ||
| 3rd Trimester | 18 (2.0%) | 14 (2.0%) | ||
| Number of PNC visits | 1-3 visits | 140 (15.4%) | 105 (14.5%) | 0.427 |
| 4-8 visits | 278 (30.4%) | 211 (29.1%) | ||
| 9+ visits | 495 (54.2%) | 407 (56.3%) | ||
| Risk Status at Baseline | ||||
| Active smoking | Yes | 198 (21.7%) | 152 (21.0%) | 0.745 |
| ETSE | Yes | 755 (82.7%) | 599 (82.9%) | 0.904 |
| Depression | Yes | 463 (50.7%) | 367 (50.7%) | 0.985 |
| IPV | Yes | 336 (36.8%) | 274 (37.9%) | 0.650 |
| Number of risks | 1 | 363 (39.8%) | 288 (39.9%) | 0.889 |
| 2 | 299 (32.7%) | 230 (31.9%) | ||
| 3 | 213 (23.3%) | 174 (24.1%) | ||
| 4 | 38 (4.1%) | 30 (4.1%) | ||
among women not currently working
among women with a previous pregnancy.
among women with a previous live birth.
Significant reductions (p<0.001) between baseline and postpartum were observed for ETSE (82.7% to 54.8%), depression (50.7% to 27.3%), and IPV (36.8% to 9.9%). Consistent with postpartum relapse reported in previous studies, there was considerable recidivism in cigarette smoking at postpartum (21.7% to 26.5%, p=0.021). The number of risks reported at postpartum was lower than that at baseline. Overall, 28.5% of women with risk at baseline reported none at postpartum (p<0.001).
There were no significant differences in reporting of risks at baseline between the IG and UCG (Table 3). Comparing risk-specific change between baseline and the postpartum period in the two groups, the resurgence in smoking rates is significantly higher in the UCG (p<0.001), while the reduction in ETSE is significantly higher in the IG (p=0.011). No significant differences were seen in the change for depression and IPV between the two groups.
Table 3. Individual Risk Factor Change in the Intervention and Usual Care Groups Comparing Baseline to Postpartum (N=913) (Combined Five Imputed Datasets#).
| Characteristics | Intervention (N=452) |
Usual Care (N=461) |
P-value (IG vs. UCG) |
|---|---|---|---|
| Smoking at baseline interview | 106 (23.4%) | 92 (20.0%) | 0.205 |
| Smoking at postpartum interview | 116 (25.6%) | 126 (27.3%) | 0.580 |
| Change in percent† | 2.2% | 7.3% | <0.001 |
| ETSE at baseline interview | 372 (82.2%) | 383 (83.1%) | 0.712 |
| ETSE at postpartum interview | 233 (51.6%) | 267 (57.9%) | 0.055 |
| Change in percent† | -30.6% | -25.2% | 0.011 |
| Depression at baseline interview | 229 (50.6%) | 234 (50.8%) | 0.961 |
| Depression at postpartum interview | 115 (25.5%) | 134 (29.0%) | 0.303 |
| Change in percent† | -25.1% | -21.8% | 0.100 |
| IPV at baseline interview | 169 (37.4%) | 167 (36.2%) | 0.727 |
| IPV at postpartum interview | 39 (8.6%) | 52 (11.3%) | 0.216 |
| Change in percent† | -28.8% | -24.9% | 0.074 |
Frequencies are averaged over the five imputations and rounded to the nearest integer.
A positive percentage indicates increased risk from baseline to postpartum while a negative percentage indicates a decreased risk.
Table 4 presents number of risks at baseline and postpartum. While there were no significant differences at baseline, but at postpartum the IG and UCG differed significantly (p=0.033). Additional analyses comparing the two groups in the postpartum period revealed a significant difference in the distribution of risks. In the IG, 32.1% reported no risk compared to 24.9% of the UCG (p=0.031). For the IG, 8.6% reported three or more of the designated risks combined, compared to 12.3% of the UCG (p=0.074).
Table 4. Comparison of Risk Factors in the Intervention and Usual Care Groups at Baseline and Postpartum Interviews for Women with At Least One Risk (Combined Five Imputed Datasets#).
| Characteristic | Category | Intervention (N=452) |
Usual Care (N=461) |
P-value## (IG vs. UCG) |
|---|---|---|---|---|
| Number of risks at baseline (smoking, ETSE, depression, IPV) |
1 | 173 (38.3%) | 190 (41.2%) | 0.526 |
| 2 | 154 (34.1%) | 144 (31.3%) | ||
| 3 | 105 (23.2%) | 108 (23.5%) | ||
| 4 | 20 (4.4%) | 18 (3.9%) | ||
| Number of risks at postpartum (smoking, ETSE, depression, IPV) |
0 | 145 (32.1%) | 115 (24.9%) | 0.033 |
| 1 | 158 (34.9%) | 178 (38.7%) | ||
| 2 | 110 (24.4%) | 111 (24.1%) | ||
| 3 | 31 (6.8%) | 49 (10.6%) | ||
| 4 | 8 (1.8%) | 8 (1.7%) |
Frequencies are averaged over the five imputations and rounded to the nearest integer.
Using Generalized Linear Modeling.
In Table 5, bivariate analyses compare risk resolution by care group. RA or RS for risks encountered at baseline are compared to RN. Comparison of the three categories of risk resolution in an unadjusted generalized linear model for the IG and UCG was significant (p=0.005). The IG shows a significantly higher percentage of RA or RS (63%) as compared to the UCG (54%). Resolution of RA versus RN and RS versus RN are compared separately, with significant differences between groups (p=0.007, p=0.009, respectively). The number needed to treat (NNT) (average number of mothers needed to receive the intervention for one additional woman to resolve risks over usual care mothers) is 14 women (95% CI: 7,139) to resolve some risks, 9 (95% CI: 5,31) to resolve all risks, and 11 (95% CI: 7,43) to resolve some or all risks.
Table 5. Risk Resolution between Baseline and Postpartum Interviews by Care Group (Combined Five Imputed Datasets).
| Resolution | Category | Intervention (N=452) |
Usual Care (N=461) |
P-value# (IG vs. UCG) | Odds Ratio (95% CI) |
Absolute Risk Difference | Number Needed to Treat (95% CI) |
|---|---|---|---|---|---|---|---|
| A. Distribution of Risk Resolution | |||||||
| Risks resolved | None (or increased): RN | 166 (36.6%) | 212 (46.1%) | 0.005 | |||
| Resolved Some: RS | 141 (31.3%) | 134 (29.0%) | 1.34 (0.97, 1.86) |
0.06 | 14 (7, 139) |
||
| Resolved All: RA | 145 (32.1%) | 115 (24.9%) | 1.62 (1.15, 2.29) |
0.10 | 9 (5, 31) |
||
| B. Comparison of Risk Resolution | |||||||
| Resolved all risks vs. none (RA vs. RN) |
Yes | 145 (46.7%) | 115 (35.1%) | 0.007 | 1.62 (1.15, 2.29) |
0.12 | 9 (5, 31) |
| No | 166 (53.3%) | 212 (64.9%) | |||||
| Resolved some or all risks vs. none (RA or RS vs. RN) |
Yes | 287 (63.4%) | 248 (53.9%) | 0.009 | 1.48 (1.10, 1.98) |
0.10 | 11 (7, 43) |
| No | 166 (36.6%) | 212 (46.1%) | |||||
Note: Column totals do not add up because of rounding of the five imputations to the nearest integer.
Using Generalized Linear Modeling.
Table 6 presents the results of multiple logistic regression models predicting risk resolution, either RA or RS. Participation in the IG was associated with RA vs. RN (OR=1.86, 95% CI: 1.25-2.75, NNT=7, 95% CI: 4, 19) and RA or RS vs. RN (OR=1.60, 95% CI: 1.15-2.22, NNT=9, 95% CI: 6, 29) in the postpartum period. Resolution of risk was associated in both models with depression, IPV, or ETSE documented in participants at baseline. In contrast, cigarette smoking at baseline reduced the odds of RA (p<0.001). Use of illicit drugs during pregnancy as reported at baseline reduced the odds of RA (p=0.014); alcohol use during pregnancy at baseline had a similar effect on reduction of RS (p=0.018). Employment during pregnancy was associated with increased odds ratio of RA (p=0.020).
Table 6. Results of Multiple Logistic Regression Models# Predicting Various Risk Resolution Categories at Postpartum Interview.
| Parameters | Category | P-value | Odds Ratio | 95% CI for OR |
|---|---|---|---|---|
|
A. Women resolved all risks vs. women resolved no risks at postpartum (RA vs. RN) (N=638) | ||||
| Intercept | <.001 | 0.22 | (0.12, 0.41) | |
| Care group | IG vs. UCG | 0.002 | 1.86 | (1.25, 2.75) |
| Current employment | Y vs. N | 0.020 | 1.53 | (1.07, 2.19) |
| Illicit drugs (baseline interview) | Y vs. N | 0.014 | 0.39 | (0.19, 0.82) |
| Active smoking at baseline | Y vs. N | <.001 | 0.32 | (0.18, 0.59) |
| ETSE at baseline | Y vs. N | 0.020 | 1.74 | (1.09, 2.77) |
| Depression at baseline | Y vs. N | 0.028 | 1.55 | (1.05, 2.30) |
| IPV at baseline | Y vs. N | <.001 | 2.61 | (1.66, 4.11) |
|
B. Women resolved at least one risk vs. women resolved no risks at postpartum (RA or RS vs. RN) (N=913) | ||||
| Intercept | <.001 | 0.15 | (0.09, 0.25) | |
| Care group | IG vs. UCG | 0.005 | 1.60 | (1.15, 2.22) |
| Alcohol use (baseline interview) | Y vs. N | 0.018 | 0.58 | (0.38, 0.91) |
| ETSE at baseline | Y vs. N | <.001 | 3.46 | (2.25, 5.33) |
| Depression at baseline | Y vs. N | <.001 | 3.62 | (2.59, 5.06) |
| IPV at baseline | Y vs. N | <.001 | 4.82 | (3.31, 7.02) |
Covariates screened for significance include maternal age; education; marital status; employment status; enrollment in Medicaid and WIC; illicit drug and alcohol use at baseline; previous premature delivery, pregnancy loss, or live birth; early initiation of prenatal care (PNC); gestational age at baseline; smoking, ETSE, depression and IPV at the time of the Baseline interview.
Discussion
This study recruited minority women with psychosocial and behavioral risks seeking health care at urban PNC sites. These mothers brought other challenges to their pregnancies that could not or were not addressed by the intervention, including unmet economic needs, a low level of education, and associated behavioral challenges, including alcohol and drug use. Despite their poor reproductive profile (with high rates of miscarriage, stillbirth, and previous preterm delivery) and the low level of intendedness for the pregnancy, they sought PNC relatively early and used the prenatal health care delivery system at least 4 times in 85% of cases. Despite these constraints and the challenges of an overwhelmed health care system, we were able to demonstrate the efficacy of a cognitive behavioral intervention during the postpartum period.
The prenatal intervention as designed could be delivered during pregnancy at a minimum of four visits, well within a manageable range for this population. The mean time of 36 minutes for an individual intervention fits easily into the waiting periods between services provided. An important element for intervention feasibility was a judicious selection of interventions from a spectrum of risks that could be effectively addressed without being overwhelming to either patients or providers.
This intervention was successful in risk reduction without addressing associated alcohol and drug use. Both factors showed significant interference with the intervention effect, suggesting the importance of addressing them in future multiple risk factor interventions. Employment at the time of recruitment enhanced the intervention effect. This finding could be related to underlying characteristics of women who sought and secured employment, such as education, life skills, and self-efficacy, or a reflection of the employment status itself, including income, access to care, and overall level of organization and lifestyle.
We demonstrated the utility and feasibility of using an innovative, integrated approach. It is important to consider the fact that the intervention showed postpartum efficacy on a combination of risks that included smoking, a behavior with known recidivism. Postpartum effects of this intervention would very likely have been even higher if this risk factor were excluded. It is also possible that these effects could wane with time, causing the re-emergence of risks. Women in the IG may hypothetically retain coping and behavioral modification skills learned during the intervention, which could assist them in maintaining their behavioral gains for a longer period. We would recommend, in future studies, following these mothers for longer intervals to measure sustained effects.
A limitation of this study was the inability to deliver the minimum number of intervention sessions to 46% of the participants. Whether intervention delivery would be further compromised when tested for effectiveness under non-experimental conditions remains to be seen. The delivery of the postpartum booster sessions was limited to one to two sessions only, which may not have been adequate, especially in the case of depression. Successful cognitive behavioral therapy interventions addressing postpartum depression were much more intensive (up to 8 weekly home visits).54 More modest interventions, including a single encounter with a mental health provider, did not show efficacy.55 Our decision to exclude anxiety as a targeted risk factor may also have affected the efficacy of the intervention. There is growing evidence in the literature that anxiety and depression show significant overlap and are best addressed simultaneously.1,56 Future intervention design may include antidepressants. Including fathers could have improved efficacy, especially in smoking and ETSE. Including partners has also been shown to improve the chance of postnatal effects of interventions addressing postpartum distress and depression17. Finally these intervention effects may apply only to high risk minority pregnant women. It would be important to test this intervention in other racial or socio-demographic groups to show whether results can be generalized. Another significant limitation is the high rate of loss-to-follow-up (20% of participants). Our effort to adjust through implementation of multiple imputation methodology can only partially compensate for attrition in a study designed to measure outcomes based on an intent-to-treat approach.
This study suggests that a multiple risk factor intervention that addresses both psychosocial and behavioral risks can be effective in reducing those risks when delivered during pregnancy and reinforced during the postpartum period. This intervention was delivered within the constraints of the existing PNC delivery system and confirms the willingness of high-risk, urban minority populations to participate in such interventions. The sustained postpartum effects were only tested on average 10 weeks postnatally. But even within that time period, the intervention appears to have benefited these mothers in terms of risk behaviors in the short run, and could ultimately influence the health and wellbeing of their infants long-term. Extending similar services within existing outreach programs (e.g., Healthy Families USA) may ensure better delivery of such interventions and require minimal additional infrastructural support.
Acknowledgments
This study was supported by grants no. 3U18HD030445; 3U18HD030447; 5U18HD31206; 3U18HD031919; 5U18HD036104, National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities. Some of the findings were presented at the 2007 Pediatric Academic Societies' Annual Meetings.
References
- 1.Field T, Diego M, Hernandez-Reif M, et al. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depress Anxiety. 2003;17(3):140–151. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- 2.Neggers Y, Goldenberg R, Cliver S, Hauth J. Effects of domestic violence on preterm birth and low birth weight. Acta Obste Gynecol Scand. 2004;83(5):455–460. doi: 10.1111/j.0001-6349.2004.00458.x. [DOI] [PubMed] [Google Scholar]
- 3.Phares TM, Morrow B, Lansky A, Barfield WD, Prince CB, Marchi KS, et al. Surveillance for disparities in maternal health-related behaviors—selected states, Pregnancy Risk Assessment Monitoring System (PRAMS), 2000-2001. MMWR. 2004;53(4):1–13. [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 5.Villar J, Farnot U, Barros F, Victora C, Langer A, Belizan JM. A randomized trial of psychosocial support during high-risk pregnancies. The Latin American Network for Perinatal and Reproductive Research. N Engl J Med. 1992;327(18):1266–1271. doi: 10.1056/NEJM199210293271803. [DOI] [PubMed] [Google Scholar]
- 6.Ricketts SA, Murray EK, Schwalberg R. Reducing low birthweight by resolving risks: Results from Colorado's Prenatal Plus Program. AJPH. 2005;95(11):1952–1957. doi: 10.2105/AJPH.2004.047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strecher V, Wang C, Derry H, Wildenhaus K, Johnson C. Tailored interventions for multiple risk behaviors. Health Educ Res. 2002;17:619–626. doi: 10.1093/her/17.5.619. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Goins KV, Pbert L, Ockene JK. Predictors of smoking cessation in pregnancy and maintenance postpartum in low-income women. Ma Child Health Jl. 2005;9(4):393–402. doi: 10.1007/s10995-005-0020-8. [DOI] [PubMed] [Google Scholar]
- 9.Moses-Kolko EL, Roth EK. Antepartum and postpartum depression: healthy mom, healthy baby. JAMWA. 2004;59(3):181–191. [PubMed] [Google Scholar]
- 10.Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Implications of ante-natal depression and anxiety for obstetric outcome. Obstet Gynecol. 2004;104(3):467–476. doi: 10.1097/01.AOG.0000135277.04565.e9. [DOI] [PubMed] [Google Scholar]
- 11.Pallitto CC, Campbell JC, O'Compo P. Is intimate partner violence associated with unintended pregnancy? A review of the literature. Trauma Viol Abuse. 2005;6(3):217–235. doi: 10.1177/1524838005277441. [DOI] [PubMed] [Google Scholar]
- 12.Plichta SB. Intimate partner violence and physical health consequences: Policy and practice implications. J Interpersonal Violence. 2004;19(11):1296–1323. doi: 10.1177/0886260504269685. [DOI] [PubMed] [Google Scholar]
- 13.Parker B, McFarlane J, Soeken K, Silva C, Reel S. Testing an intervention to prevent further abuse to pregnant women. Res Nurs Health. 1999;22(1):59–66. doi: 10.1002/(sici)1098-240x(199902)22:1<59::aid-nur7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JK, Haider F, Ellis K, Hay DM, Lindow SW. The prevalence of domestic violence in pregnant women. Int J Obstet Gynaecol. 2003;110(3):272–275. [PubMed] [Google Scholar]
- 15.Sockrider MM, Hudmon KS, Addy R, Dolan Mullen P. An exploratory study of control of smoking in the home to reduce infant exposure to environmental tobacco smoke. Nicot Tob Res. 2003;5(6):901–910. doi: 10.1080/14622200310001615240. [DOI] [PubMed] [Google Scholar]
- 16.Logsdon MC, Davis DW. Social and professional support for pregnant and parenting women. Am J Mat Child Nurs. 2003;28(6):371–376. doi: 10.1097/00005721-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Matthey S, Kavanagh DJ, Howle P, Barnett B, Charles M. Prevention of postnatal distress or depression: an evaluation of an intervention at preparation for parenthood classes. J Affect Disord. 2004;79(1-3):113–126. doi: 10.1016/S0165-0327(02)00362-2. [DOI] [PubMed] [Google Scholar]
- 18.Lando HA, Valanis BG, Lichtenstein E, Curry SJ, McBride CM, Pirie PL, et al. Promoting smoking abstinence in pregnant and postpartum patients: a comparison of 2 approaches. Am J Managed Care. 2001;7(7):685–693. [PubMed] [Google Scholar]
- 19.EI-Khorazaty MN, Johnson AA, Kiely M, El-Mohandes AAE, Subramanian S, Laryea HA, Murray KB, Thornberry JS, Joseph JG. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for primary care. Behav Res Ther. 1997;35(8):785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. BDI-FastScreen for medical patients. San Antonio, TX: Psycho Corp, Harcourt Assessment Inc.; 2000. [Google Scholar]
- 22.Melvin CL, Tucker P. Smoke-Free Families Common Evaluation Measures for Pregnancy and Smoking Cessation Projects Working Group. Measurement and definition for smoking cessation intervention research: the Smoke-Free Families experience. Tob Control. 2000;9 III:iii87–iii90. doi: 10.1136/tc.9.suppl_3.iii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams PF, Benson V. Current estimates from the National Health Interview Survey, 1990. National Center for Health Statistics. Vital Health Stat. 1991;10(181) [PubMed] [Google Scholar]
- 24.LeClere FB, Wilson JB. Advance data from vital and health statistics. no. 288. Hyattsville, Maryland: National Center for Health Statistics; 1997. Smoking behavior of recent mothers, 18–44 years of age, before and after pregnancy: United States, 1990. [PubMed] [Google Scholar]
- 25.National Center for Health Statistics. 1990 National Health Interview Survey of Health Promotion and Disease Prevention. Pregnancy and Smoking Tape Documentation. 1991 [Google Scholar]
- 26.Yu SM, Park CH, Schwalberg RH. Factors associated with smoking cessation among U.S. pregnant women. Mat Child Health J. 2002;6(2):89–97. doi: 10.1023/a:1015412223670. [DOI] [PubMed] [Google Scholar]
- 27.Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children's exposure to environmental tobacco smoke: randomised controlled trial. BMJ. 2000;321:337–42. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiClemente CC, Prochaska JO, Fairhurst S, Velicer WF, Rossi JS, Velasquez M. The process of smoking cessation: An analysis of precontemplation, contemplation and contemplation/action. J Consult Clin Psychol. 1991;59:295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 29.Velicer WF, Fava JL, Prochaska JO, Abrams DB, Emmons KM, Pierce J. Distribution of smokers by stage in three representative samples. Prev Med. 1995;24:401–411. doi: 10.1006/pmed.1995.1065. [DOI] [PubMed] [Google Scholar]
- 30.Prochaska JO, Velicer WF, DiClemente CC, Fava JL. Measuring the processes of change: Applications to the cessation of smoking. J Consult Clin Psychol. 1988;56:520–528. doi: 10.1037//0022-006x.56.4.520. [DOI] [PubMed] [Google Scholar]
- 31.Derogatis LR, Lipman RS, Coci L. SCL-90, an outpatient psychiatric rating scale-preliminary report. Psychopharmacol Bull. 1973:13–28. [PubMed] [Google Scholar]
- 32.Derogatis L, Rickels K, Uhlenhuth EH, Covi L. The Hopkins symptom checklist: a measure of primary symptom dimensions. In: Pichot P, editor. Psychological Measurements in Psychopharmacology: Problems in Psychopharmacology. Basel: Kargerman; 1974. pp. 79–110. [DOI] [PubMed] [Google Scholar]
- 33.Straus MA. Manual for the Conflict Tactics Scales. Durham, NH: Family Research Laboratory, University of New Hampshire; 1995. [Google Scholar]
- 34.Straus MA, Gelles RJ. Physical Violence in American Families: Risk Factors and Adaptations to Violence in 8,145 Families. New Brunswick, NJ: Transaction Pub; 1990. [Google Scholar]
- 35.Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scale (CTS2): Development and preliminary psychometric data. J Fam Issues. 1996;17(3):283–316. [Google Scholar]
- 36.McFarlane J, Parker B. Preventing abuse during pregnancy: An assessment and intervention protocol. Am J Mat Child Nurs. 1994;19:321–324. [PubMed] [Google Scholar]
- 37.Soeken K, McFarlane J, Parker B, Lominack MC. The abuse assessment screen: A clinical instrument to measure frequency, severity, and perpetrator of abuse against women. In: Campbell JC, editor. Empowering survivors of abuse: health care for battered women and their children. Thousand Oaks, CA: Sage Publications; 1998. [Google Scholar]
- 38.McFarlane J, Malecha A, Gist J, Watson K, Batten E, Hall I, et al. Increasing the Safety-Promoting Behaviors of Abused Women. Am J Nurs. 2004;104(3):40–50. doi: 10.1097/00000446-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Bandura A. Self-Efficacy: Toward a unifying theory of behavior change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 40.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 41.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. AmJ Health Promot. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 42.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increase abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62(1):141–6. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 43.Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94(5):685–95. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- 44.Sykes CM, Marks DF. Effectiveness of a cognitive behaviour therapy self-help programme for smokers in London, UK. Health Prom Int. 2001;16(3):255–60. doi: 10.1093/heapro/16.3.255. [DOI] [PubMed] [Google Scholar]
- 45.Windsor RA, Cutter G, Morris J, et al. The effectiveness of smoking cessation methods for smokers in public health maternity clinics: A randomized trial. AJPH. 1985;75:1389–1392. doi: 10.2105/ajph.75.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Windsor RA. Counseling smokers in Medicaid maternity care: the SCRIPT project. Tob Control. 2000;9 1:i62. doi: 10.1136/tc.9.suppl_1.i62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Windsor RA, Woodby L, Miller T, et al. Effectiveness of agency for health care policy and research clinical practice guideline and patient education methods for pregnant smokers in Medicaid maternity care. Am J Obstet Gynecol. 2000;182(1):68–75. doi: 10.1016/s0002-9378(00)70492-3. [DOI] [PubMed] [Google Scholar]
- 48.Prochaska JO. Psychology of smoking cessation programs: Lessons from Pathways to Change. Progress with Nicotine Replacement Products for Smoking Cessation: Perspectives on Newer Products and Controversial Issues. Scientific Therapeutics Information. 1995 [Google Scholar]
- 49.Miranda J, Munoz R. Intervention for minor depression in primary care patients. Psychosom Med. 1994;56(2):136–141. doi: 10.1097/00006842-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Dutton MA. Empowering and healing battered women. New York: Springer; 1992. [Google Scholar]
- 51.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Method. 2001;27(1):85–95. [Google Scholar]
- 52.Raghunathan TE, Solenberger P, Van Hoewyk J. Ann Arbor, MI: Survey Methodology Program, University of Michigan; 2002. IVEware: Imputation and Variance Estimation Software: User Guide. http://www.isr.umich.edu/src/smp/ive/ [Google Scholar]
- 53.SAS Institute Inc. SAS/STAT Software. Version 9.1. Cary, NC: SAS Institute Inc.; 2003. [Google Scholar]
- 54.Chabrol H, Teissedre F, Saint-Jean M, Teisseyre N, Roge B, Mullet E. Prevention and treatment of post-partum depression: a controlled randomized study on women at risk. Psychol Med. 2002;32(6):1039–1047. doi: 10.1017/s0033291702006062. [DOI] [PubMed] [Google Scholar]
- 55.Gamble JA, Creedy DK, Webster J, Moyle W. A review of the literature on debriefing or non-directive counseling to prevent postpartum emotional distress. Midwifery. 2002;18(1):72–79. doi: 10.1054/midw.2001.0287. [DOI] [PubMed] [Google Scholar]
- 56.Field T, Diego M, Henandez-Reif M, Salman F, Schanberg S, Kuhn C, Yando R, et al. Prenatal anger effects on the fetus and neonate. Obstet Gynaecol. 2002;22(3):260–266. doi: 10.1080/01443610220130526. [DOI] [PubMed] [Google Scholar]