Abstract
A large number of variables have been identified which appear to influence macrophage phenotype within the tumour microenvironment. These include reciprocal chemical and physical interactions with tumour cells and with non-malignant cells of the tumour microenvironment, tissue oxygen tension, and the origin and prior experience of the particular macrophage population. In this review we outline the key evidence for these influences and consider how macrophage phenotype is acquired and the relevance of the TLR/NF-κB pathway.
Introduction to macrophage biology
Macrophages are part of the innate immune system, recognising, engulfing and destroying many potential pathogens. Macrophages can also recognise autologous tumour cells, virus-infected cells and benign cells undergoing programmed cell death (apoptosis) [1, 2]. Aside from their roles in innate immunity, macrophages function as regulator and effector cells in both humoral and cell mediated immune responses [3]. Upon phagocytosis, macrophages degrade proteins and process antigens for presentation on MHC molecules [4], where T-cells can recognise the substances as “foreign”. When activated in an immune response, macrophages acquire microbicidal and tumouricidal activities involving reactive-oxygen species and reactive-nitrogen metabolites [5].
Macrophages possess great potential to orchestrate the microenvironment of normal tissues and contribute to many aspects of physiological homeostasis. During embryonic development, macrophages play an important role in scavenging dying cells and clearing areas of apoptosis, and thereby contribute to organogenesis [6]. The macrophage lineage is involved in wound healing, tissue repair, bone remodelling, graft-versus-host reactions, acute and chronic inflammation and cancer-related inflammation. Solid tumours consist of neoplastic cells, non-malignant stromal cells and migratory haematopoietic cells. Complex interactions between the different cell types in this microenvironment regulate inflammation, tissue remodelling, tumour growth, progression, metastasis and angiogenesis [7, 8]. There is strong evidence that the tumour microenvironment is inflammatory and that activation of the innate immune system plays a role in the progression of cancer [3, 7]. Indeed, plasticity of macrophage function is well described, and evidence suggests that TAM are involved in complex chemical cross talk with tumour cells and other cells of the tumour microenvironment [9-11]. This interplay has the potential to modulate macrophage phenotype which may in turn change the ability of the tumour to thrive. Several publications in recent years demonstrated the importance of the ‘right’ phenotype at the ‘right’ time [12]. There is an extensive body of literature showing that myeloid cells, such as macrophages are not only abundant in epithelial cancers but also involved in promoting tumour development and spread. These conclusions are based not only on association studies, but also on experiments showing that ablation of macrophage function, or inhibition of their infiltration into experimental tumours, inhibits growth and metastases [13]. Additionally gene array studies of diagnostic lymph node specimens in follicular lymphoma have shown that genes associated with a strong ‘macrophage’ signature are associated with a poorer prognosis, independently of clinical variables or of gene expression of the tumour cells themselves [14].
The paradigms of macrophage plasticity
Macrophage activation depends on signals from the surrounding microenvironment and can be strongly regulated by the products of T-lymphocytes and natural killer cells. Interferon-gamma and a cytokine network involving interleukin-4, interleukin-10, interleukin-12, interleukin-13 appear to be particularly important. In this regard we will appraise whether the frequently-quoted classification of activated macrophages as M1 or M2 phenotype, referring to the Th1/Th2 paradigm, remains valid and relevant. In vitro data established that peripheral blood monocyte-derived macrophages could be polarized into M1 and M2 phenotypes. This has been extensively validated as an in vivo phenomenon for M1 macrophages which are activated by IFN-γ or by bacterial cell wall derived Lipopolysaccharide (LPS). Less well defined is the alternative activation of M2 macrophages [15]. IL-4 and IL-13 have common, as well as regulatory functions in type-1 and type-2 responses.
As initially described, M1 macrophages are activated by microbial products or IFN-γ, produce large amounts of proinflammatory cytokines, express high levels of MHC molecules, and are implicated in the killing of pathogens and tumour cells [1]. M2 macrophages moderate the inflammatory response, can promote angiogenesis and tissue remodelling in cancer [8, 16]. Stimulation with IL-4, IL-13, IL-10, immune-complexes, glucocorticoid hormones, and agonists of Toll-like receptors (TLR) or the IL-1R, drives macrophages toward an M2 phenotype [17]. TAM mainly display an IL-10high, IL-12low phenotype with expression of mannose (MR) and scavenger receptor class A (SR-A) [2] and approximate an M2 phenotype. The tumour environment is thought to ‘educate’ TAM [18] towards a tumour-promoting M2 phenotype but the mechanisms underlying this phenomenon are not fully understood. According to Condeelis and Pollard [19], TAM are obligate partners for malignant cell migration, invasion and metastases in many different cancers. There is a growing body of pre-clinical and clinical evidence associating abundance of TAM with poor prognosis [20].
In relation to TAM it may be particularly confusing to refer to a binary differentiation between M1 and M2 phenotypes. This scheme derived from attempts to classify ‘M2’ activation as the phenotype that will result from exposure to cytokines (IL-4, IL-13) that are produced generally in Th2-type responses, particularly in allergic, cellular and humoral responses to parasitic and extracellular pathogens [1, 15]. Several studies postulated the effects of these cytokines on macrophages, together with the effects of IL-10, as being deactivating. However, it has been evident for some time that IL-4 and IL-13 induce overlapping cell-surface and other phenotypic changes that are distinct from those induced by IFN-γ and IL-10 [1, 15]. Macrophage interactions are influenced markedly by cytokines and adhesive interactions with the extracellular matrix. The specific composition of the activating signal leads to a variety of macrophage functions, which differ significantly.
Attempting to classify all activated macrophage phenotypes as simply either M1 or M2 risks oversimplifying the true in vivo situation, and may lead to misleading conclusions being drawn. Macrophage phenotype and activation status needs to be seen in the context of their physical, chemical and cellular environment. Indeed, there is compelling in vitro evidence that not only is activation of TAM required to promote tumour growth, but that defects in the regulation of such activated macrophages might also be necessary. By observing immune synapses formed between Natural Killer lymphocytes and macrophages, Nedvetzki and colleagues [21] showed that macrophage-lytic synapses developed between these cell types if the macrophages were in a state of activation. This indicates a role for NK cells in eradicating activated macrophages, and suggests important interactions between immune cell populations. Defects in this auto-regulation of activated macrophage numbers may contribute to tumour progression. Additionally antibody-dependent cellular phagocytosis, mediated by macrophages contributes to antitumour activity in vitro [22]. As such, the functional significance of macrophage activation is likely to depend on many factors in addition to macrophage phenotype alone. Several publications have highlighted an important – but not fully understood - role of macrophages in promoting cancer. Innate immune cells, such as macrophages, drive malignant progression through the production of proinflammatory mediators such as TNF-α and IL-6 [23] [24] [25].
Macrophage activation in the gastro-intestinal tract
GI tract macrophage populations exemplify the influence of surrounding stromal cells and their products on macrophage phenotype. Furthermore, they provide evidence for phenotypic variation between resident and recruited macrophage populations. Such complex interactions provide tantalising clues as to how tissue-specific TAM phenotypes may be acquired in the context of gastro-intestinal and other malignancies.
The gastrointestinal (GI) tract is not only a site of direct interaction with the external environment but is also a site that is populated by commensal microbial flora.The local immune cells must be able to mount effective immune responses to external pathogenic agents and also maintain tolerance to gut flora. Consequently, the GI tract harbours a particular subset of innate and adaptive immune cell populations that display phenotypic properties that are specific to and conditioned by their tissue localisation. Lamina propria (LP) macrophages have a particular non-inflammatory phenotype, which is characterised by the reduced expression of innate immune receptors, low co-stimulatory activity and pro-inflammatory cytokine production [26]. In this respect they approximate to the described M2 phenotype. On the other hand, LP macrophages seem to retain normal and even enhanced phagocytic and anti-bacterial activity, albeit without the ensuing production of the pro-inflammatory cytokines IL-1, IL-6, IL-8 and TNF-α [27]. Human LP macrophages and peripheral blood monocytes express similar levels of CD33, CD68 and CD13 (myeloid markers) as well as HLA-DR (MHCII) [28]. However, LP macrophages have reduced expression of CD14, Fcα (CD89) and Fcγ (CD16/CD32/ CD64) receptors for IgA and IgG, respectively, complement receptors 3 (CD11b/CD18) and 4 (CD11c/CD18), LFA-1 integrin (CD11a/CD18) and TREM (‘triggering receptor expressed on myeloid cells”) [28, 29]. In addition, intestinal macrophages are also deficient for the production of cytokines such as IL-1, IL-6, IL-8, IL-10, IL-12, RANTES, TGF-β and TNF-α [27, 28]. These reduced levels of expression have functional consequences as LP macrophages have a limited response to LPS and IgA, for example, which probably contributes to the absence of an immune response to intestinal bacteria under baseline conditions and maintenance of gut homeostasis [28]. In addition, the potential antigen presenting function of LP macrophages is also compromised by their reduced expression of molecules that participate in the activation of antigen-specific T cells such as CD80, CD86 and CD40 [30]. Interestingly, peripheral blood monocytes can acquire a similar phenotype to LP macrophages when they are exposed to intestinal stromal-conditioned media, exemplifying their plastic nature. Intestinal stromal cells produce IL-10 and TGF-β, and the latter seems to be an important mediator of the conditioning effect of stromal cell products on peripheral blood monocytes as targeting this cytokine with blocking antibodies abrogates the change in phenotype [27]. IL-10 also plays an important role in maintenance of intestinal homeostasis that is made evident in IL-10-deficient mice, in which intestinal macrophages differentiate abnormally into cells that produce high levels of IL-12 and IL-23 and contribute to the spontaneous development of colitis [31]. In addition to these regulatory soluble factors, direct cell interactions are likely to be required for the development of the complete LP macrophage phenotype. Resident intestinal macrophages, are characterised by low or absent CD14 expression [28] but in settings of intestinal inflammation, such as inflammatory bowel disease (IBD) or Crohn’s disease, the number of CD14hi cells increases and there is evidence this represents pro-inflammatory blood monocytes recruited into sites of inflammation. In Crohn’s disease, increased expression of CD40 can also be detected, which co-localises with CD68 [32]. In fact, increased expression of CD34 (L-selectin ligand) is observed in mucosal endothelial cells from Crohn’s disease and ulcerative colitis patients and is likely to contribute to monocyte recruitment into tissues [33]. The significance of macrophage recruitment into inflamed mucosa was also evident in a spontaneous model of ileitis in which increased expression of P-selectin glycoprotein-1 (PSGL-1), P-selectin and vascular cell adhesion molecule-1 (VCAM-1) was detected. Blocking of any of these three adhesion proteins was shown to inhibit the adhesion of CD14+ monocytes and importantly PSGL-1 blocking could also inhibit T cell adhesion and had a positive therapeutic effect [34]. In addition to the expression of CD14, the infiltrating monocytes also express the co-stimulator protein CD80 while the majority of resident macrophages upregulate CD86 [30]. There is also upregulation of TREM-1 in intestinal macrophages of patients with Crohn’s disease and ulcerative colitis, which contributes to the amplification of the inflammatory phenotype [35] (Figure 1).
Figure 1.
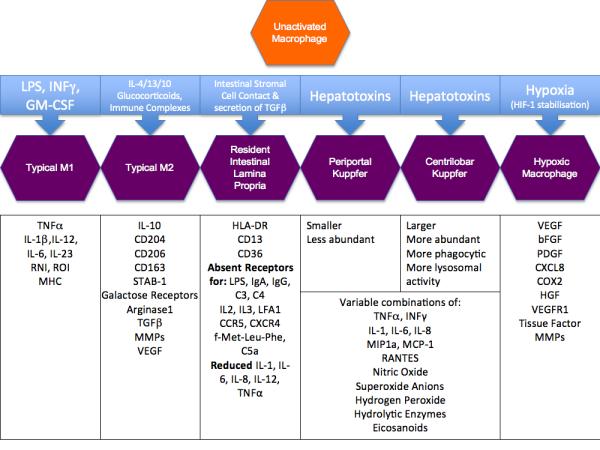
Ways of activation and polarization of LP macrophages, Kupffer cell, periportal/centrilobal macrophages and the phenotype-modulating triggers.
Macrophages in the Liver
The majority of the hepatic blood supply is provided by the portal vein, which carries gut-derived nutrients, toxins and antigens, as well as substances derived from the spleen. This unique location allows molecules absorbed by the intestine to transit the liver, where they are metabolized, or degraded if toxic. This function also implies that the liver is continuously exposed to antigens and cells of the immune system. Therefore Kupffer cells are continuously exposed to endotoxins and in chronic state of low-level activation (Figure 1) [36].
Liver damage is caused by variety of chemical, viral and microbial pathogens. It is thought that inflammation not only precedes but is also needed to generate damage of the hepatocytes. Hepatotoxins lead to Kupffer cell activation and the release of superoxide anions, hydrogen peroxide, nitric oxide, hydrolytic enzymes, eicosanoids, cytokines (IL-1, IL-6, IL-8, TNF-α), and chemokines (MIP1a, MCP-1, RANTES, IL-8), which can induce liver injury either by acting directly on the liver cells or via chemoattraction of extra-hepatic cells (e.g monocytes, neutrophils, lymphocytes). Like other mononuclear phagocytes Kupffer cells have the capacity to act as antigen presenting cells for the induction of T-lymphocyte response and are known to release a number of different immunoregulatory cytokines such as IL-1, IL-6, TNF-α, TGF-β and IFN-γ. Kupffer cell functional and phenotypic heterogeneity seems to be closely related with location. Location (periportal or centrilobular) of Kuppfer cells is key to their main function. Periportal Kupffer cells are more abundant, are larger, possess greater lysosomal enzyme activities, and demonstrate more phagocytic activity. This heterogeneity is also reflected in their maturity with more mature macrophages expressing higher levels of lysosomal enzymes, TNF-α, IL-1 and Prostaglandin E whereas the more immature population is characterized by the release of nitric oxide and superoxide anions and they exhibit increased cytotoxic activity towards tumour cells. The liver provides examples of how macrophage activation may be physiological and beneficial to the host, or may be potentially maladaptive and harmful. This appears to depend upon the location and maturity of the macrophages, and so by inference, the composition of their prior and ongoing antigen exposure. This provides further support to the concept that macrophage activation is not simply a binary M1/M2 phenomenon, but is almost as variable as the activating stimuli that direct it. Some of the key molecular events underlying macrophage activation are examined below.
Toll-like receptors in tumour-macrophage communication
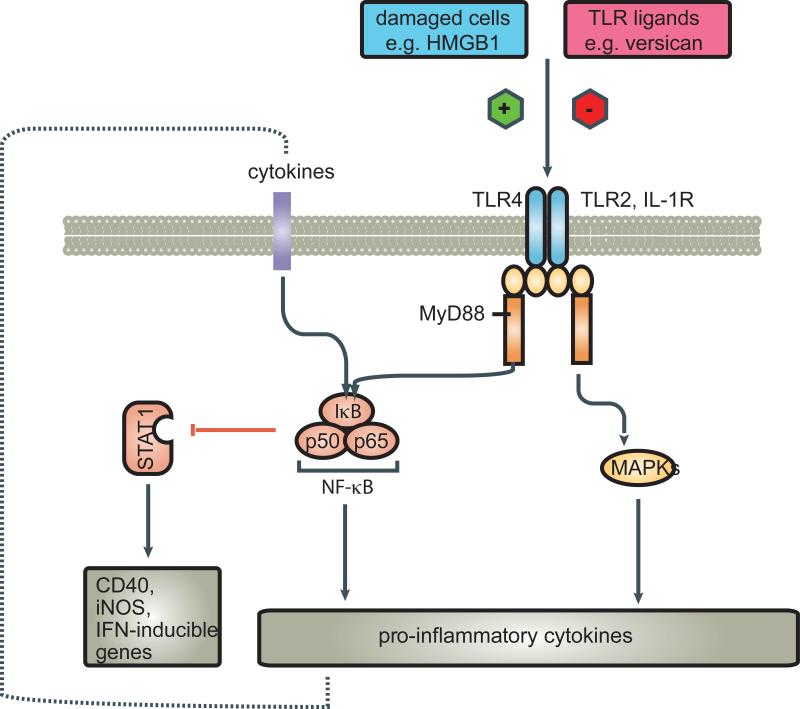
In 1863 Virchow noted leukocytes in neoplastic tissues and made a connection between inflammation and cancer [37, 38]. Deidier observed a positive correlation between infection and remission of malignant disease in the 18th century [39]. It has long been suggested that there may be common pathways of inflammation shared by responses to infection, and to malignancy. We present evidence that Toll-like receptors (TLR) on macrophages may be critical elements in these common pathways. TLR are classically described as a family of transmembrane receptors that recognize conserved molecular patterns of microbial origin (Figure 2). Accumulating evidence indicates that TLRs also have an important role in tissue repair and tissue injury-induced inflammation, as well as cancer progression. TLR ligands in this case can be either microbial or host-derived [40]. Signalling pathways via TLRs originate from the conserved Toll/IL-1 receptor (TIR) domain. The TIR domain-containing MyD88 acts as a common adaptor that induces inflammatory cytokines; however, there exists a MyD88-independent pathway that induces type I IFNs in TLR4 and TLR3 signalling [41, 42] (Figure 2).
Figure 2.
Macrophage – Tumour cell interaction. There are a variety of factors in the microenvironment, tumour cell or stroma derived which can polarize macrophages towards alternatively activation. IKKβ [60, 61] maintains the alternative activation of macrophages which contributes to tumour progression. Inhibition of macrophage IKKβ signalling, in the presecence of a physiological ligand, increases STAT1 activation and leads to recruitment of NK cells and disease stabilization.
A recent study by Naugler and colleagues [43] using a mouse model of chemically induced liver cancer, suggests cell injury may also lead to the release of endogenous factors that activate innate immune cells. These authors showed that dead hepatocytes activate liver macrophages (Kupffer cells) through the molecule MyD88, which is an essential adaptor for Toll like receptor (TLR) signaling [43]. The TLRs are pathogen recognition molecules that are hard-wired to trigger activation of innate immunity upon recognition of pathogen-associated molecular patterns (PAMPs). TLRs have an important role in driving the inflammatory response but also in priming adaptive immunity through the activation and maturation of antigen-presenting cells, including dendritic cells (DCs) and macrophages.
Apetoh et al. [44] have revealed an interesting role of TLR signalling in cancer therapy. The authors studied the immune-stimulatory properties of dying tumour cells after chemotherapy or radiation therapy. Using TLR4 and MyD88-deficient DCs, they show that TLR4 signaling is required for crosspresentation of antigens from apoptotic tumour cells on MHC class I to generate antitumour cytotoxic T cell (CTL) responses. Apetoh et al. [45] also identified a “danger signal” from dying tumour cells, the nuclear protein highmobility group box 1 protein (HMGB1, Figure 2) that triggers this protective immune response through activation of TLR4. According to their work, the interaction of high mobility group box 1 protein (HMGB1) released from dying tumour cells with Toll-like receptor 4 (TLR4) on dendritic cells is required for the crosspresentation of tumour antigens and the promotion of tumour specific cytotoxic T-cell responses [45]. Furthermore, mutation of TLR4 in mice reduces the efficacy of both chemotherapy and radiation therapy and a mutation in the human TLR4 gene is associated with an increased frequency of metastasis in breast cancer patients after conventional chemotherapy.
HMGB1 is an abundant nuclear protein tightly associated with chromatin and serves both as a transcription factor and as an inflammatory cytokine [46]. HMGB1 binds to two receptor systems, Toll-like receptors (TLRs) 2, 4, and 9 and RAGE [47]. HMGB1 is released by necrotic cells and by apoptotic tumour cells when they are killed by NK or T cells [48, 49].
However, despite this seemingly positive role of TLR signalling in tumour immune responses activation of TLR signalling can also enhance tumour development through various mechanisms. In a mouse model of transplanted metastatic cancers, activation of TLR4 by intraperitoneal injection of bacterial LPS stimulated the growth of lung metastases [50, 51]. TLR4 activation of host macrophages resulted in the production of several different inflammatory cytokines that influenced tumour growth. TNF-α was identified as the major host-produced factor that enhances the growth of lung metastases in this mouse model, in part through activation of NF-κB in the tumour cells [51]. However, TLR4 signalling also induces cytokines (IFN) that have antitumour effects [51] by induction of TRAIL, a potent inducer of tumour cell death [51].
A recent publication by Kim and colleagues [52] showed another side of TLR function. They describe that Lewis lung carcinoma (LLC) cells secreted potent macrophage activators leading to production of IL-6 and TNF-α through activation of the TLR family members TLR2 and TLR6, exclusively. Their studies indicated that a tumour derived extra-cellular matrix proteoglycan, versican, is necessary for TLR2-mediated (but not TLR4) myeloid cell activation and consequently metastatic spread. TLR2 is known to recognise gram positive bacteria-derived lipoteichoic acid and lipoprotein and appears to function cooperatively with a variety of other receptors (such as TLR1, TLR6 and CD14) under certain conditions. Indeed, there is evidence to suggest that TLR2 can cooperate with a variety of different co-receptors, to different extents,–depending on the stimulating antigen. The observations by Kim et al [52] were independent from HMGB1. It has previously been suggested that the activity of HMGB1 as an activator of the immune system is regulated by post-translational modifications. The modifications discussed in this context were acetylation, methylation, and phosphorylation. In particular, hyperacetylated HMGB1, as isolated from actively secreting immune cells, was suggested to be more active as an adjuvant than purified recombinant protein [46]. Kazama et al [53] found that the regulation lies not simply in the release of HMGB1 from necrotic cells, but rather in the oxidative state of the HMGB1 that is released. The post-translational modifications might be responsible for the observed differences in these two studies. This will raise the question how the different forms of HMGB1 affect the variety of cell types, such as macrophages and DCs, and how different forms of HGMB1 regulate innate immunological functions.
This process might contribute to the plasticity of macrophage phenotype and possibly in determining the diverse functions of macrophages. Better knowledge could potentially be used to modulate macrophage activities and interfere with unwanted immunological activation.
Dr. William Coley, in the late 19th century, applied this philosophy to treat cancer and developed what became known as Coley’s toxin, which was in fact a soup of killed Streptococcus pyogenes and Serratia marcescens [54]. Coley extrapolated the association of postoperative infections with improved clinical outcome. The approach is still used today in the form of bacillus Calmette-Guerin (BCG) for treatment of bladder cancer [55]. Researchers have tried in vain to identify the “active” component of both Coley’s vaccine and BCG for treatment of cancer. However, it is likely these agents trigger an innate immune response through multiple TLRs, which may provide both direct tumouricidal activity and a platform for the development of anti-tumour immunity but on the other hand may provide an additional stimulus to further promote tumour progression. BCG and Coley’s toxins probably trigger a ‘good’ inflammatory response, via TLRs, that not only stimulates macrophages to kill tumour cells but also promotes development of sustained and effective adaptive immunity to the tumour. This type of response may also contribute to successful chemotherapy or radiotherapy, according to recent data from Apetoh et al [44]. In the light of the data from Kim et al [52] it will be important to establish how best to stimulate TLRs to change a tumour-promoting microenvironment to a tumour-inhibiting state and to understand the signalling mechanisms involved. Many TLRs share similar signalling pathways, and it is likely that the strength of overall TLR ligation is significant in determining downstream events, but the above data suggest that this is also influenced by the pattern of TLR subtypes stimulated and the antigens ligating them.
Coley’s work remains controversial and few have been able to reproduce the beneficial effects that Coley obtained. We can only guess the macrophage phenotype in Coley’s experiments, as fever seemed to be a critical determinate in the historic case reports. Therefore the biologically active agent in Coley’s Toxin was thought to be LPS with consequent lymphocyte activation and increase in pro-inflammatory cytokines such as TNF-α, IL-6, IL-12.
Downstream of TLR signalling, Nuclear Factor-Kappa B (NF-κB) – the crucial link?
The activation of TLR2 and TLR4 results in the activation of inflammatory cells and initiation of host responses directed to eliminate and kill invading organisms [56, 57]. However, inadequate pathogen eradication, prolonged inflammatory signalling, and defects in anti-inflammatory mechanisms can all lead to chronic inflammation and benefit tumour development [58]. Both TLR2 and TLR4 trigger NF-κB activation, leading to cell survival and pro-inflammatory responses. Recent studies in mouse models of colon and liver cancer have defined an important role for NF-κB activation in driving cancer-associated inflammation [23, 59] and data from our own group described an additional role for NF-κB in maintaining the immunosuppressive phenotype of alternatively activated macrophages [60, 61]. NF-κB is a transcription factor with a pivotal regulatory role in a variety of cellular processes. It appears to be a key regulator of macrophage activation pathways and is critical for macrophage activation responses to inflammatory stimuli including TLR ligands [62, 63]. Activation of NF-κB in macrophages has been shown to be required for the onset of tumour development in several inflammation-induced cancer models [23, 59, 64]. Various cytokines are produced by innate immune cells as a result of NF-κB activation during tumour development and progression. These cytokines include TNF-α and IL-6 which, although they participate in a wide variety of complex interacting pathways, have been implicated in providing not only pro-survival signals in tumour cells (through NF-κB or STAT3 activation in these cells) but also support for tumour growth and progression [16]. Experimental validation for this concept has come from genetic studies of murine hepatocellular carcinoma (HCC) and colitis associated colon cancer (CAC) [23, 59, 64]. In these examples, targeted gene deletion of key elements of the NFkB pathway such as IKKβ[23] or inhibition of regulatory cytokines such as TNF-α [65] resulted in reduced tumour volume over time. These studies emphasise the essential requirement of NF-κB activation in both TAMs and tumour cells in perpetuating the inflammatory pathways promoting tumour initiation and growth. In particular, NF-κB activation in TAMs shapes their pro-tumoural repertoire including the release of TNFα, IL-6 and IL-1β which sustain tumour cell growth/survival [7, 66, 67]. It is interesting to note these convincingly pro-tumoural activities of TNFα, given its early descriptions as a “tumour necrotizing factor” [68], a consequence of destructive effects on tumour vasculature [69], which occurs only under certain circumstances and does not accurately represent our current understanding of its multitude of complex pathophysiological roles [68].
The plasticity of macrophage activation over time has been indicated by such studies of inflammation induced cancer. Macrophages appear to initially respond to acute inflammatory signals with a pro-inflammatory repertoire, which changes in established tumours to an alternative pro-tumour phenotype [2, 62]. High levels of nuclear p50/p50 homodimers lead to a defective expression of NF-κB-inducible genes and the IL-10high/IL-12low M2-like phenotype [70, 71]. Saccani and colleagues demonstrated that depletion of p50 in TAMs resulted in decreased tumour growth and prolonged host survival due to p50−/− TAMs showed an anti-tumoural, pro-inflammatory M1 phenotype with increased IL-12p40 and TNF-α, but decreased IL-10 production [71]. Redirecting M2-like TAMs (and dendritic cells) toward an M1-like phenotype (by reactivating NF-κB using the TLR9 ligand, CpG and an IL-10 receptor-specific antibody) induced significant anti-tumour immunity in a murine mammary carcinoma model [72].
Tissue specific gene targeting of IKKβ to study the role of NF-κB in inflammation and innate immunity showed that targeted deletion of IKKβ expression in epithelial cells inhibits inflammation and immunity to infection. However, deletion IKKβ and NF-κB activation in macrophages has the opposite effect and results in increased inflammation and conferring resistance to infection. These experiments have shown a role for IKKβ in suppressing ‘classical’ macrophage activation in the context of bacterial infection [60]. A similar role for IKKβ was described in tumour-associated macrophages. This parallel study showed that targeting IKKβ in tumour-associated macrophages (TAMs) prevents their polarisation to an ‘alternative’ activation phenotype and reverses their tumour promoting activity [61]. Both studies showed that IKKβ and NF-κB inhibits ‘classical’ macrophage activation through negative cross-talk with STAT-1 (Figure 3) [60, 61]. Targeted deletion or inhibition of IKKβ in alternatively activated macrophages increased their tumouricidal activity through elevated NOS2 expression and IL-12-dependent recruitment and activation of NK cells. This implies that inhibition of the IKKβ/NF-κB pathway promotes an M1-like phenotype, while intact IKKβ/NF-κB activation maintains these cells in an alternative, tumour-promoting activation state. Saccani et al. [71] showed that overexpression of p50 (an NF-κB subunit) was associated with the M2-like TAM phenotype in a mouse fibrosarcoma model. Collectively these studies suggest an NF-κB dependent mechanism for inhibition of the M1 phenotype in macrophages. Parallel studies have also described an NF-κB dependent mechanism for inhibition of STAT-1 in M1 macrophages using LPS as a stimulus [60], it would be interesting to see if p50−/− macrophages have elevated STAT-1 activation. Although p50 is essential to the expression of many NF-κB target genes, as a binding partner for RelA (p65) and cRel, p50 homodimers have also been described to inhibit NF-κB regulated genes due to the lack of a transactivation domain. This mechanism has been described in the experimental phenomenon of LPS-induced tolerance and particularly inhibition of TNF-α expression [73]. Although this mechanism has not been formally demonstrated in TAM, Saccani et al. [71] suggest p50-mediated repression of IL-12 may offer an alternative explanation for the role of NF-κB in the maintaining the TAM phenotype. The p50 subunit can recruit co-activators that are essential for expression of certain NF-κB target genes and knockout of p50 results in loss of expression [74-76]. Furthermore, the affinity of RelA:p50 heterodimers for NF-κB target gene promoters is much higher than that of p50:p50 homodimers [77], therefore elevation of p50 levels in the nucleus may not necessarily result in repression of NF-κB target genes. Saccani et al. [71] have clearly shown that p50 levels are elevated in TAM, this may result in repression of certain NF-κB target genes, but equally p50 may increase expression of genes required to maintain the TAM phenotype.
Figure 3.
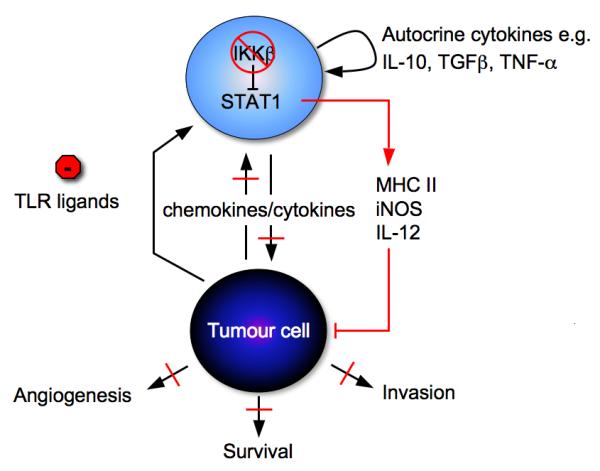
Tumour cell damage, as may result from chemotherapy or radiotherapy, leads to the release of molecules termed danger signals. These include HMGB1, as well as other chemokines and cytokines from the necrotic and damaged cells. They act as chemo-attractants, drawing monocytes, T-lymphocytes, and dendritic cells to the tumour microenvironment, and also act on the resident immune cells. Activation of TLR4 by HMGB1 on dendritic cells induces cross-presentation and the generation of antigen-specific T-lymphocytes [44, 45].
There also appear to be physiological TLR ligands such as versican [52] which lead to similar down-stream activation of pro-inflammatory pathways promoting tumour progression. Activated dendritic cells or macrophages can also release these tumour-promoting proinflammatory cytokines upon TLR activation.
As mentioned before, the phenotype of TAMs can differ markedly between different tumour types [66] and different stages of tumour development [67]. These divergent consequences of NF-κB modulation and inhibition could be accounted for by differences in the methods of deriving macrophages and thus highlights the importance of the origins and prior activation status of any given macrophage population in determining their resultant activated phenotype.
Hypoxia and the TAM phenotype
Hypoxia is a typical feature of tumour microenvironment and, in addition to being positively correlated with the extent of TAM infiltration, promotes the pro-tumour TAM phenotype. HIF-1 (hypoxia-inducible factor-1) α has a key role in controling the cellular response to hypoxia. Hypoxia stabilizes the inducible α-subunit, preventing post-translational hydroxylation and subsequent degradation via the proteasome.Persistent α-subunits attach β-subunits in the nucleus before binding hypoxic response elements (HREs) of oxygen-sensitive genes, altering their expression [78]. In this way, hypoxia stimulates TAM to secrete proangiogenic cytokines and enzymes, immunosuppressive agents and mitogens [79]. In vitro work has identified a distinct hypoxia induced pro-angiogenic human macrophage phenotype, with upregulation of VEGF, bFGF, CXCL8, COX2, HGF, VEGFR1, tissue factor (F3) and MMP12 [80, 81]. In human breast carcinoma, TAM express VEGF almost exclusively in hypoxic and/or perinecrotic areas, and upregulate VEGF on migrating to hypoxic perinecrotic areas of human breast tumour spheroids [82].
In recent years, clear evidence has emerged that HIF-1α is also responsive to many stimuli under normoxic conditions, including thrombin, growth factors, vasoactive peptides, insulin, LPS and cytokines such as TNF-α. One putative mechanism underlying these responses is the transcriptional regulation of HIF-1α by NF-κB. More recently NF-κB has been shown to regulate HIF-1α expression under hypoxic conditions [83]. Rius et al. [83] showed, with the use of mice lacking IKKβ in different cell types, that NF-κB is a critical transcriptional activator of HIF-1α and that basal NF-κB activity is required for HIF-1α protein accumulation under hypoxia. IKKβ deficiency results in defective induction of HIF-1α target genes, including VEGF, which are critical for tumour angiogenesis.
Conclusions
Macrophages are one of the major antigen-presenting cells present in tumours and in certain cases these innate immune cells may account for as much as half the tumour mass. There is increasing evidence that tumour associated macrophages express an immunosuppressive phenotype and display several tumour-promoting functions, including promotion of angiogenesis and matrix remodelling [18, 84]. The phenotype of TAMs has been suggested to promote tumour antigen tolerance through production of immunosuppressive factors rather than by priming a protective immune response [2].
The M1/M2 phenotypic paradigm of macrophage activation has provided a framework for studying the complex in vivo behaviour of TAMs. There is strong evidence of plasticity of macrophage phenotype, which appears to be influenced by multiple environmental factors acting over time on macrophages of different origins on a background of prior exposures and activation states. As such, different macrophage phenotypes are observed not only in different tissues, but in different locations in the same tissue, and at different times in the same tissue. Complex interactions between macrophages and the surrounding cells underlie the variety of phenotypes observed in activated macrophages. Thus, it is not entirely surprising that TAMs do not conform to a single phenotype. Studies illuminating the pathways of macrophage activation, and particularly involving NF-κB, may well provide a clearer mechanistic understanding of the pro-tumour modelling of macrophage function by cancer cells.
It is likely that NF-κB activation in innate immune cells contributes to tumour promotion by a variety of mechanisms. It is clear that NF-κB dependent genes expressed by macrophages, such as TNF-α and IL-6, can promote malignant cell survival and invasion. In addition, there are NF-κB dependent mechanisms to suppress the anti-tumour activity of macrophages; this may be driven by autocrine and paracrine production of cytokines such as TNF-α and IL-1 [85] [61]. Recent evidence also suggests that NF-κB regulates the hypoxic response in macrophages [83], which is thought to be critical to their tumour-promoting phenotype.
From the perspective of translational research, a major purpose for classifying macrophage populations should be to identify cells with common in vivo biological traits reflecting the very pathways of activation which may ultimately be amenable to therapeutic intervention. This information may be exploited with the use of adjuvants or inhibitors to enhance tumour immunogenicity in conjunction with conventional cancer therapy. So, this is the big question: How can we exploit macrophage plasticity to maximise their therapeutic potential?
References
- 1.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 4.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A. Macrophage diversity and polarization: in vivo veritas. Blood. 2006;108:408–409. [Google Scholar]
- 6.Hume DA. Bring out your dead. Nat Immunol. 2008;9:12–14. doi: 10.1038/ni0108-12. [DOI] [PubMed] [Google Scholar]
- 7.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 10.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-{alpha} dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 11.Sica A, Saccani A, Bottazzi B, Bernasconi S, Allavena P, Gaetano B, et al. Defective expression of the monocyte chemotactic protein-1 receptor CCR2 in macrophages associated with human ovarian carcinoma. J Immunol. 2000;164:733–738. doi: 10.4049/jimmunol.164.2.733. [DOI] [PubMed] [Google Scholar]
- 12.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dave SD, Wright G, Tan B, Rosenwald A, Gasoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 19.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 21.Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vely F, Pende D, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 22.Oflazoglu E, Stone IJ, Gordon KA, Grewal IS, van Rooijen N, Law CL, et al. Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood. 2007;110:4370–4372. doi: 10.1182/blood-2007-06-097014. [DOI] [PubMed] [Google Scholar]
- 23.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- 25.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 27.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–2656. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 29.Schenk M, Bouchon A, Birrer S, Colonna M, Mueller C. Macrophages expressing triggering receptor expressed on myeloid cells-1 are underrepresented in the human intestine. J Immunol. 2005;174:517–524. doi: 10.4049/jimmunol.174.1.517. [DOI] [PubMed] [Google Scholar]
- 30.Rugtveit J, Bakka A, Brandtzaeg P. Differential distribution of B7.1 (CD80) and B7.2 (CD86) costimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD) Clin Exp Immunol. 1997;110:104–113. doi: 10.1046/j.1365-2249.1997.5071404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 32.Sawada-Hase N, Kiyohara T, Miyagawa J, Ueyama H, Nishibayashi H, Murayama Y, et al. An increased number of CD40-high monocytes in patients with Crohn’s disease. The American journal of gastroenterology. 2000;95:1516–1523. doi: 10.1111/j.1572-0241.2000.01938.x. [DOI] [PubMed] [Google Scholar]
- 33.Seidelin JB, Vainer B, Horn T, Nielsen OH. Circulating L-selectin levels and endothelial CD34 expression in inflammatory bowel disease. The American journal of gastroenterology. 1998;93:1854–1859. doi: 10.1111/j.1572-0241.1998.538_f.x. [DOI] [PubMed] [Google Scholar]
- 34.Inoue T, Tsuzuki Y, Matsuzaki K, Matsunaga H, Miyazaki J, Hokari R, et al. Blockade of PSGL-1 attenuates CD14+ monocytic cell recruitment in intestinal mucosa and ameliorates ileitis in SAMP1/Yit mice. J Leukoc Biol. 2005;77:287–295. doi: 10.1189/jlb.0204104. [DOI] [PubMed] [Google Scholar]
- 35.Mulligan G, Mitsiades CS, Bryant B, Zhan F, Chng WJ, Roels S, et al. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood. 2007;109:3177–3188. doi: 10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]
- 36.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the ’liver tolerance effect’. Immunol Cell Biol. 2002;80:84–92. doi: 10.1046/j.0818-9641.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- 37.Virchow R. Die krankhaften Geschwülste. Dreissig Vorlesungen, gehalten während des Wintersemesters 1862-1863. Lithographie als Frontispiz Berlin: A Hirschwald 1863. 1863;Bd. I:543. [Google Scholar]
- 38.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 39.Garay RP, Viens P, Bauer J, Normier G, Bardou M, Jeannin JF, et al. Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help. European journal of pharmacology. 2007;563:1–17. doi: 10.1016/j.ejphar.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 41.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 42.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 43.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 44.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 45.Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68:4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 46.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 47.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 48.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 49.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature Reviews Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 50.Pidgeon GP, Harmey JH, Kay E, Da Costa M, Redmond HP, Bouchier-Hayes DJ. The role of endotoxin/lipopolysaccharide in surgically induced tumour growth in a murine model of metastatic disease. Br J Cancer. 1999;81:1311–1317. doi: 10.1038/sj.bjc.6694369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coley WB. Treatment of inoperable malignant tumors with toxins of erysipelas and the bacillus prodigiosus. Trans Am Surg Assn. 1894;12:183–212. [Google Scholar]
- 55.Bassi P. BCG (Bacillus of Calmette Guerin) therapy of high-risk superficial bladder cancer. Surg Oncol. 2002;11:77–83. doi: 10.1016/s0960-7404(02)00008-7. [DOI] [PubMed] [Google Scholar]
- 56.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 57.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 58.Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6:1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 59.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 60.Fong C, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, et al. An anti-inflammatory role for IKKβ through the inhibition of ‘classical’ macrophage activation. J Exp Med. 2008 doi: 10.1084/jem.20080124. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- 63.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 64.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 66.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 67.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 68.Balkwill F. TNF and Cancer: a Timeline. Nature Reviews Cancer. 2009 in press. [Google Scholar]
- 69.Daniel D, Wilson NS. Tumor necrosis factor: renaissance as a cancer therapeutic? Curr Cancer Drug Targets. 2008;8:124–131. doi: 10.2174/156800908783769346. [DOI] [PubMed] [Google Scholar]
- 70.Cao S, Zhang X, Edwards JP, Mosser DM. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem. 2006;281:26041–26050. doi: 10.1074/jbc.M602222200. DOI M602222200 [pii] 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 72.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 73.Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, et al. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Invest. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-kappa B-mediated inhibition. Nature. 1992;359:339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- 75.Leung TH, Hoffmann A, Baltimore D. One nucleotide in a kappaB site can determine cofactor specificity for NF-kappaB dimers. Cell. 2004;118:453–464. doi: 10.1016/j.cell.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. Embo J. 2003;22:5530–5539. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phelps CB, Sengchanthalangsy LL, Malek S, Ghosh G. Mechanism of kappa B DNA binding by Rel/NF-kappa B dimers. J Biol Chem. 2000;275:24392–24399. doi: 10.1074/jbc.M003784200. [DOI] [PubMed] [Google Scholar]
- 78.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. DOI S0006295202011681 [pii] [DOI] [PubMed] [Google Scholar]
- 79.Lewis C, Murdoch C. Macrophage responses to hypoxia. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White JR, Harris RA, Lee SR, Craigon MH, Binley K, Price T, et al. Genetic amplification of the transcriptional response to hypoxia as a novel means of identifying regulators of angiogenesis. Genomics. 2004;83:1–8. doi: 10.1016/s0888-7543(03)00215-5. DOI S0888754303002155 [pii] [DOI] [PubMed] [Google Scholar]
- 81.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–1243. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. 1096–9896. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 83.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 85.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]