Abstract
Background
Adenosine triphosphate-sensitive potassium (KATP) channels in brain are involved in neuroprotective mechanisms. Pharmacologic activation of these channels is seen as beneficial, but clinical exploitation by using classic K+ channel openers is hampered by their inability to cross the blood–brain barrier. This is different with the inhalational anesthetic xenon, which recently has been suggested to activate KATP channels; it partitions freely into the brain.
Methods
To evaluate the type and mechanism of interaction of xenon with neuronal-type KATP channels, these channels, consisting of Kir6.2 pore-forming subunits and sulfonylurea receptor-1 regulatory subunits, were expressed in HEK293 cells and whole cell, and excised patch-clamp recordings were performed.
Results
Xenon, in contrast to classic KATP channel openers, acted directly on the Kir6.2 subunit of the channel. It had no effect on the closely related, adenosine triphosphate (ATP)-regulated Kir1.1 channel and failed to activate an ATP-insensitive mutant version of Kir6.2. Furthermore, concentration–inhibition curves for ATP obtained from inside-out patches in the absence or presence of 80% xenon revealed that xenon reduced the sensitivity of the KATP channel to ATP. This was reflected in an approximately fourfold shift of the concentration causing half-maximal inhibition (IC50) from 26 ± 4 to 96 ± 6 μm.
Conclusions
Xenon represents a novel KATP channel opener that increases KATP currents independently of the sulfonylurea receptor-1 subunit by reducing ATP inhibition of the channel. Through this action and by its ability to readily partition across the blood–brain barrier, xenon has considerable potential in clinical settings of neuronal injury, including stroke.
-
What We Already Know about This Topic
• Adenosine triphosphate-sensitive potassium (KATP) channels are important to neuroprotection, but drugs activating these do not cross into the brain
• Xenon is neuroprotective and has been proposed to act on KATP channels
-
What This Article Tells Us That Is New
• In cell cultures, xenon activated KATP channels by a mechanism distinct from that of most drugs acting at this channel, which may in part explain its neuroprotective effects
Introduction
Adenosine triphosphate-sensitive K+ (KATP) channels are critical for many processes in which electrical activity has to match metabolic constraints.1 Within the central nervous system, KATP channels have been suggested to be key mediators in neuroprotective and preconditioning pathways. They are widely expressed in brain,2 and recent studies have demonstrated that global genetic ablation of the pore-forming subunit Kir6.2 of the KATP channel increases neuronal vulnerability to acute hypoxia significantly,3 thus suggesting that KATP channel opening in brain has a protective effect during hypoxia–ischemia equivalent to that observed for cardiac tissue.4,5 In support of this concept, transgenic overexpression of the regulatory subunit of the channel, the sulfonylurea receptor (SUR)-1, in the forebrain of mice improved their resistance to kainic acid-induced seizures.6 These studies highlight the beneficial effects of KATP channel opening for neuronal survival under pathophysiologic conditions and suggest that this protective potential should be explored under clinical conditions.
Current KATP channel openers such as diazoxide, pinacidil, and nicorandil are used clinically to suppress inappropriate release of insulin or to treat angina or hypertension,7,8 but poor penetration of the blood–brain barrier has limited their use as neuroprotectants; although some beneficial effect is seen in vivo, presumably attributable to their vasodilatory action on cranial vasculature.9
The search for novel KATP channel openers led us to explore inhalational anesthetics. These drugs easily partition across the blood–brain barrier, and some of them have neuroprotective and preconditioning properties.10 One of the most promising anesthetics in this respect is the noble gas xenon.11 In fact, we have recently demonstrated that the preconditioning action of xenon in vitro requires functional KATP channels; however, it remained unclear whether xenon interacts directly with the channel and how its action relates to that of classic KATP channel openers.12
In this study, we intended to analyze the effect of xenon on KATP channels in detail. We hypothesize that xenon interacts with KATP channels directly, and we aim to determine whether xenon acts on the pore-forming Kir6.2 subunit or the SUR subunit. We also postulate that xenon might activate the channel by interfering with adenosine triphosphate (ATP) inhibition.
Materials and Methods
Mutagenesis and Transfection
HEK293 cells were cultured and transiently transfected as described previously.12 For most experiments, cells were transfected with pcDNA3 containing green fluorescent protein, mouse Kir6.2 (wild-type or mutant Kir6.2-K185Q), and rat SUR1. Additional experiments were performed on cells transfected with green fluorescent protein and Kir6.2ΔC26 or green fluorescent protein and rat Kir1.1 (in pcDNA6). Kir6.2-K185Q was generated by site-directed mutagenesis using standard techniques (QuikChange system; Stratagene, Agilent Technologies UK Ltd., Stockport, Cheshire, United Kingdom) and verified by direct sequencing. Cells were incubated in a humidified incubator at 37°C for 24–96 h before use.
Solutions
Stock solutions of tolbutamide (50 mm) and diazoxide (20 mm) were prepared in 100 mm NaOH. Solutions for the xenon experiments were prepared by bubbling with pure gases (xenon, nitrogen, and oxygen) at a rate of 30 ml/min for 20 min, producing saturated solutions for the individual gases.13 The test solutions with fractional gas concentrations were then obtained by mixing adequate volumes of the individual solutions with maintaining 20% oxygen-saturated solution and the balance (80%) being made up by varying amounts of nitrogen- and xenon-saturated solutions. Immediately after mixing, these solutions were transferred to glass syringe barrels containing a polypropylene float.13 All other drugs were added directly to the experimental solutions. Xenon was obtained from Air Products (Basingstoke, Hampshire, United Kingdom), and nitrogen and oxygen were from BOC Gases (Guildford, Surrey, United Kingdom). All other chemicals were from Sigma (Poole, Dorset, United Kingdom).
Electrophysiology
Electrophysiologic recordings were performed on cells that exhibited green fluorescent protein fluorescence. Cells were held at 20°–24°C and constantly superfused at 3–5 ml/min of either extracellular solution containing (in millimolar): 118 NaCl, 3 KCl, 1 MgCl2, 1.5 CaCl2, 25 HEPES (pH adjusted to 7.4 with NaOH) for whole cell recordings or solution containing (in mm): 107 KCl, 2 MgCl2, 1 CaCl2,10 EGTA, 10 HEPES (pH 7.2 with KOH; final [K+] ~140 mm) for inside-out macropatches. For magnesium-free conditions, EGTA was replaced with EDTA and MgCl2 was omitted.
Patch pipettes were pulled from thin-walled borosilicate glass (GC150TF; Harvard Apparatus, Edenbridge, United Kingdom) and had resistances of 3–5 MΩ when filled with pipette solution. Currents were recorded with an EPC9 patch-clamp amplifier (HEKA Elektronik, Lambrecht, Germany). For whole cell recordings, the pipette solution contained (in millimolar) 120 KCl, 1 NaOH, 1 MgCl2, 1 CaCl2, 5 EGTA, 5 HEPES, and 0.3 K2ATP (pH adjusted to 7.3 with KOH; final [K+] ~140 mm). Currents were recorded during 700-ms hyperpolarizing voltage ramps from −20 to −120 mV every 15 s. For inside-out macropatches, the pipette (external) solution contained (in millimolar) 140 KCl, 1.2 MgCl2, 2.6 CaCl2, and 10 HEPES (pH 7.4 with KOH), and currents were recorded in response to 700-ms voltage ramps from −110 to +100 mV. Rapid exchange of solutions was achieved by positioning the patch in the mouth of one of a series of adjacent inflow pipes placed in the bath. Recordings were filtered at 1 kHz, digitized at 3 kHz, and analyzed using Pulse/Pulsefit software (HEKA Elektronik).
Data Analysis
The slope conductance was measured by fitting a straight line to the current–voltage relationship between −20 and −100 mV: the average of five consecutive ramps was calculated in each solution. ATP concentration–inhibition curves for Kir6.2/SUR1 currents were fit to the modified Hill equation:
| (1) |
where G is the conductance in the presence of ATP, Gc is the conductance in the absence of ATP, [ATP] is the ATP concentration, IC50 is the ATP concentration at which inhibition is half maximal, and h is the Hill coefficient (slope factor). Fits were obtained using Origin Pro software (Origin Lab Corporation, Northampton, MA).
Statistical analysis was performed using Origin Pro software. All data were expressed as mean ± SEM. To test for differences between groups, two-tailed unpaired t test or one-way ANOVA followed by Tukey multiple comparison test were used as appropriate. A P value of less than 0.05 was considered significant.
Results
Xenon Enhances KATP Currents in Intact Cells
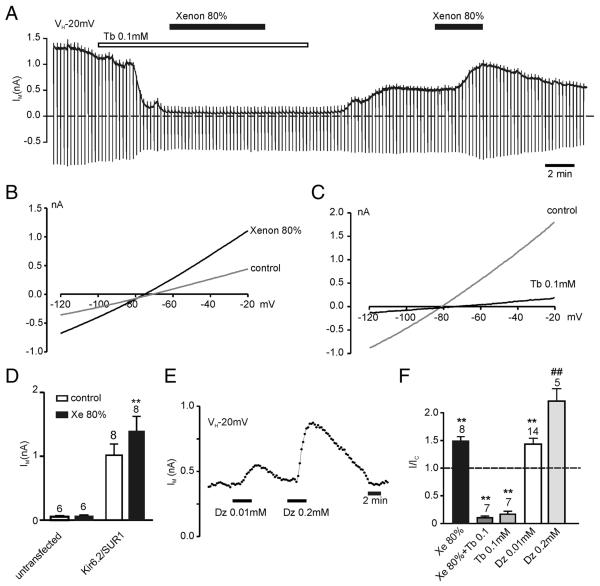
Whole cell recordings were performed on HEK293 cells transiently transfected with Kir6.2 and SUR1. These cells exhibited a large-standing outward current at a holding potential of −20 mV, a zero-current potential of approximately −75 mV, and sensitivity to tolbutamide, consistent with the expression of KATP channels (fig. 1). Xenon was applied at a concentration of 80%, which achieves anesthesia in humans.14 At this concentration, xenon increased the outward current by 49 ± 8% (n = 7) compared with control (80% N2; figs. 1A, B, F). The reversal potential of this current (fig. 1B) indicated it to be a K+ current. Furthermore, 80% xenon had no effect when the current was preblocked with 0.1 mm tolbutamide (n = 7) or in untransfected HEK293 cells (n = 6; figs. 1A, D, F). These results strongly suggest that the xenon-induced K+ current was mediated by Kir6.2/SUR1 channels. For comparison, diazoxide, the traditional KATP channel opener, was also tested. As expected, diazoxide increased the whole cell Kir6.2/SUR1 current concentration dependently (figs. 1E, F).
Fig. 1.
KATP channels are activated by xenon. (A) Whole cell recording (holding potential [VH] −20 mV) from an HEK293 cell-expressing Kir6.2/SUR1 channels demonstrating reversible current activation by 80% xenon and its preclusion by tolbutamide (Tb). Drugs were bath applied as indicated by horizontal bars. Dashed line is zero-current level. (B, C) Current–voltage relationships demonstrating KATP current activation by xenon and block by tolbutamide. (D) Mean current amplitude at −20 mV for untransfected and Kir6.2/SUR1-transfected cells in the absence or presence of 80% xenon (Xe). (E) Effects of the SUR1-specific K-channel opener diazoxide (Dz) on Kir6.2/SUR1 whole cell currents. Plotted is the mean holding current at −20 mV every 15 s. (F) Mean effects of the K-channel openers xenon and diazoxide and the blocker tolbutamide on Kir6.2/SUR1 currents. Expressed is the holding current at −20 mV in the presence of the drug as a fraction of the current in the absence of the drug. Xe + Tb: current in the presence of xenon and tolbutamide. Numbers (N) are given above the bars. **P < 0.01 compared with control. ##P < 0.01 compared with lower drug concentration.
KATP Channel Activation by Xenon Is Independent of the SUR Subunit
To probe which subunit of the KATP channel is critical for xenon activation, we investigated the effect of xenon on the pore-forming subunit of the KATP channel alone. This was achieved by using Kir6.2ΔC26, a truncated version of Kir6.2 lacking the C-terminal 26 amino acid residues. Kir6.2ΔC26 forms functional channels without the need to assemble with SUR1.15 Xenon increased Kir6.2ΔC26 currents to the same extent as wild-type (Kir6.2/SUR1) currents (figs. 2A, B), whereas diazoxide and tolbutamide, which require the SUR1 subunit to open and close the channel, were ineffective (fig. 2B). These results provided evidence that xenon activates KATP channels mainly through its interaction with the Kir6.2 subunit.
Fig. 2.
Xenon does not require the SUR1 subunit to activate KATP channels. (A) Xenon activation of whole cell Kir6.2ΔC26 currents. Plotted is the mean holding current at −20 mV every 15 s. Note the enhanced rundown compared with channels containing the SUR1 subunit.15,19 Dashed line is approximation of slower phase of rundown. (B) Mean data from recordings performed on Kir6.2ΔC26, Kir1.1, and K185Q/SUR1 currents summarizing the effects of activators and inhibitors of these channels. Expressed is the holding current at −20 mV in the presence of the drug as a fraction of the current in the absence of the drug. Note the lack of effect of tolbutamide (Tb) and diazoxide (Dz) on Kir6.2ΔC26 currents. (C) Whole cell Kir6.2-K185Q/SUR1 current is sensitive to tolbutamide but not xenon. Numbers (N) are given above the bars. *P < 0.05 compared with control. **P < 0.01 compared with control.
Xenon Does Not Enhance the Activity of Kir1.1 Channels
Next, we tested whether xenon also activates other inward rectifier K+ channels that are not highly ATP sensitive. Experiments were performed with Kir1.1, which shares 36% sequence identity with Kir6.2 and is the only inward rectifier outside the Kir6.x subfamily that has been reported to be ATP regulated, albeit in the millimolar range.16,17 Whole cell Kir1.1 current amplitude at −20 mV was 672 ± 96 pA (n = 8), and 80% xenon had no significant effect: the amplitude decreased by 4 ± 9% (n = 4; fig. 2B). As expected, Kir1.1 was not inhibited by 0.1 mm tolbutamide either (1 ± 1% inhibition; n = 5) but was sensitive to 1 mm BaCl2 (80 ± 6% inhibition; n = 4; fig. 2B) as described previously.16 These results suggested that the activation of K+ currents is not a nonspecific effect seen with all inward-rectifier K+ channels.
Xenon Activation Is Abolished in ATP-Insensitive Kir6.2 Mutant
The hallmark feature that distinguishes Kir6.x channels from other inward rectifiers is their strong inhibition by adenine nucleotides. To test the hypothesis that xenon activation is related to ATP inhibition, we explored the effects of xenon on a mutant Kir6.2 (Kir6.2-K185Q),15 which is ATP insensitive, because the interaction of the channel with the β-phosphate of ATP is disrupted.18 This mutant was chosen because it not only disrupts ATP inhibition but also leaves the intrinsic open probability of the channel virtually unchanged, thus excluding changes in ligand sensitivity secondary to intrinsic gating.19,20 When coexpressed with SUR1, this channel exhibited large whole cell currents, but addition of 80% xenon had no further significant effect (107 ± 3% of control; n = 6; figs. 2B, C). In contrast, diazoxide enhanced (n = 4) and tolbutamide inhibited (n = 5) these whole cell currents (figs. 2B, C).
Xenon Activates KATP Currents in Inside-out Macropatches
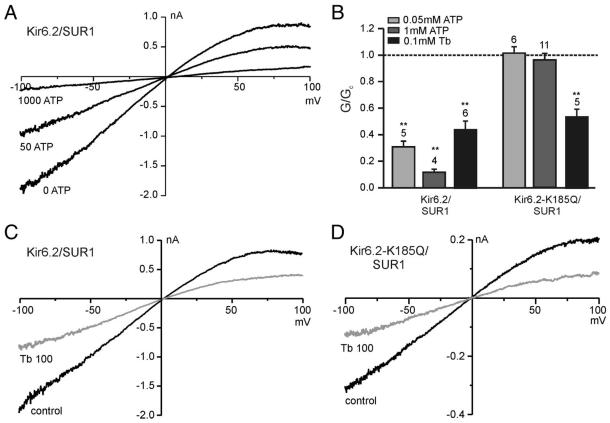
The results thus far indicate that SUR1 is not essential for xenon activation, activation is not universal between inwardly rectifying K+ channels, and xenon activation requires the integrity of ATP inhibition to stimulate KATP currents. However, these results do not exclude the possibility that xenon exerts its effect indirectly by altering the ATP concentration within the cell. Consequently, inside-out patch-clamp recordings were used to (A) separate the channels from cell metabolism and (B) to allow exposure to defined ATP concentrations to further explore the relationship between ATP inhibition and xenon activation of KATP currents. Figure 3A shows typical current–voltage relationships for Kir6.2/SUR1 currents in inside-out macropatches. The wild-type Kir6.2/SUR1 current was highly sensitive to ATP, with 50 μm ATP inhibiting the current by approximately 70% and 1 mm ATP causing virtually complete inhibition. In contrast, Kir6.2-K185Q/SUR1 currents were not reduced by 50 μm or 1 mm ATP (fig. 3B). However, both wild-type and mutant channels were sensitive to tolbutamide (figs. 3B-D). In line with earlier observations, tolbutamide inhibition was less pronounced in excised patches compared with whole cell recording conditions.21,22
Fig. 3.
Properties of wild-type and mutant KATP channels in inside-out patches. (A) Current–voltage relationships obtained from inside-out patches excised from cells expressing Kir6.2/SUR1 revealed macroscopic inwardly rectifying adenosine triphosphate (ATP)-sensitive currents in symmetrical K+ concentrations. (B) Mean slope conductance (G) in the presence of ATP or tolbutamide (Tb) expressed as a fraction of the conductance in the absence of the drug (Gc). ATP inhibition was concentration dependent for Kir6.2/SUR1 and absent for Kir6.2-K185Q/SUR1 currents. Numbers (N) are given above the bars. **P < 0.01 compared with control. (C, D) Current–voltage relationships obtained for Kir6.2/SUR1 and Kir6.2-K185Q/SUR1 currents, respectively, in the absence and presence of 0.1 mm tolbutamide.
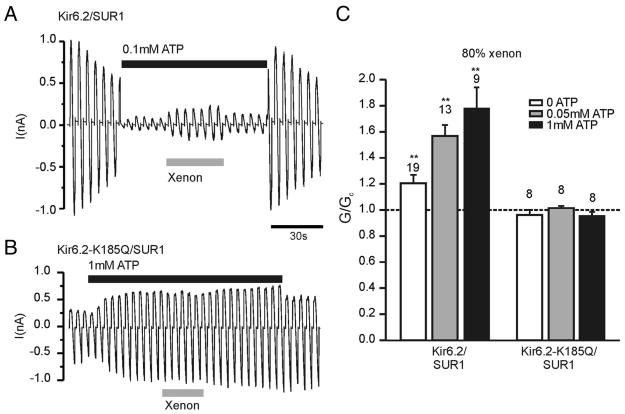
Similar to the observation in whole cell recordings, xenon enhanced Kir6.2/SUR1 currents but not mutant Kir6.2-K185Q/SUR1 currents (fig. 4). This discrepancy remained at various ATP concentrations (fig. 4C). In the presence of 50–1000 μm ATP, xenon enhanced Kir6.2/SUR1 currents by more than 50% (fig. 4A, C). This is comparable to whole cell recordings where similar increases were achieved by xenon. In contrast, in the nominal absence of ATP, xenon increased the slope conductance of the Kir6.2/SUR1 current by approximately 20%, which is significantly less than that in the presence of 50 μm or 1 mm ATP (P < 0.01; fig. 4C). These results further support the hypothesis that xenon interferes with ATP inhibition of Kir6.2.
Fig. 4.
Xenon activates wild-type but not mutant KATP channels in inside-out patches. (A, B) Xenon enhanced Kir6.2/SUR1 currents, but not Kir6.2-K185Q/SUR1 currents, in the presence of adenosine triphosphate (ATP). Note that 1 mm ATP increased Kir6.2-K185Q/SUR1 currents because of MgATP-dependent refreshment15,23 and because of its interaction with the SUR1 subunit in the presence of MgCl2.24 (C) Mean data from recordings as shown in A and B. The slope conductance (G) in the presence of ATP and xenon is expressed as a fraction of the conductance in the absence of the drug (Gc). Xenon activation of Kir6.2/SUR1 increased with increasing ATP concentrations but was absent in the Kir6.2-K185Q mutant both in the absence and presence of ATP. Numbers (N) are given above the bars. ** P < 0.01 compared with control.
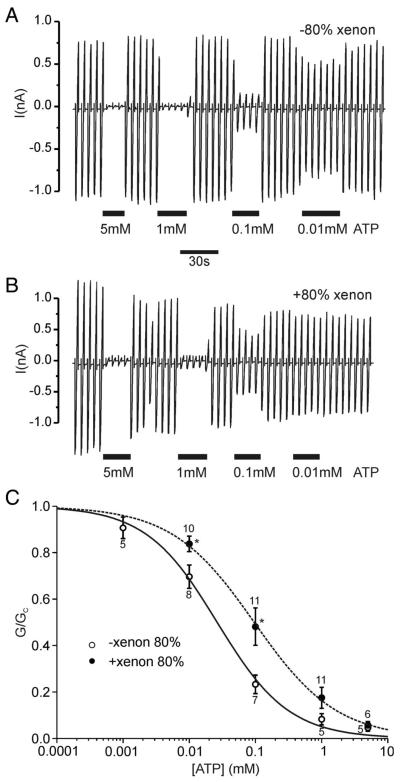
Finally, ATP concentration–response curves were obtained in the presence and absence of xenon (fig. 5A, B). These recordings were performed in magnesium-free solution to minimize the effect of the SUR subunit on ATP inhibition of KATP currents.25 Kir6.2/SUR1 currents were inhibited by ATP in a concentration-dependent manner. Figures 5A and B show that at each ATP concentration applied, the remaining current is larger in the presence of 80% xenon. ATP concentration–inhibition curves, obtained in the presence and absence of 80% xenon established that the IC50 for ATP inhibition shifted from 26 ± 4 μm (n = 5) in the absence to 96 ± 6 μm (n = 6) in the presence of xenon (fig. 5C). These results demonstrate that xenon decreases the ATP sensitivity of the KATP channel.
Fig. 5.
Xenon reduces ATP inhibition of inside-out patches. (A, B) Inside-out patch-clamp recordings of Kir6.2/SUR1 currents in the absence (A) or presence (B) of 80% xenon. Recordings were performed in Mg2+-free solution (note the absence of refreshment) and adenosine triphosphate (ATP) was applied as indicated by the bars. (C) ATP concentration–inhibition curves for Kir6.2/SUR1 currents in the absence of MgCl2 as obtained from experiments as shown in A and B. Experiments were performed either in the absence (open circles) or in the presence of 80% xenon (filled circles). Solutions containing ATP were alternated with ATP-free solutions. Slope conductance (G) in the presence of the drug is expressed as a fraction of the slope conductance in its absence (Gc). The dotted lines and solid lines are the best fit to the data using the modified Hill equation (Eq. 1). Numbers (N) are given above the data points. *P < 0.05 compared with same ATP concentration in the absence of xenon.
Discussion
Xenon, a Novel K-channel Opener
Our study demonstrates that the noble gas xenon acts as a KATP channel opener, but unlike other KATP channel openers, xenon works on the Kir6.2 pore-forming subunit of the KATP channel. Xenon enhances KATP currents when applied to either the outside or the inside of the plasma membrane. This is not surprising because, similar to other inhalational anesthetics, it easily penetrates biologic membranes. In fact, xenon is the first KATP channel opener that readily partitions into the brain and is thus of great interest as a potential neuroprotectant.
Mechanisms of KATP Channel Activation by Xenon
Xenon Activation Does Not Require the SUR Subunit of the KATP Channel
Classic KATP channel openers, such as diazoxide, require the SUR subunit to potentiate KATP currents. In addition, activation by these drugs is abolished in the absence of Mg2+ or when the nucleotide-binding domains of SUR are functionally disabled.26 In contrast, xenon potentiated Kir6.2ΔC26 currents in the absence of SUR and did not require Mg2+. Furthermore, xenon was unable to activate an ATP-insensitive mutant of Kir6.2 even in the presence of SUR1, further supporting the notion that it is the pore-forming Kir6.2 subunit and not the regulatory SUR subunit that is crucial for the effect of xenon. This again is different from the observations with diazoxide and further strengthens the conclusion that xenon is a novel KATP channel opener.
Xenon Activation Is Not Dependent on Intracellular Signaling Cascades or Metabolism
We have demonstrated that xenon enhances KATP currents not only in intact cells but also in inside-out macropatches in which ATP concentrations are dictated by the bath solution. These findings rule out that xenon exerts its effect indirectly via affecting cellular metabolism and thus intracellular ATP content. Rather, the data are highly suggestive of a direct effect of xenon on the channel, although membrane-delimited signaling cascades cannot be excluded. Xenon has been reported to activate selected two-pore-domain potassium channels. It activates TREK-1 but not TASK-3 expressed in intact HEK293 cells.13 Similarly, in our study, it activated Kir6.2 but not the closely related ATP-regulated Kir1.1 channel. This argues that xenon activation is a highly specific property displayed only by few select channels and is not widely shared between inward rectifiers or even more generally between K+ channels.
Molecular Mechanism of Xenon Enhancement of KATP Currents
The most obvious difference between Kir6.2 and Kir1.1 is that the former, but not the latter, is inhibited by micromolar concentrations of ATP.15,27 Furthermore, we observed that xenon activation of KATP currents in inside-out patches was more pronounced in the presence of ATP than in its nominal absence, and xenon shifted the Ki for ATP inhibition to an approximately fourfold higher concentration. These observations would be consistent with xenon either interfering with ATP inhibition of the KATP channel, potentially as a competitive antagonist, or simply requiring a preblocked channel to cause activation. However, because tolbutamide prevents xenon activation, the latter possibility is unlikely. To certify that xenon is a competitive ATP antagonist, measurements of the Ki of ATP inhibition would be required over a large xenon concentration range. Given the concentration of xenon needed to achieve the effect described here, this is experimentally not feasible.
In support of the hypothesis that xenon interferes with ATP inhibition, it failed to enhance Kir6.2-K185Q/SUR1 currents both in intact cells and in inside-out patches. The residue K185 interacts with the β-phosphate of ATP and thus contributes to the ATP-binding site.18,28 This mutant has a significantly reduced ATP sensitivity but unchanged intrinsic open probability.20 This is important because ligand binding to the KATP channel tends to be state dependent.19,29,30 A number of additional residues have been shown to contribute to the ATP-binding site on Kir6.2 by interacting with the adenine and ribose ring of ATP, but these were not tested with xenon because they have a more profound effect on intrinsic open probability.28 With the available data, we cannot distinguish whether xenon activation is lost in the mutant channel because K185 forms part of the xenon interaction site or whether lack of ATP inhibition simply prevented xenon enhancement. In any case, it is clear that xenon works differently from other KATP channel openers, as diazoxide activation remains unchanged by the K185Q mutation.
On first sight, xenon activation is reminiscent of phospholipid activation of the KATP channel.31,32 Activation by PIP2 also reduces ATP sensitivity, and the phospholipid-binding site has been located close to the ATP-binding site on Kir6.2.31-33 However, PIP2 activation is not precluded by tolbutamide; on the contrary, PIP2 decreases the efficacy of tolbutamide and other sulfonylureas.30,34 Furthermore, phospholipid activation is common among inward rectifiers.35 Thus, xenon activation seems to be different, although a definite answer would require crystallization of the channel in the presence of xenon (and ATP).
Summary and Potential Clinical Implications
Most importantly, we have demonstrated that xenon is a novel KATP channel opener. It is the first KATP channel opener that readily crosses the blood–brain barrier, thus providing a neuroprotective effect in addition to enhancing cerebral blood flow.36 Second, it is safe to use. Years of research into its potential as an anesthetic agent has shown that it has a benign side effect profile.11 Furthermore, the fact that the sulfonylurea tolbutamide prevents its actions suggests that xenon can be used by diabetic patients because it does not interfere with the channel-blocking capabilities of tolbutamide, and hence, a reduced efficacy of sulfonylureas in terms of insulin release is not expected. However, even diabetic patients are likely to retain the neuroprotective benefits of xenon because sulfonylureas do not easily partition into the brain. Finally, in addition to the acute neuroprotection afforded by opening of KATP channels, xenon has also been demonstrated to act as a lasting neuroprotectant in a preconditioning paradigm.37 We have recently provided evidence that this preconditioning effect of xenon is also critically dependent on the opening of KATP channels.12 Here, xenon is different from other inhalational anesthetics because in that study we found only xenon to be capable of inducing neuronal preconditioning through a KATP channel-dependent mechanism. In contrast, we demonstrated that halothane, isoflurane, and sevoflurane are weak KATP channel inhibitors.12 This leaves the preconditioning effect exerted by these compounds unlikely to be mediated by KATP channel opening. This conclusion was substantiated by our finding that sevoflurane preconditioned neuronal-glial cell cocultures in a KATP channel-independent fashion.12 We conclude that xenon could be applied successfully in patients with increased risk of stroke and that it might be a promising drug for neuroprotection in high-risk patients, both perioperatively and when given immediately after cardiovascular incidents such as stroke.
Acknowledgments
The authors thank Raquel Yustos, M.Sc., Technician, Biophysics Section, Blackett Laboratory, Imperial College London, London, United Kingdom, for expert technical assistance.
Supported by the Medical Research Council, London, United Kingdom, and the Westminster Medical School Research Trust, London, United Kingdom. Dr. Maze is a coinventor of a series of patents relating to the use of xenon as a neuroprotectant; the inventions were owned by Imperial College who formed a spinout company (Protexeon) that was acquired by Prodair (a wholly-owned subsidiary of Air Products, Allentown, Pennsylvania). He has received consultancy fees, grants, and xenon supplies from Air Products in consideration for helping to commercially exploit xenon as a neuroprotectant. Were xenon to be marketed as a neuroprotectant, Dr. Maze would receive royalties on these sales. These patents are owned by Imperial College and form the basis of a “Developmental Pathway Grant” from the Medical Research Council, London, United Kingdom, for preclinical work for the exploitation of xenon for organ transplantation.
References
- 1.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 2.Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of K(ATP) channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- 3.Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–6. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- 4.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:H2106–13. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–16. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Sanchez C, Basile AS, Fedorova I, Arima H, Stannard B, Fernandez AM, Ito Y, LeRoith D. Mice transgenically overexpressing sulfonylurea receptor 1 in fore-brain resist seizure induction and excitotoxic neuron death. Proc Natl Acad Sci U S A. 2001;98:3549–54. doi: 10.1073/pnas.051012898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashcroft FM. ATP-sensitive potassium channelopathies: Focus on insulin secretion. J Clin Invest. 2005;115:2047–58. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura T, Miki T. ATP-sensitive K+ channel openers: Old drugs with new clinical benefits for the heart. Curr Vasc Pharmacol. 2003;1:251–8. doi: 10.2174/1570161033476646. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblum WI. ATP-sensitive potassium channels in the cerebral circulation. Stroke. 2003;34:1547–52. doi: 10.1161/01.STR.0000070425.98202.B5. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Traystman RJ, Murphy SJ. Inhalational anesthetics as preconditioning agents in ischemic brain. Curr Opin Pharmacol. 2008;8:104–10. doi: 10.1016/j.coph.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preckel B, Schlack W. Inert gases as the future inhalational anaesthetics? Best Pract Res Clin Anaesthesiol. 2005;19:365–79. doi: 10.1016/j.bpa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics—Evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110:986–95. doi: 10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–52. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 14.Rossaint R, Reyle-Hahn M, Schulte Am Esch J, Scholz J, Scherpereel P, Vallet B, Giunta F, Del Turco M, Erdmann W, Tenbrinck R, Hammerle AF, Nagele P. Multicenter randomized comparison of the efficacy and safety of xenon and isoflurane in patients undergoing elective surgery. Anesthesiology. 2003;98:6–13. doi: 10.1097/00000542-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–83. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 16.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–8. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 17.Vanoye CG, MacGregor GG, Dong K, Tang L, Buschmann AS, Hall AE, Lu M, Giebisch G, Hebert SC. The carboxyl termini of K(ATP) channels bind nucleotides. J Biol Chem. 2002;277:23260–70. doi: 10.1074/jbc.M112004200. [DOI] [PubMed] [Google Scholar]
- 18.Trapp S, Haider S, Jones P, Sansom MS, Ashcroft FM. Identification of residues contributing to the ATP binding site of Kir6.2. EMBO J. 2003;22:2903–12. doi: 10.1093/emboj/cdg282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trapp S, Proks P, Tucker SJ, Ashcroft FM. Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. J Gen Physiol. 1998;112:333–49. doi: 10.1085/jgp.112.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. EMBO J. 1998;17:3290–6. doi: 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: A reinterpretation. J Physiol. 1997;504(Pt 1):35–45. doi: 10.1111/j.1469-7793.1997.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence CL, Proks P, Rodrigo GC, Jones P, Hayabuchi Y, Standen NB, Ashcroft FM. Gliclazide produces high-affinity block of KATP channels in mouse isolated pancreatic beta cells but not rat heart or arterial smooth muscle cells. Diabetologia. 2001;44:1019–25. doi: 10.1007/s001250100595. [DOI] [PubMed] [Google Scholar]
- 23.Xie LH, Horie M, Takano M. Phospholipase C-linked receptors regulate the ATP-sensitive potassium channel by means of phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A. 1999;96:15292–7. doi: 10.1073/pnas.96.26.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gribble FM, Tucker SJ, Haug T, Ashcroft FM. MgATP activates the beta cell KATP channel by interaction with its SUR1 subunit. Proc Natl Acad Sci U S A. 1998;95:7185–90. doi: 10.1073/pnas.95.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–94. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- 26.Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–52. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–71. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 2005;24:229–39. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enkvetchakul D, Loussouarn G, Makhina E, Shyng SL, Nichols CG. The kinetic and physical basis of K(ATP) channel gating: Toward a unified molecular understanding. Biophys J. 2000;78:2334–48. doi: 10.1016/S0006-3495(00)76779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krauter T, Ruppersberg JP, Baukrowitz T. Phospholipids as modulators of K(ATP) channels: Distinct mechanisms for control of sensitivity to sulphonylureas, K(+) channel openers, and ATP. Mol Pharmacol. 2001;59:1086–93. [PubMed] [Google Scholar]
- 31.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–4. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 32.Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–41. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 33.Haider S, Tarasov AI, Craig TJ, Sansom MS, Ashcroft FM. Identification of the PIP2-binding site on Kir6.2 by molecular modelling and functional analysis. EMBO J. 2007;26:3749–59. doi: 10.1038/sj.emboj.7601809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koster JC, Sha Q, Nichols CG. Sulfonylurea and K(+)-channel opener sensitivity of K(ATP) channels. Functional coupling of Kir62 and SUR1 subunits. J Gen Physiol. 1999;114:203–13. doi: 10.1085/jgp.114.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–6. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 36.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 37.Ma D, Hossain M, Pettet GK, Luo Y, Lim T, Akimov S, Sanders RD, Franks NP, Maze M. Xenon preconditioning reduces brain damage from neonatal asphyxia in rats. J Cereb Blood Flow Metab. 2006;26:199–208. doi: 10.1038/sj.jcbfm.9600184. [DOI] [PubMed] [Google Scholar]