Abstract
Objective
To comprehensively ascertain the extent and severity of clinical features in multiple affected individuals from two large families with proven heterozygous mutations in the CYLD locus and to correlate these findings with the three appendageal tumor predisposition syndromes, familial cylindromatosis (FC), Brooke-Spiegler syndrome (BSS), and multiple familial trichoepitheliomas (MFT) known to be associated with such germline mutations.
Design
Inter- and intra-familial observational study.
Setting
Tertiary genetic and dermatology referral centre.
Participants
32 individuals were recruited from two large multigenerational families with CYLD mutations. Clinical details, history and tumor maps were obtained from all participants whilst 18 were further corroborated with detailed clinical examination.
Main outcome measures
Severity of tumor density, distribution and histology, associated medical conditions, patient symptoms and impact of disease on quality of life.
Results
We demonstrate a wide variation in clinical presentation seen in individuals from the same family. In addition, we provide clinical evidence that correlates with hormonally stimulated hair follicles being particularly vulnerable to loss of heterozygosity and tumor induction.
Conclusion
In view of our findings, we propose that the burden of disease at sites other than the head and neck is underreported in the literature, but impacts greatly on quality of life. The differentiation between the clinical diagnoses has little prognostic or clinical utility in genetic counselling even within individuals from the same family. Thus, we suggest an encompassing diagnosis of “CYLD cutaneous syndrome”. Finally, our results relating to the clinical distribution of tumors suggest hormonal factors may play an important role in tumor induction in these patients.
Keywords: Cylindroma, trichoepithelioma, spiradenoma, pubic distribution, CYLD, androgen stimulation, ultraviolet radiation
INTRODUCTION
Heterozygous mutations within the CYLD gene locus have been identified as the cause of three clinically distinct dermatological phenotypes: Familial cylindromatosis (OMIM 313270), Brooke-Spiegler syndrome (OMIM 605041) and multiple familial trichoepitheliomas (OMIM 601606)1-3. These conditions are unified by a predisposition to inherited skin appendage tumors, the diagnostic hallmark being the presence of cylindromas. Although rare in the population, these disorders are associated with a high level of morbidity, which can impact greatly on the quality of life of the affected individual. In particular, these highly disfiguring tumors often affect the face and scalp, 4 which in some cases culminate in entire scalp removal.
Cylindromas are purported to arise from hair follicle stem cells within the bulge region of the hair follicle 5, a model supported by the numerous tumors seen on hair bearing sites. As the wildtype CYLD allele is lost in 70% of tumors, it has been postulated that CYLD has a classical tumor suppressor role in cylindroma formation3. Ultraviolet radiation (UVR) has been suggested as the major predisposing factor for loss of heterozygosity (LOH) and tumor initiation 6, based on the presence of face and scalp tumors. However, careful clinical mapping of the distribution of the tumors does not exist to support this hypothesis.
Four CYLD knockout mouse models have been derived, which may help inform clinical phenotypes associated with germline CYLD mutations. The phenotypes seen in CYLD null mice include: an increased sensitivity to cutaneous squamous papilloma formation6; immunological defects involving T-cell maturation7and B-cell responses8; an increased predisposition to inducible colitis and colorectal tumor formation9; and a protective effect against lethal S. Pneumoniae infections10.
The CYLD locus encodes a ubiquitin hydrolase 11. This has been implicated in the negative regulation of cell proliferation through a direct role in both the nuclear factor kappa beta (NFkB), and c-JUN pathways 11, 12. Recently, studies have identified a group of drugs including aspirin that can inhibit the NFkB pathway downstream of CYLD's putative point of action13. Excitingly, this opens up new avenues for chemoprevention and potential non-surgical approaches to the treatment of tumors in these patients.
Previous work (Table 1) investigating clinical phenotypes across multiple families has shown little phenotype-genotype correlation with regard to the position of a germline CYLD mutation and the tumors types seen in each family 1. We were interested in examining clinical phenotypes, tumor distribution, and the burden of disease associated with the diagnosis of germline CYLD mutations in two large multigenerational families. This information is a prerequisite for future accurate genetic counselling for individuals both within and outwith these families.
TABLE 1. Review of pedigrees and correlation with genotype.
Review of series of published pedigrees and correlation with genotype including from this paper. This table indicates: 1) The female preponderance described in the literature is based largely on small pedigrees, 2) The reported penetrance can vary between pedigrees 3) Where genotypes are available, there is no correlation with phenotype.
| Author | Pedigree size |
Proportion Affected |
Affected Females |
Affected Males |
F:M ratio |
Clinical Diagnosis |
Genotype |
|---|---|---|---|---|---|---|---|
| Gerretsen35 | 237 | 13% | 19/119 | 11/118 | 1.71 | BSS | NG |
| Rajan | 133 | 26% | 18/66 | 16/67 | 1.14 | FC, BSS, MFT |
2460delC |
| Welch25 | 115 | 25% | 15/51 | 14/64 | 1.34 | BSS | NG |
| Rajan | 77 | 21% | 9/42 | 7/35 | 1.07 | FC, MFT | 2469+1G>A |
| Welch25 | 67 | 16% | 9/41 | 2/26 | 2.85 | BSS | NG |
| Zheng G31 | 36 | 56% | 10/19 | 10/17 | 0.89 | MFT | 2822A>T |
| Saunders 36 | 32 | 25% | 5/14 | 3/18 | 2.14 | BSS | NG |
| Zhang G29 | 29 | 31% | 6/20 | 3/9 | 0.90 | MFT | 2272C>T |
| Salhi 30 | 29 | 45% | 6/17 | 7/12 | 0.61 | MFT | 2104-2105 ins C |
| Young 1 | 24 | 46% | 7/14 | 4/10 | 1.25 | FC, MFT | 2806 C>T |
| Liang 37 | 23 | 26% | 4/10 | 2/13 | 2.60 | MFT | 2241_2242 del AG |
| Liang37 | 20 | 30% | 2/12 | 4/8 | 0.33 | MFT | 1826+2T>G |
| Hu38 | 17 | 88% | 7/8 | 8/9 | 0.98 | MFT | 2240A>G |
| Zhang X39 | 15 | 60% | 6/7 | 3/8 | 2.29 | MFT | 2355-2358 del CAGA, |
| Burrows40 | 14 | 29% | 3/6 | 1/8 | 4.00 | BSS | NG |
| Zheng G31 | 14 | 79% | 7/9 | 4/5 | 0.97 | MFT | 1462delA |
| Zheng G31 | 11 | 55% | 5/9 | 1/2 | 1.11 | MFT | 2128C>T |
| Burrows40 | 9 | 44% | 3/4 | 1/5 | 3.75 | BSS | NG |
| Fenske41 | 9 | 33% | 2/5 | 1/4 | 1.60 | FC, MFT | NG |
| Bumgardner42 | 8 | 50% | 3/4 | 1/4 | 3.00 | BSS | NG |
| Poblitez- Guirrez43 |
7 | 57% | 3/4 | 1/3 | 2.25 | BSS | 2253 del G |
| Susanne | 6 | 67% | 1/3 | 3/3 | 0.33 | FC | NG |
| Oiso44 | 4 | 50% | 1/2 | 1/2 | 1.00 | FC | 2272C>T |
Pedigrees with only 1 member were discounted; Not all 50 mutations are shown.
Key: NG - Not genotyped
MATERIALS AND METHODS
PATIENT COHORT
Appropriate approval from the local ethical review board was obtained (REC 06/1059) and written informed consent was obtained from patients.
Two large families with known germline mutations3 within the CYLD locus were identified. Family A has a frameshift mutation (c.2460delC), resulting in a premature termination codon, whilst Family B has a splice site mutation (c.2469+1G>A). In total, twenty-six affected individuals as determined by a dermatologist or geneticist were surveyed, of which eighteen were clinically examined by the same dermatologist (N.R.). Eight unaffected relatives, five from family A and three from family B, provided blood samples to exclude the presence of a non-penetrant mutation.
QUESTIONNAIRE AND TUMOR MAPPING
A questionnaire was designed following a search for all publications in PubMed for the text: CYLD, cylindroma, Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepitheliomas. The phenotypes seen in CYLD knockout associated models and all clinical phenotypic data was collated into a clinical questionnaire. The questionnaire aimed to gather clinical data on patient signs and symptoms, disease severity, hormonal exposure in females, associated diseases and response to existing aspirin or NSAID therapy.
Due to the extensive geographical spread of the families, a template was designed, and affected members were asked to self-report the exact location of tumors. However, as the majority of patients lived in the North East of England, detailed examination of eighteen patients was performed to ensure corroboration. Tumor mapping done in the clinic corroborated well with the template data on the torso and face. Detailed location on the scalp was accurate when there were less than approximately ten tumors; accurate resolution was not possible when confluent tumors were present.
Eight affected patients who were not examined due to geographical reasons, were interviewed by telephone. These patients self-reported lesion locations on a template and supplemented this subjective description with confirmatory digital photographic images. Histological reports were reviewed to confirm the nature of the tumors where available. The distribution of tumors on each patient was ultimately marked on a template and a composite image was created by overlaying the templates according to gender using Adobe Photoshop™.
DNA SEQUENCING
Genomic mutation analysis for the known familial CYLD germline mutations were undertaken in affected and unaffected individuals within both families. This was performed by the NHS molecular diagnostic laboratory based in the Institute of Human Genetics, Newcastle upon Tyne.
RESULTS
Penetrance of tumor phenotype
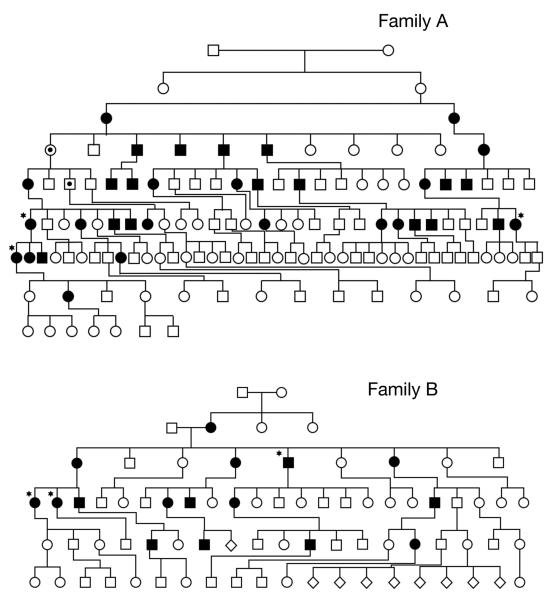
The pedigrees of both families are shown in Fig. 1. They both demonstrate an autosomal dominant pattern of inheritance across 7 generations in family A with 34 out of 133 individuals being reported as affected, and 5 generations in family B with 16 out of 77 individuals being reported as affected. In total, 50 members of both families out of 210 were reported as clinically affected, 27 female and 23 male. This result is significantly less than would be expected with an autosomal dominant inheritance pattern and could be accounted for by either non penetrance of the mutation or a milder presentation of the condition which was under reported especially in the earlier generations. In particular, female IV-1 and male V-3 in family A, obligate carriers on family history for the CYLD mutation, were reported as unaffected. Neither of these patients were alive to confirm clinical status.
Figure 1.
The pedigrees of family A (7 generations) and family B (5 generations), with patients who have had their scalps removed indicated with a star, and the obligate carriers indicated by a dot.
To address the possibility of non-penetrance within these families, we undertook CYLD mutation analysis in all available living affected individuals and contacted non-affected individuals to offer them testing within both families. 9 members of family A, in whom a diagnosis of BSS and FC had been made, were shown to have the familial mutation by sequencing. 5 unaffected members (age range 33-64) were shown not to have inherited the mutation. Family B were similarly examined, with 3 affected members with an FC phenotype, found to have the familial mutation and 3 unaffected members (age range 40-54) shown not to have the mutation.
Although numbers are small, we found no indication for non penetrance of either CYLD mutation. The unaffected obligate individuals present in this family most likely indicate a variation in CYLD mutation expression with family A.
Severity of phenotype and variety of expression
In our population, 5 female patients and 1 male patient developed severe scalp disease which necessitated entire scalp removal and repair with split thickness skin grafts. The mean age for this was 55 (50-60) years. Patients who had undergone this procedure were all noted to have multiple tumors on the trunk and genital areas. Other markers of severity include use of wigs preoperatively (23%) and painful tumors (52%).
Although there is the suggestion of clustering within the pedigree of individuals with a severe tumor burden, relatives of these patients were often noted to have only mild disease. These patients had a few tumors, that were only apparent on close inspection of the scalp. Conversely, one patient in family A (V-14), who had only three tumors on the face, fathered 4 children who each presented with more than 30 tumors on the face. In addition, unaffected obligate carrier V-3 had 9 affected progeny over three further generations, one of whom required total scalp removal.
Tumor histology and distribution
We have summarised the clinical and tumor data in Table 2. The average age of onset of tumor formation was 16 years (range 8 - 30), consistent with tumor initiation occurring after adrenarche. In both families, the commonest site for the presenting tumor was the scalp. The clinically diagnosed tumor phenotypes were supported by histology in 10/26 patients.
TABLE 2. Summary of Clinical Findings.
Summary of clinical findings in our patient dataset.
| TUMOR LOCATION | MARKERS OF SEVERITY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Pt No. | Mutation | M/F | Onset (Yrs) |
Tumor Type |
Clinical Diag. |
Scalp | Trunk | Pubic | Ear | Wig Use |
Scalp Removed |
Painful Tumor |
| A-1 | 2460 | F | 25 | Cy | FC | + | + | + | + | + | + | + |
| A-2 | 2460 | F | 12 | Cy,Es, Te |
BSS | + | + | + | + | + | + | + |
| A-3 | 2460 | F | 15 | Cy, Es, Te |
BSS | + | + | + | + | + | + | + |
| A-4 | 2460 | F | 9 | Cy,Es, Te |
BSS | + | + | + | + | − | − | + |
| A-5 | 2460 | F | 8 | Cy | FC | + | + | − | − | − | − | + |
| A-6 | 2460 | F | 15 | Cy | FC | + | + | − | − | − | − | − |
| A-7 | 2460 | F | 25 | Cy | FC | + | − | − | − | − | − | + |
| A-8 | 2460 | F | 12 | Cy | FC | + | + | − | − | − | − | − |
| A-9 | 2460 | F | 11 | Cy, Te, Mi |
BSS | + | + | + | + | − | − | + |
| A-10 | 2460 | F | 13 | Te | MFT | − | − | − | − | − | − | − |
| A-11 | 2460 | F | 12 | Te | MFT | − | + | − | − | − | − | − |
| A-12 | 2460 | M | 15 | Te | MFT | − | − | − | − | − | − | − |
| A-13 | 2460 | M | 12 | Cy, Es | BSS | + | + | + | − | − | − | + |
| A-14 | 2460 | M | 30 | Cy,Es, Mi |
BSS | + | − | − | − | − | − | + |
| A-15 | 2460 | M | 15 | Cy,Te | BSS | + | + | − | − | − | − | − |
| A-16 | 2460 | M | NR | Cy,Te | BSS | − | − | − | − | − | − | − |
| A-17 | 2460 | M | 15 | Cy | FC | + | + | − | − | − | − | NR |
| B-18 | 2469 | F | 18 | Cy | FC | + | + | + | − | + | + | + |
| B-19 | 2469 | F | 18 | Cy, Es | FC | + | + | + | − | + | + | + |
| B-20 | 2469 | F | 23 | Cy | FC | + | + | + | + | + | − | + |
| B-21 | 2469 | M | NR | Cy | FC | + | + | + | − | NR | + | NR |
| B-22 | 2469 | M | 17 | Cy | FC | + | + | − | − | − | − | − |
| B-23 | 2469 | M | 16 | Te | MFT | − | − | − | − | − | − | − |
| B-24 | 2469 | M | NR | Cy,Te | FC | + | − | − | − | − | − | NR |
| B-25 | 2469 | M | 18 | Cy | FC | + | + | + | + | − | − | − |
| B-26 | 2469 | M | 15 | Cy | FC | + | − | − | − | − | − | − |
| 12 M 14 F |
Avg. 16 yrs |
Total % | 81% | 69% | 42% | 27% | 24% | 23% | 52% | |||
Abbreviations: Cy- Cylindroma, Es - Eccrine Spiradenoma, Te- Trichoepithilioma, Mi - Milia, + Present, − Absent, 2460 - Family A (c.2460 del C), 2469 - Family B (c.2469+1 G>A), NR - Not Recorded
Family A presented with classical scalp cylindromas, eccrine spiradenomas, trichoepithiliomas and milia. This wide variation in tumor type and clinical presentation in family A supported a clinical diagnosis of BSS within the majority of patients. However, some individuals with exclusively facial trichoepithiliomas, if seen in an isolated setting, would have been diagnosed with MFT.
In contrast, family B presented almost exclusively with cylindromas, supporting a diagnosis of FC in the majority of these individuals.
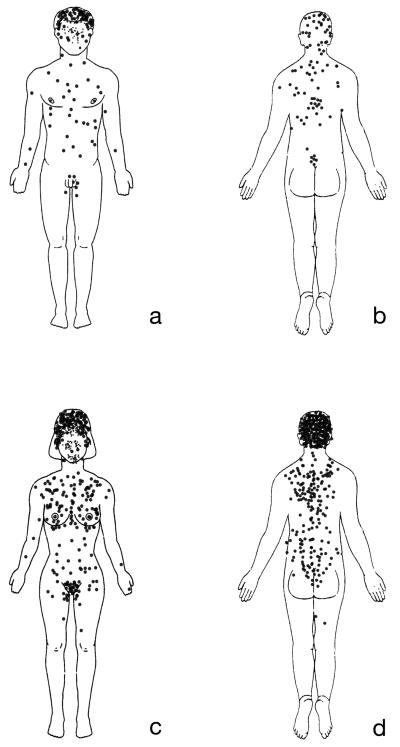
The detailed tumor distribution maps comprising in total 14 female and 12 male from both families were combined to produce composite maps (Fig. 3). From these maps, it is clear that the tumors extend beyond the head and neck, with the trunk being involved in 69% of patients. The tumor distribution forms a “V-shaped” distribution on the trunk with tumors being more concentrated in the midline. Noticeably, tumors are highly concentrated on the hair bearing pubic and genital areas, with a relative absence on other areas of the body such as lower legs and axillary skin. Tumors were not seen on hairless areas of the body such as the palms and soles.
Figure 3.
Tumor locations in 12 male and 14 female patients in the two families.
In addition, although not depicted on the tumor maps, patients with advanced scalp tumors (Family A: VI-1; Family B: IV-1, IV-2 and IV-3,) showed a higher density of tumors over areas which are predisposed to androgenic alopecia (Hamilton, 1951) (Fig. 2d). Although in these patients tumors did occur on the occipital and temporal scalp, they were smaller and not confluent, suggesting this to be a true predisposition, rather than one that was biased due to balding. Furthermore this distribution was also seen in female patients. Interestingly, one woman with severe disease reported an increase in number of tumors during pregnancy and also when hormone replacement therapy was started. As this had been previously noted anecdotally 14, the remaining 13 affected females were directly questioned. No relationship was seen between number of pregnancies, hormonal contraception, hormone replacement therapy or duration of menarche-menopause in other patients.
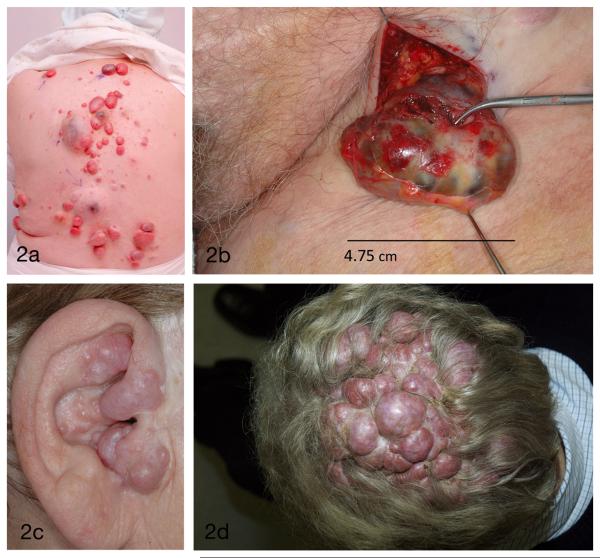
Figure 2.
Typical lesions at different sites: 2a Midline distribution of tumors on back (spiradenomas arrowed); 2b Pubic eccrine spiradenoma; 2c Occluded ear canal resulting in conductive deafness; 2d Scalp demonstrating larger confluent tumors clustering in areas predisposed to androgenetic alopecia.
CYLD mutation phenotypes have a severe impact on quality of life
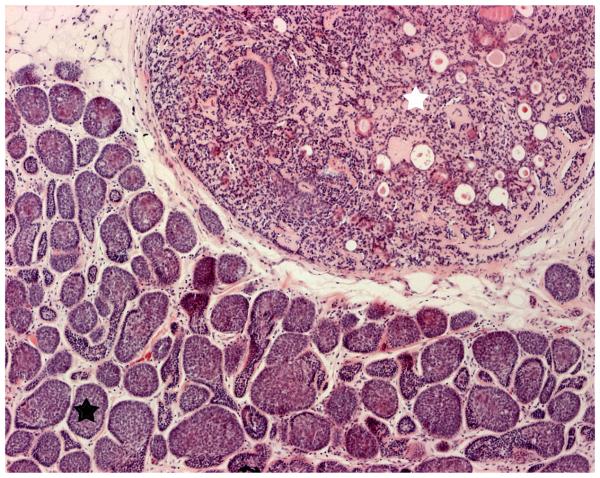
Pain and disfigurement were commonly reported in individuals from both families as major signs and symptoms. Lesion-associated pain was a major complaint in over 50% of affected individuals, and attributed by the patient to both histologically confirmed cylindromas as well as spiradenomas. Whilst eccrine spiradenomas could be distinguished clinically as they were usually painful on light pressure and had a deep blue hue (Fig. 2a), some painful cylindromas were also noted to have blue regions which corellated histologically with areas of spiradenomatous differentiation (Fig. 4). This finding of regions within the same tumor showing spiradenomatous and cylindromatous patterns has been previously reported 15
Figure 4.
Haematoxylin and eosin stain displaying the characteristic pattern suggestive of cylinders in cross section which gave rise to the term cylindroma (lower left corner; black star), with an adjacent region (upper right corner; white star) within the tumor displaying a large ball of basophillic cells with areas of ductal differentiation, consistent with an eccrine spiradenoma (Original magnification 10x).
Apart from pain, tumors on the back which were compressed during sitting or sleeping were susceptible to pressure necrosis, which caused problems due to ulceration and chronic discharge. Tumors in the pubic area were found in 42% of affected individuals and resulted in sexual dysfunction (Fig. 2b). 27% of affected individuals were noted to have numerous confluent cylindromas in the ear canal resulting in a conductive deafness when occlusion of the ear canal occurred (Fig. 2c).
Taken together, the impact on quality of life due to repeated surgery, scalp removal, painful tumors, deafness and sexual dysfunction appears to be underreported in the literature and a cause of major unaddressed morbidity within families.
Associated co-morbidities
In keeping with a previously reported association16 one individual was also diagnosed with bilateral parotid gland tumors and underwent bilateral parotid surgical excision. (Family B: III-5). Unfortunately we were unable to obtain the histology report on these tumors.
Due to the immunodysregulation observed in CYLD null mouse models, affected patients were also questioned as to symptoms associated with either an immunodeficiency or autoimmune phenotype. The results revealed no evidence of a predisposition to recurrent infections, autoimmune disease, colitis, infertility and non-cutaneous cancers. One patient reported vitilligo, whilst other diseases reported included ischaemic heart disease and osteoarthritis.
DISCUSSION
Patients with CYLD mutations experience significant clinical morbidity
Here, we provide the most comprehensive study of the clinical features associated with germline heterozygous mutations within the CYLD gene locus.
In addition to the well recognised morbidity associated with face and scalp cylindromas in these families, we highlight that the tumor predisposition associated with this condition extends beyond the face and scalp affecting both the trunk and genital areas. These less well recognised sites of tumor formation can impact greatly on the quality of life experienced by these patients. Unless actively sought these complications may be under-diagnosed and not treated.
In our patient cohort, we found only a slight female preponderance of the disease. However, the impact of this disease appears to be more severe in females with 5 women, compared to only one male, undergoing entire scalp removal. Although clinical photographs support the underlying clinical severity that warranted this procedure, it is also feasible that the psychological impact and social disability has a greater impact on females.
Basal cell adenomas and adenocarcinomas of the parotid glands and minor salivary glands have been reported in association with this disorder 16. In our families only one such case was reported, suggesting that this is an uncommon event in these families.
None of our patients developed any of the clinical features associated with the CYLD null (CYLDnull) mouse models 7-10, 17. Conversely, none of the CYLDnull mice developed cutaneous cylindromas. This phenotypic difference may be due to the germline molecular heterozygosity found in the patients compared to the homozygote status of the CYLDnull mouse models, and would suggest that either loss of the second CYLD allele in humans is a rare event outwith the hair follicle or that somatic loss of the second CYLD allele in cells from other tissues may be non-viable. However, this explanation does not account for the observation that there is over-representation of mutations within the 3′ end of CYLD gene in all published human cohorts ascertained on their predisposition to cutaneous tumors.
Thus, an intriguing alternative explanation for the phenotypic difference between CYLDnull mice and the patients is that the human mutations represent hypomorphic alleles, which could potentially result in the production of a truncated protein with the potential for tissue-specific dominant negative pathogenic interactions. Evidence underpinning this theoretical possibility comes from recent murine models in which truncating mutations (CYLDtruncated) were engineered into the mCYLD locus. In direct contrast to the viable phenotype seen in the CYLDnull models7-10, 17, the CYLDtruncated mouse models died shortly after birth18, 19. However, in humans, mechanistic data is still limited. In cylindroma tissue, a truncated form of the CYLD protein has not been visualised on immunohistochemistry despite the antibody recognising the N-terminus of the CYLD protein 6. In addition, the only heterozygous full CYLD gene deletion reported to date is in a 14-year-old girl with a large heterozygote chromosome deletion encompassing the CYLD locus20. Although the preliminary report does not indicate the presence of cutaneous tumors, she is currently too young to exclude this as a possible future complication. Further work is necessary before precise mechanisms of the pathogenicity of the mutated CYLD locus can be formulated.
Tumor initiation
The pluripotent hair follicle stem cell as the probable cell of origin of cylindromas 6 is supported by our clinical data. The occurrence of multiple, different, rare 21skin appendage tumors in these patients suggest tumors may arise from a cell which is not fully committed to a lineage of differentiation. Furthermore, sites recognized to have an absence of hair follicles such as the palms and soles are spared. Further evidence for a pluripotent cell of origin comes from direct histological examination of excised tumors showing evidence of a mixed differentiation lineage, with features of cylindroma and eccrine spiradenoma seen within the same tumor (Fig. 4). As the hair follicle stem cell compartment becomes better defined 22, it is to be hoped that the cell of origin of cylindromas will become clearer.
One key event in familial cancer tumorigenesis is loss of the wildtype allele in a previously heterozygous cell (LOH). Due to the high incidence of tumor formation on the face and scalp in patients with a CYLD mutation, it has been postulated that UVR associated DNA damage may be the main initiating factor in the development of cylindromas 17, 23. Our data supports this hypothesis, as all patients have a tumor on the face or scalp, and the highest number of tumors is at these sites. In contrast however, we demonstrate a high incidence of genital and trunk cylindromas and spiradenomas in more than half of the affected patients. Tumors at lifelong light protected sites such as the pubic skin indicate that other mechanisms independent of UVR must play a role tumor initiation. There is further supportive data from a study of patients with nevoid basal cell carcinoma syndrome (NBCCS), where the cutaneous tumors are also thought to arise from hair follicles. This study 24 showed that patients with NBCCS (who carry PTCH mutations) have a disproportionately high proportion (59-65%) of tumors on the trunk, compared to the general population (9-12%) who present with basal cell carcinoma. There was no correlation to sun exposure behavior, (though numbers were small) and tumors were seen at genital sites as well as in highly pigmented skin types. Taken together, the distribution of skin tumors resulting from germline mutations in 2 known tumor suppressor genes suggest alternative, non-UVR mediated mechanisms of tumor induction are pertinent. One feature of human hair that may highlight such mechanisms is the differential sensitivity of follicles at different body sites to hormonal stimulation. Puberty highlights follicles that change from fine vellus hair to coarse hair in response to hormonal stimulation in some areas such as the beard area, axilla and pubic region. Another follicular respone seen is acne, where hormonally driven proliferation of the opening of the hair follicle at certain sites results in comedones, inflammatory pustules and papules. The detailed mapping of tumor distribution in our patient cohort demonstrates an increased incidence of tumors in areas associated with hormonally stimulated hair follicles such as the pubic hair and upper trunk. The distribution on the torso correlates well with the distribution of hair follicles affected by acne at puberty. The converse of this, a relative absence of tumors on unstimulated sites such as distal limbs, palms and mucous membranes is also seen. Further evidence that the tumors are more likely to arise from hair follicles that are hormonally stimulated come from the observation that the onset of tumors occurs after adrenarche 25, and the larger size and confluency of scalp tumors in areas predisposed to androgenic alopecia (male pattern balding is thought to reflect follicles that preferentially senesce following androgen stimulation26). Finally, recent preliminary data in mouse follicles has shown that hair follicle cycling is dependent on CYLD 27. Whilst the precise mechanisms remain undetermined, the clinical data is supportive of hormonally sensitive hair follicles being vulnerable to LOH at the CYLD locus and tumor induction.
Genetic counselling issues
The data we present here confirms that even within one family, mutations within the CYLD locus give rise to a wide variation of clinical phenotypes. 50 mutations have now been identified in CYLD, with the majority found towards the carboxyl terminus of the protein, the position of the catalytic residues of ubiquitin hydrolase 2, 23. Clustering around the catalytic residues of ubiquitin hydrolase suggests the loss of deubiquitinating activity is important for the development of a cutaneous phenotype. The poor genotype-phenotype correlation with regard to tumor histology, and wide variation in clinical expression that we demonstrate in our families has been noted by other authors 1, 28, 29. In contrast, there have been families in China with up to 4 generations affected 30, 31with predominantly MFT, where the same mutation in other populations have resulted in heterogenous phenotypes, highlighting the possibility of population-specific modifier genes.
This wide variation in clinical phenotype means that genetic counselling and prognostication in families with known CYLD mutations is not straight forward. The presence of two obligate carriers in our pedigrees who were reported as unaffected by their relatives, but had severely affected children, underlines the importance of all ‘at risk’ individuals within a family being offered predictive testing if available or examined by a specialist with a prior knowledge of the condition. A review of the literature places the oldest patient to develop a first tumor at 42 years 32. Taken with our data of unaffected patients of the pedigree who were sequenced, this suggests that unaffected individuals in families that reach this age are unlikely to be carriers of a mutation33. As most couples have children prior of this age, predictive testing for a known familial mutation rather than clinical examination of the ‘at risk’ individual would be required if reassurance concerning future or an ongoing pregnancy was requested. Our results also indicate the impact on the lives of future generations of affected individuals cannot be gauged by the clinical severity of previously affected individuals.
Based on the variation seen in our European families, we suggest that the clinical diagnosis of BSS, MFT and FC have no prognostic, diagnostic or descriptive value and should be abandoned. Individuals found to have a germline CYLD mutation should be given the diagnosis of CYLD cutaneous syndrome, as the eventual clinical phenotype may be uncertain. This nomenclature will also avoid confusion with regard to possible genetic heterogeneity.
Novel therapeutic approaches are needed for this devastating condition
The need for non-surgical approaches in the treatment of these families is emphasised by the repeated surgical procedures individuals in both families underwent, the frequencies often only being restrained by available clinical resources.
Our current understanding of CYLD's function as a negative regulator of the nuclear factor kappa beta (NFkB) pathway 11, highlighted a role for aspirin in the treatment of these patients 13. However the clinical efficacy of aspirin in one trial of topical aspirin was limited, with only 2 out of 12 tumors showing complete remission after 24 weeks34.
However, this preliminary work does suggest that further studies are required to analyse the dose, timing and efficacy of NFkB modifiying drugs, perhaps at an earlier stage of tumorigenesis or prophylactically in these patients.
CONCLUSION
Here, we highlight the wide variation in both clinical severity and tumor type present in two large multigenerational families with CYLD cutaneous syndrome. The histological heterogeneity of the tumors adds weight to the argument that a hair follicle stem cell is the cell of origin. Significantly, the distribution of tumors suggest that hormonally sensitive hair follicles may be predisposed to tumor formation. Further understanding of the role of CYLD in regulating pathways such as NFkB may help develop non-surgical treatments of this devastating condition.
Acknowledgment
We are indebted to the families who made this study possible and to David Bourn for his technical help in the work.
Funding/ Support: This study was supported in part by the North East Skin Research Fund and Cancer Research UK.
Footnotes
Financial Disclosure: None reported.
References
- 1.Young AL, Kellermayer R, Szigeti R, Teszas A, Azmi S, Celebi JT. CYLD mutations underlie Brooke-Spiegler, familial cylindromatosis, and multiple familial trichoepithelioma syndromes. Clin Genet. 2006 Sep;70(3):246–249. doi: 10.1111/j.1399-0004.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 2.Saggar S, Chernoff KA, Lodha S, et al. CYLD Mutations in Familial Skin Appendage Tumors. J Med Genet. 2008 Jan 30; doi: 10.1136/jmg.2007.056127. [DOI] [PubMed] [Google Scholar]
- 3.Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000 Jun;25(2):160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- 4.Evans CD. Turban tumour. Br J Dermatol. 1954 Dec;66(12):434–443. doi: 10.1111/j.1365-2133.1954.tb12575.x. [DOI] [PubMed] [Google Scholar]
- 5.Jahoda C, Reynolds A. Skin stem cells - a hairy issue. Nature medicine. 2000 Oct;6(10):1095–1097. doi: 10.1038/80418. [DOI] [PubMed] [Google Scholar]
- 6.Massoumi R, Podda M, Fassler R, Paus R. Cylindroma as tumor of hair follicle origin. J Invest Dermatol. 2006 May;126(5):1182–1184. doi: 10.1038/sj.jid.5700218. [DOI] [PubMed] [Google Scholar]
- 7.Reiley WW, Zhang M, Jin W, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006 Apr;7(4):411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- 8.Jin W, Reiley WR, Lee AJ, et al. Deubiquitinating enzyme CYLD regulates the peripheral development and naive phenotype maintenance of B cells. J Biol Chem. 2007 May 25;282(21):15884–15893. doi: 10.1074/jbc.M609952200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Stirling B, Temmerman ST, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006 Nov;116(11):3042–3049. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim JH, Stirling B, Derry J, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007 Aug;27(2):349–360. doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003 Aug 14;424(6950):801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 12.Reiley W, Zhang M, Sun SC. Negative regulation of JNK signaling by the tumor suppressor CYLD. J Biol Chem. 2004 Dec 31;279(53):55161–55167. doi: 10.1074/jbc.M411049200. [DOI] [PubMed] [Google Scholar]
- 13.Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003 Aug 14;424(6950):797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 14.Given K, Pickrell K, Smith D. Dermal cylindroma (turban tumor). Case report. Plast Reconstr Surg. 1977 Apr;59(4):582–587. [PubMed] [Google Scholar]
- 15.Crain RC, Helwig EB. Dermal cylindroma (dermal eccrine cylindroma) Am J Clin Pathol. 1961 Jun;35:504–515. doi: 10.1093/ajcp/35.6.504. [DOI] [PubMed] [Google Scholar]
- 16.Jungehulsing M, Wagner M, Damm M. Turban tumour with involvement of the parotid gland. J Laryngol Otol. 1999 Aug;113(8):779–783. doi: 10.1017/s0022215100145190. [DOI] [PubMed] [Google Scholar]
- 17.Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006 May 19;125(4):665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Ermolaeva M, S H, Courtois G, Pasparakis M. Early postnatal lethality in knock-in mice expressing a truncated catalytically inactive form of CYLD. NF-kB: 20 Years on the Road from Biochemistry to Pathology Keystone Symposium. 2006 [Google Scholar]
- 19.Trompouki E, KDaMG Targeted inactivation of the cylindromatosis tumor suppressor gene causes perinatal lethality in mice. NF-kB: 20 Years on the Road from Biochemistry to Pathology Keystone Symposium. 2006 [Google Scholar]
- 20.DECIPHER DatabasE of Chromosomal Imbalance and Phenotype in Humans using Ensembl Resources. 2009. https://decipher.sanger.ac.uk/perl/application/patient/2856. Accessed 20/03/09, 2009. [DOI] [PMC free article] [PubMed]
- 21.Harwood CA, McGregor JM, Swale VJ, et al. High frequency and diversity of cutaneous appendageal tumors in organ transplant recipients. J Am Acad Dermatol. 2003 Mar;48(3):401–408. doi: 10.1067/mjd.2003.97. [DOI] [PubMed] [Google Scholar]
- 22.Ohyama M, Terunuma A, Tock CL, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006 Jan;116(1):249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massoumi R, Paus R. Cylindromatosis and the CYLD gene: new lessons on the molecular principles of epithelial growth control. Bioessays. 2007 Dec;29(12):1203–1214. doi: 10.1002/bies.20677. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein AM, Bale SJ, Peck GL, DiGiovanna JJ. Sun exposure and basal cell carcinomas in the nevoid basal cell carcinoma syndrome. J Am Acad Dermatol. 1993 Jul;29(1):34–41. doi: 10.1016/0190-9622(93)70148-m. [DOI] [PubMed] [Google Scholar]
- 25.Welch JP, Wells RS, Kerr CB. Ancell-Spiegler cylindromas (turban tumours) and Brooke-Fordyce Trichoepitheliomas: evidence for a single genetic entity. J Med Genet. 1968 Mar;5(1):29–35. doi: 10.1136/jmg.5.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahta AW, Farjo N, Farjo B, Philpott MP. Premature Senescence of Balding Dermal Papilla Cells In Vitro Is Associated with p16(INK4a) Expression. J Invest Dermatol. 2007 Nov 8; doi: 10.1038/sj.jid.5701147. [DOI] [PubMed] [Google Scholar]
- 27.Moriwaki K, Sugawara K, Kobayashi H, Massoumi R, Ishii M. A role of CYLD in hair cycling mouse. Journal of Investigative Dermatology. 2007;127:s107–s107. 207. [Google Scholar]
- 28.Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res. 2006 Feb 1;12(3 Pt 1):950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Huang Y, Yan K, et al. Diverse phenotype of Brooke-Spiegler syndrome associated with a nonsense mutation in the CYLD tumor suppressor gene. Exp Dermatol. 2006 Dec;15(12):966–970. doi: 10.1111/j.1600-0625.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- 30.Salhi A, Bornholdt D, Oeffner F, et al. Multiple familial trichoepithelioma caused by mutations in the cylindromatosis tumor suppressor gene. Cancer Res. 2004 Aug 1;64(15):5113–5117. doi: 10.1158/0008-5472.CAN-04-0307. [DOI] [PubMed] [Google Scholar]
- 31.Zheng G, Hu L, Huang W, et al. CYLD mutation causes multiple familial trichoepithelioma in three Chinese families. Hum Mutat. 2004 Apr;23(4):400. doi: 10.1002/humu.9231. [DOI] [PubMed] [Google Scholar]
- 32.Martins C, Bartolo E. Brooke-Spiegler syndrome: treatment of cylindromas with CO2 laser. Dermatol Surg. 2000 Sep;26(9):877–880. doi: 10.1046/j.1524-4725.2000.00034.x. discussion 881. [DOI] [PubMed] [Google Scholar]
- 33.Biggs PJ, Wooster R, Ford D, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995 Dec;11(4):441–443. doi: 10.1038/ng1295-441. [DOI] [PubMed] [Google Scholar]
- 34.Oosterkamp HM, Neering H, Nijman SM, et al. An evaluation of the efficacy of topical application of salicylic acid for the treatment of familial cylindromatosis. Br J Dermatol. 2006 Jul;155(1):182–185. doi: 10.1111/j.1365-2133.2006.07224.x. [DOI] [PubMed] [Google Scholar]
- 35.Gerretsen AL, Beemer FA, Deenstra W, Hennekam FA, van Vloten WA. Familial cutaneous cylindromas: investigations in five generations of a family. J Am Acad Dermatol. 1995 Aug;33(2 Pt 1):199–206. doi: 10.1016/0190-9622(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 36.Saunders H, Tucker P, Saurine T, Watkins F. Pedigree of multiple benign adnexal tumours of Brooke-Spiegler type. Australas J Dermatol. 2003 May;44(2):144–148. doi: 10.1046/j.1440-0960.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- 37.Liang YH, Gao M, Sun LD, et al. Two novel CYLD gene mutations in Chinese families with trichoepithelioma and a literature review of 16 families with trichoepithelioma reported in China. Br J Dermatol. 2005 Dec;153(6):1213–1215. doi: 10.1111/j.1365-2133.2005.06960.x. [DOI] [PubMed] [Google Scholar]
- 38.Hu G, Onder M, Gill M, et al. A novel missense mutation in CYLD in a family with Brooke-Spiegler syndrome. J Invest Dermatol. 2003 Oct;121(4):732–734. doi: 10.1046/j.1523-1747.2003.12514.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XJ, Liang YH, He PP, et al. Identification of the cylindromatosis tumor-suppressor gene responsible for multiple familial trichoepithelioma. J Invest Dermatol. 2004 Mar;122(3):658–664. doi: 10.1111/j.0022-202X.2004.22321.x. [DOI] [PubMed] [Google Scholar]
- 40.Burrows NP, Jones RR, Smith NP. The clinicopathological features of familial cylindromas and trichoepitheliomas (Brooke-Spiegler syndrome): a report of two families. Clin Exp Dermatol. 1992 Sep;17(5):332–336. doi: 10.1111/j.1365-2230.1992.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 41.Fenske C, Banerjee P, Holden C, Carter N. Brooke-Spiegler syndrome locus assigned to 16q12-q13. J Invest Dermatol. 2000 May;114(5):1057–1058. doi: 10.1046/j.1523-1747.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- 42.Bumgardner AC, Hsu S, Nunez-Gussman JK, Schwartz MR. Trichoepitheliomas and eccrine spiradenomas with spiradenoma/cylindroma overlap. Int J Dermatol. 2005 May;44(5):415–417. doi: 10.1111/j.1365-4632.2005.02044.x. [DOI] [PubMed] [Google Scholar]
- 43.Poblete Gutierrez P, Eggermann T, Holler D, et al. Phenotype diversity in familial cylindromatosis: a frameshift mutation in the tumor suppressor gene CYLD underlies different tumors of skin appendages. J Invest Dermatol. 2002 Aug;119(2):527–531. doi: 10.1046/j.1523-1747.2002.01839.x. [DOI] [PubMed] [Google Scholar]
- 44.Oiso N, Mizuno N, Fukai K, Nakagawa K, Ishii M. Mild phenotype of familial cylindromatosis associated with an R758X nonsense mutation in the CYLD tumour suppressor gene. Br J Dermatol. 2004 Nov;151(5):1084–1086. doi: 10.1111/j.1365-2133.2004.06231.x. [DOI] [PubMed] [Google Scholar]