Abstract
Ticks deposit saliva at the site of their attachment to a host in order to inhibit haemostasis, inflammation and innate and adaptive immune responses. The anti-haemostatic properties of tick saliva have been described by many studies, but few show that tick infestations or its anti-haemostatic components exert systemic effects in vivo. In the present study, we extended these observations and show that, compared with normal skin, bovine hosts that are genetically susceptible to tick infestations present an increase in the clotting time of blood collected from the immediate vicinity of haemorrhagic feeding pools in skin infested with different developmental stages of Rhipicepahlus microplus; conversely, we determined that clotting time of tick-infested skin from genetically resistant bovines was shorter than that of normal skin. Coagulation and inflammation have many components in common and we determined that in resistant bovines, eosinophils and basophils, which are known to contain tissue factor, are recruited in greater numbers to the inflammatory site of tick bites than in susceptible hosts. Finally, we correlated the observed differences in clotting times with the expression profiles of transcripts for putative anti-haemostatic proteins in different developmental stages of R. microplus fed on genetically susceptible and resistant hosts: we determined that transcripts coding for proteins similar to these molecules are overrepresented in salivary glands from nymphs and males fed on susceptible bovines. Our data indicate that ticks are able to modulate their host’s local haemostatic reactions. In the resistant phenotype, larger amounts of inflammatory cells are recruited and expression of anti-coagulant molecules is decreased tick salivary glands, features that can hamper the tick’s blood meal.
Keywords: Rhipicephalus (Boophilus) microplus, Haemostasis, Clotting time, Ticks, Bos taurus taurus, Bos taurus indicus, Saliva, Haematophagy, Transcriptome
1. Introduction
Ticks have adapted to blood feeding on vertebrate hosts who avoid blood loss to these ecto-parasites by vasoconstriction, platelet aggregation and coagulation. These steps are under control of many factors and enzymes that are responsible for the amplification and propagation of coagulation, fibrinolysis, inflammation and tissue repair (Francischetti et al., 2008a). In order to overcome these defences ticks deposit saliva at the site of their attachment on hosts. By means of in vitro assays several investigators have shown that tick saliva contains several molecules that inhibit responses associated with homeostasis of host physiological systems such as haemostasis, inflammation and adaptive immune responses (Ribeiro, 1995). Saliva from all haematophagous arthropods studied to date contains molecules that inhibit platelet aggregation, coagulation proteases and/or thrombin (reviewed in Ribeiro and Francischetti (2003)). Tick saliva is no exception (reviewed in Mans and Neitz (2004)) and there are reports that saliva from several species of ticks also contains molecules that inhibit thrombin (Zhu et al., 1997; Nienaber et al., 1999; Horn et al., 2000; Ciprandi et al., 2006; Nakajima et al., 2006; Koh et al., 2007), serine proteases (Imamura et al., 2005) and aggregation of platelets (Mans et al. 1998).
Despite of the variety of anti-haemostatic molecules identified in ticks, few studies have examined their in vivo effects on haemostasis. The first study showed that a recombinant tick anti-coagulant peptide, when infused into monkeys with disseminated intravascular coagulation, inhibited the formation of fibrinopeptide A by thromboplastin (Neeper et al., 1990). Another study examined the in vivo effects of ixolaris, a two-Kunitz salivary gland protein identified in Ixodes scapularis that is similar to Tissue Factor Pathway Inhibitor and binds to FXa or FX. Using a model of venous thrombosis in rats, Nazareth et al. (2006) showed that administration of ixolaris caused a dose-dependent, rapid and prolonged reduction in thrombus formation. Finally, Reck et al. (2008) showed that by the end of a parasitic cycle of the cattle tick Rhipicephalus (Boophilus) microplus on bovines, collagen- and ADP-induced platelet aggregation had decreased and activated partial thromboplastin and prothrombin times had increased.
The study by Reck et al. (2008) did not compare haemostasis in contrasting host phenotypes of infestations. This aspect is important because among certain specific tick/host combinations some hosts are able to inhibit the tick’s blood meal. Furthermore, Reck Jr. et al. did not examine the effect of tick bites on local haemostasis, i.e., at the site where the haemorrhagic feeding pool is formed, and neither did they examine the effect of different development stages of this parasite upon local haemostasis in vivo. In the present study, we examined the effect of feeding by different developmental stages of R. microplus on local clotting time in tick-infested skin of contrasting phenotypes of tick infestations in bovines. We also examined if the inflammatory profile of tick-infested skin was associated with alterations in local haemostasis. Finally, we examined the expression profiles of transcripts for putative anti-haemostatic proteins identified in cDNA libraries constructed using salivary glands from the different developmental stages of R. microplus fed on genetically susceptible and resistant hosts.
2. Materials and methods
2.1. Larvae
Engorged females of R. microplus ticks were collected from bovines, incubated at 28 °C, with 90–95% relative humidity until oviposition. On day 3 of oviposition the females were removed and egg masses were measured. Aliquots of eggs (500 mg), which are equivalent to a batch of 10,000 larvae, were incubated under the same conditions as for oviposition until eclosion of larvae.
2.2. Animals and infestations with R. microplus
R. microplus-resistant (Bos taurus indicus, Nelore; N = 4) and - susceptible (Bos taurus taurus, Holsteins; N = 4) bovines were housed in pens and were free of exposure to ticks. All animals were artificially infested four times with 10,000 15-day-old unfed larvae of R. microplus. The larvae fed and developed into nymphs and then adults on the two groups of hosts. Each successive infestation was performed after completion of the previous parasitic cycle. Engorged females larger than 4 mm in length were counted on one side of each host at the end of the parasitic cycle in order to confirm the phenotypes of high and low infestations. The average numbers of engorged females found on one side of susceptible and resistant hosts were 220 ± 73 and 13 ± 10, respectively. The experiments described in this work were approved by the institutional Animal Ethics Committee of the Ribeirão Preto Medical School.
2.3. Collection of blood samples and measurement of clotting time
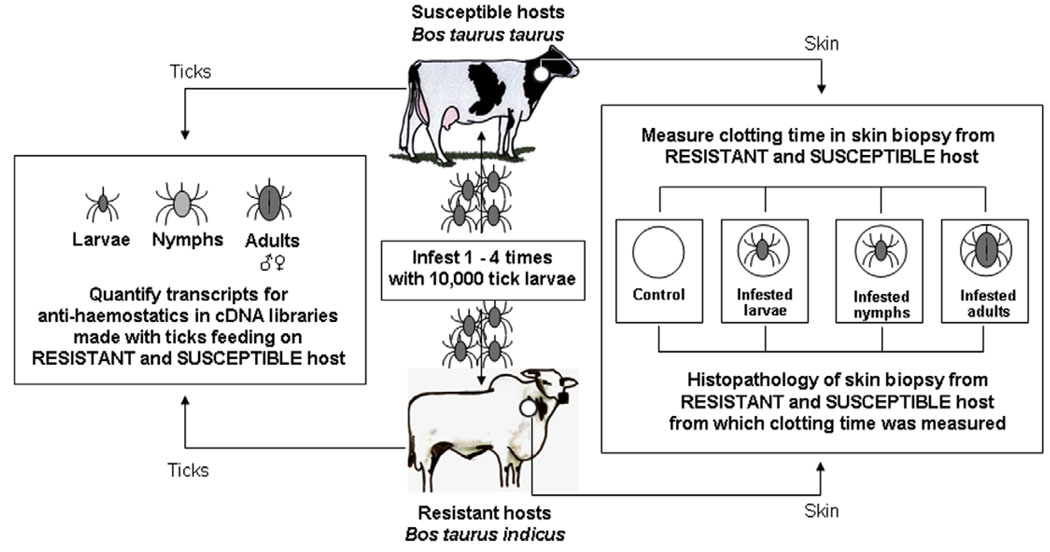
The experimental design is depicted in Fig. 1. Skin biopsies (6 mm) and peripheral blood (by internal puncture of the jugular vein with Vaccutainer® tubes) and were collected when animals were infested with larvae, nymphs or adults. Skin contained a tick in the centre and a paired control was collected from a non-infested, contra-lateral site of the same animal. Skin samples were collected without anaesthesia in order to avoid artefacts on the clotting system. All samples were collected at the following time points of each of the four parasitic cycles: 48 h after larvae were released on the hosts and 7 and 14 days later for nymph- and adult-infested skin, respectively. Visual inspection of the ticks was also done in order to confirm their developmental stage.
Fig. 1.
Experimental design to evaluate the effect of host genotype on local haemostatics in tick infestations. Skin biopsies, with a feeding larva, nymph or female attached in the centre, were collected with a 6 mm punch. Skin from a contra-lateral site of the same animal, but without feeding ticks was collected as a paired control. Data for clotting times of different experimental samples (i.e., non-infested, larvae, nymph- and adult-infested skin) were pooled for four successive infestations. In the case of adult-infested skin from susceptible bovines, data were also pooled for 1–2 times and 3–4 times infested hosts. Ticks (unfed larvae and salivary glands from feeding nymphal, male and female ticks) originating from susceptible and resistant hosts were collected and the number of transcripts for genes encoding tick anti-haemostatic proteins was determined.
Systemic and local clotting times were measured immediately after sample collection. Data for clotting times from the four infestations were pooled for samples with the same characteristics (i.e., tick-free, control skin; larvae-, nymph- or adult-infested skin from susceptible or resistant bovines, respectively). In order to account for possible effects of the host adaptive immune response against parasitic anti-haemostatic molecules, data were re-pooled in the following manner: clotting times of adult-infested skin samples from 1 and 2 times-infested susceptible bovines and from 3 and 4 times-infested susceptible bovines. There were no significant differences between the clotting times of normal, non-infested skin collected during any stage of the four parasitic cycles (data not shown).
Clotting time was measured by collecting blood by capillary action into a glass tube 800 mm × 1.5 mm in diameter (Accu-Glass, BD, Saint Louis, MO, USA) without anti-coagulant. The blood was collected immediately after jugular vein puncture or after skin biopsy. The ends of the capillary, which does not fill completely, were sealed using a gloved finger, while other contact with the blood was avoided. The tubes were shaken gently until the blood stopped moving (Pichotka and Reichel, 1950; García-Manzano et al., 2001). At this time a 1 cm section of the capillary tube was broken off in order to confirm the presence of a strand of fibrin. The chronometer was started immediately after the tubes were filled and stopped when the blood no longer moved in the capillary, resulting in a precise clotting time.
2.4. Histology of skin
Skin biopsies were incubated for 24 h in fixative solution (formalin 10% in 1 × PBS, pH 7.0), embedded in paraffin and 4 µm thick sections were cut using a microtome. Sections were stained with Haematoxylin–Eosin for analysis of the tissue’s features and May Grünwald–Giemsa for performing total and differential cell counts. Analysis of skin samples stained with Haematoxylin–Eosin was performed under light microscopy (objective 40×). Differential cell counts were done using a Reichart integrating graticle (Austria/PK6, 3× mn) on oil immersion fields (objective 100×). Cells from three areas (0.0625 mm2 each) surrounding the dermis immediately below the cement cone were counted and the means of each area were used for further analyses. Differential cell counts were performed on the same sections and areas used for total cell counts.
2.5. Extraction of mRNA from tick salivary glands and synthesis of cDNA libraries
Unfed larvae (UFL) of R. microplus ticks were obtained 3 days after hatching from eggs laid by females that had fed on resistant or susceptible bovines. Unfed larvae were frozen at −80 °C and stored until used. Feeding nymphs, male and female adults of different sizes were collected from naturally infested B. t. taurus and B. t. indicus to provide material for all libraries. Salivary glands were dissected from 25 females, 25 males and 40 nymphs fed on each type of host and were briefly washed in ice-cold PBS and immediately stored in RNALater storage solution (Ambion, Austin, TX, USA), kept 4 °C for 24 h and then at −80 °C until used. Poly A+ mRNA from R. microplus salivary glands from tick salivary glands and total RNA from UFL were isolated with the Micro-Fast Track™ 2.0 mRNA isolation kit (Invitrogen) according to the manufacturer’s instructions. This RNA (similar concentrations for all samples) was used to construct size fractionated, unidirectional cDNA libraries using the vector TriplEx2 following the instructions for the SMART™ cDNA Library Construction kit (Clontech, Palo Alto, California) with some modifications (Valenzuela et al., 2002) and packaged in lambda phage using the Gigapack® III Gold Packaging Extract (Stratagene, La Jolla, California).
2.6. Massive sequencing of the cDNA libraries
The cDNA libraries were plated to approximately 200 plaques per plate (150 mm petri dish) by infecting Escherichia coli XL1-Blue cells (Clontech) in log phase of growth. The plaques were randomly picked and transferred to a 96-well polypropylene plate (Novagen, Madison, Wisconsin) containing 100 µl of SM buffer or water per well. A volume of 3 µL of the phage sample was used as a template for a PCR reaction to amplify random cDNAs. The primers used for this reaction were sequences from the lambda TriplEX2 vector. PT 2F1 (5′-AAG TAC TCT AGC AAT TGT GAG C-3′) is positioned upstream of the cDNA of interest (5′ end), and PT 2R1 (5′-CTC TTC GCT ATT ACG CCA GCT G-3′) is positioned downstream of the cDNA of interest (3′ end). Taq DNA Polymerase Platinum (Invitrogen) was used for these reactions. Amplification conditions were: 75 °C for 2 min, of 94 °C for 2 min and 33 cycles of 94 °C for 1 min (denaturation), 49 °C for 1 min and 72 °C (annealing) for 1 min and 20 s (extension). The amplification was performed using a PTC-200 thermocycler (MJ Research, Waltham, MA; Valenzuela et al., 2002). Amplified products were visualized on a 1.1% agarose gel with ethidium bromide (1.5 µg/ml) and the amount of recombinants was determined.
PCR products were cleaned using the Montage® PCRμ96 Plate (Millipore, Billerica, Massachusetts) and Multi Screen 384 Vacuum Manifold (Millipore). A volume of 3 µL of the cleaned PCR product was used as a template for a cycle-sequencing reaction using the Big Dye kit (Applied BioSystems). The primer used for sequencing, PT 2F3 (5′-TCTCGGGAAGCG CGC CAT TGT-3′) is upstream of the inserted cDNA and downstream of the primer PT 2F1. Sequencing reactions were performed on a Gene Amp PCR System 9700 (Applied Biosystems). Cycling conditions were 96 °C for 2 min, 94 °C and 40 cycles at 96 °C for 10 s (denaturation), 50 °C for 20 s (annealing), and 60 °C for 4 min (extension). The sequencing reaction products were cleaned using the Multi Screen® Separation System (Millipore) and SephadexT® G50 Superfine (GE Healthcare, Uppsala, Sweden) and MultiScreen HV 96 well plates (Millipore). Samples were sequenced immediately on a Sequence Analyzer 3700 (Applied Biosystems) or stored at −30 °C. Random clones were sequenced from the 5′ direction only because successful sequencing from the 3′ end was usually lower than 40% due to polymerase slippage on the polyA stretch (Ribeiro et al., 2006).
2.7. Clustering of cDNA sequences and bioinformatics tools
Detailed description of the bioinformatic treatment of the data can be found elsewhere (Valenzuela et al., 2002; Ribeiro et al., 2006). Briefly the ESTs (raw sequences) were trimmed of primer and vector sequences. Only high quality ESTs were then clustered into related groups based on sequence similarity using the BLASTn algorithm (Altschul and Gish, 1996), each grouping was then aligned using the CAP three assembler (Huang and Madan, 1999) and then the consensus sequence of each assembled group was compared with other nucleotide or protein databases using appropriate BLAST algorithms (BLASTx, BLASTp, BLASTn or rpsBLAST). The final output was piped into a tab-delimited file imported into an Excel spreadsheet (Microsoft Excel Analysis Tools, Seattle, WA). BLAST searches were done locally from executables obtained at the NCBI FTP site (ftp://ftp.ncbi.nih.gov/blast/executables/; Altschul et al., 1997) against the Non-Redundant (NR) protein database of the NCBI and the Conserved Domains Database (CDD) (ftp://ftp.ncbi.nlm.nih.gov/pub/mmdb/cdd/) with SMART (Letunic et al., 2002) motifs. We also used a customized database of including all tick related protein sequences (Acari) found in the NR database. Since all libraries were constructed under the same conditions, using the same number of salivary glands and equivalent amounts of mRNA, we were able to combine all high quality ESTs from each library into one analysis resulting in contigs potentially containing ESTs from each individual library. To analyze the relative abundance of transcripts from each library contributing to a particular contig, we employed a customized bioinformatic program (Ribeiro et al. 2006) that determines how many ESTs in a particular contig were derived from each individual library, these numbers were used to analyze the distribution of ESTs in the contigs and libraries.
2.8. Statistical analyses
One-Way Analysis of Variance (ANOVA) and Kruskal–Wallis One-Way Analysis of Variance on Ranks were used to evaluate significance among group medians of coagulation times of bleeding from skin and of leukocyte populations in cellular infiltrates. In case of significance, Bonferroni’s t-test and Dunn’s method were performed as post hoc tests to perform multiple comparisons of infested versus normal skin. Student’s t-test and the Mann–Whitney rank sum test were used to evaluate significance among group medians of coagulation times of normal skin from susceptible and resistant bovines and of cellular infiltrates in normal and infested skin from different breeds. The χ2 test was used to analyze differences in the distribution of ESTs in the clusters and libraries. A P-value <0.05 was used to establish the level of significance. SigmaStat version 2.03 (SPSS Inc., Chicago, IL) was used to perform the statistical tests.
3. Results
3.1. R. microplus feeding affects clotting time at the site of its attachment to bovine skin
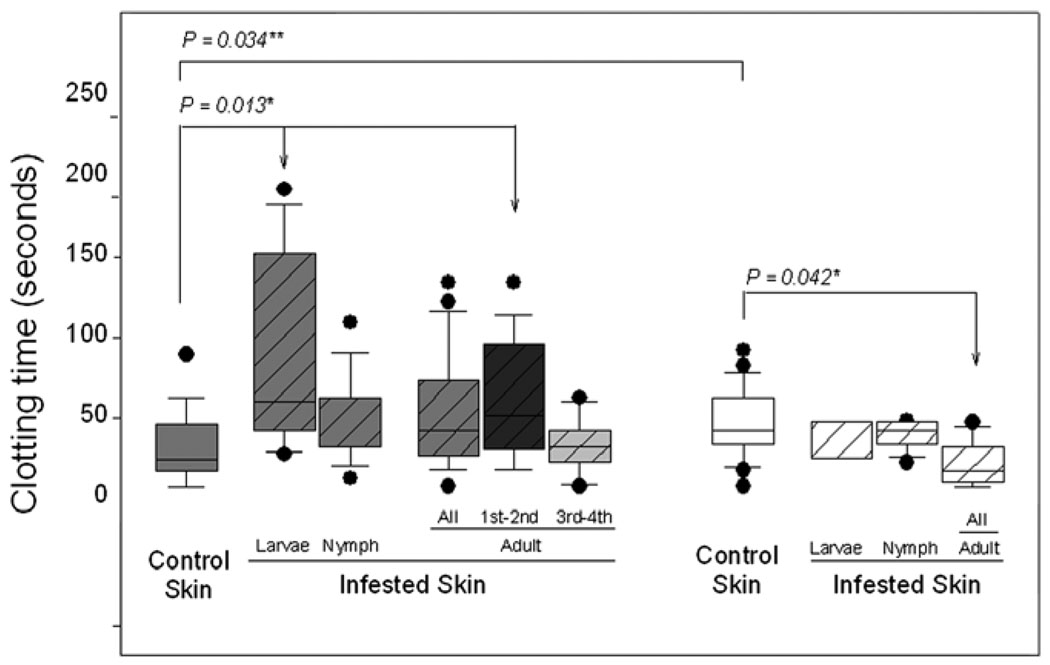
Clotting time was measured in blood from biopsies of bovine skin containing, or not, R. microplus at different stages of development. Measurements were made on susceptible animals infested one to four times. The results shown in Fig. 2 indicate that, relative to bleeding from biopsies of normal skin (clotting time = 29.95 ± SE 4.42 s), clotting time is significantly (P = 0.013) increased in blood from skin of susceptible bovines infested with larvae (clotting time = 88.75 ± SE 22.73 s); clotting time was also increased in skin infested with nymphs (clotting time = 45.10 ± SE 9.07 s) and adults (clotting time = 53.24 ± SE 8.04 s), but the difference relative to normal skin did not reach significance. An explanation for this finding could be that after several infestations susceptible hosts may have developed immunity against tick anti-haemostatic molecules. We examined this possibility by comparing clotting time of normal skin with that of one- and two-times adult-infested skin and then with clotting time of three- and four-times adult-infested skin. Indeed, clotting time in 1 and 2 times-infested susceptible bovines (88.75 ± SE 22.73 s) was now significantly (P = 0.013) longer than that of bleeding from normal skin of the same host (Fig. 2), while clotting time of blood from adult-infested skin from 3 and 4 times-infested susceptible bovines was practically the same as that of normal skin (30.80 ± SE 8.02 s). This result suggests that susceptible bovines indeed develop some resistance to tick anti-haemostatic salivary molecules (Fig. 2). Blood from larvae- and nymph-infested skin also presented with similar significant differences between once or twice infested and 3 or 4 times-infested susceptible bovines (data not shown). Clotting time of peripheral blood obtained by venipuncture of the jugular vein of infested tick-susceptible animals was within normal limits for all animals (255.92 ± SE 11.99 s).
Fig. 2.
R. microplus affects clotting time at the site of its attachment to bovine skin. Box whisker plots of clotting times measured in blood collected from punch holes made from biopsies of normal skin (empty bars) and of skin on a contra-lateral site containing a feeding tick at different developmental stages (larvae, nymph or adults; filled bars) on susceptible (grey bars: Holstein breed; N = 4) and resistant (white bars: Nelore breed; N = 4) bovines artificially infested four times with 10,000 15-day-old larvae. Data for clotting times in adult-infested skin from susceptible animals was pooled for all four infestations, for the first and second infestations and for the third and fourth infestations; data for clotting times in adult-infested skin from resistant animals was pooled for all four infestations. The boxes show the 25th and 75th percentiles, the lines within the boxes mark the median, whiskers indicate the 10th and 90th percentiles and the dots indicate outliers. *Kruskal–Wallis One-Way Analysis of Variance on Ranks followed by multiple comparisons versus control group with Dunn’s test; **Student’s t-test.
Clotting time was also measured in resistant animals. Samples from these bovines were collected only after one and two infestations because after the second infestation they became highly resistant to larvae and no feeding ticks could be found on them after the third and fourth infestations. In resistant bovines the opposite was observed (Fig. 2): tick-infested skin clotted in less time (35.00 ± SE 10.00 s; 37.37 ± SE 3.22 s; 19.37 ± SE 5.21 s for larvae, nymph- and adult-infested skin, respectively) than normal skin (44.79 ± SE 5.16 s) and in adult-infested skin, the developmental stage when female ticks rapidly engorge, the decrease in clotting time relative to that of normal skin was significant (P = 0.026). Again, clotting time of peripheral blood from tick-infested resistant bovines was within normal limits (336.54 ± SE 38.21 s). Interestingly, the clotting time of blood from normal skin from bovines of the resistant breed was significantly (P = 0.034, Student’s t-test) longer than that of normal bovine skin from bovines of the susceptible breed.
3.2. The genetic composition of the bovine host affects the inflammatory infiltrate at the tick bite site
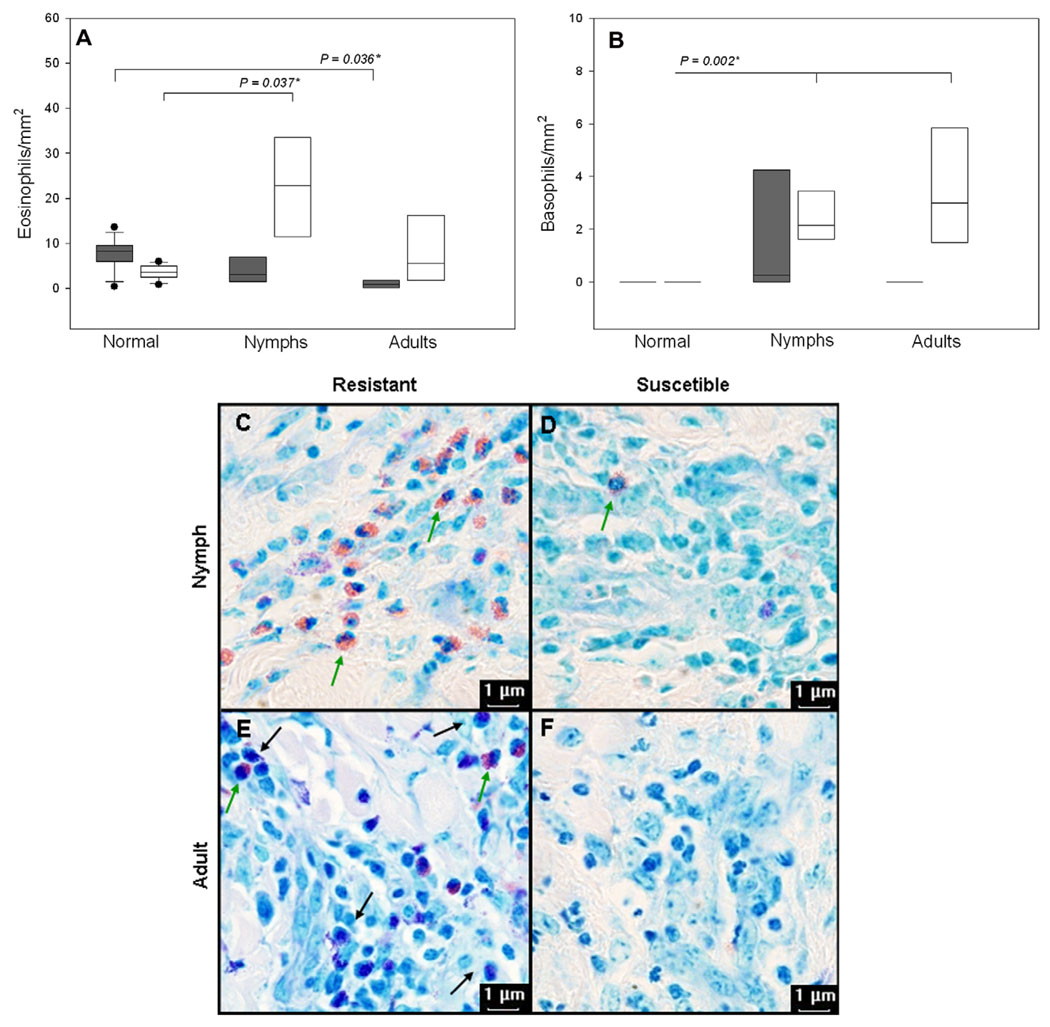
Since inflammation can affect haemostasis (Strukova, 2006; Francischetti et al., 2008a) the differences observed in clotting times of tick-infested skin of resistant and susceptible hosts may possibly be caused by differences in the profiles of local cellular inflammation elicited by tick bites. The results presented in Fig. 3 show that, indeed, resistant bovines have a better capacity to maintain and/or recruit eosinophils and basophils to sites of cutaneous inflammatory reactions to tick bites. Fig. 3A shows that relative to the cellular content of their normal skin, susceptible hosts (grey bars) maintained significantly (P = 0.024) less eosinophils in their adult-infested skin even though their normal skin contained significantly (P = 0.018) more of these leukocytes than that of resistant bovines; nymph-infested skin of susceptible hosts also contained fewer eosinophils, but the difference was not significant. Resistant hosts, on the other hand, recruited significantly (P = 0.011) more eosinophils to the nymphal bite site. The cellular infiltrates elicited by nymphs contained significantly (P = 0.047) more eosinophils in resistant hosts than in susceptible hosts. Again, relative to the cellular content of their normal skin, resistant hosts also recruited significantly (P = 0.002) more basophils to local cutaneous reactions against both nymphs and adults. Furthermore, compared to susceptible bovines, adult-infested skin from resistant hosts also contained significantly (P = 0.048) more basophils (Fig. 3B). Fig. 3C–F shows the morphological characteristics and the conspicuous qualitative differences in the composition of the inflammatory infiltrates of tick-infested skin from B. taurus and B. indicus. Several lesions in resistant, but not in susceptible bovines presented a conspicuous amount of granules and degranulated cells (data not shown). Other cellular components of local inflammation (i.e., neutrophils and mononuclear cells) were present in similar numbers in inflammatory infiltrates of both host phenotypes (data not shown). In all comparisons mast cells numbers were equal in both breeds of bovines, but were significantly lower in infested relative to normal skin at all developmental stages of R. microplus (data not shown).
Fig. 3.
Eosinophils and basophils are recruited in larger numbers to inflammatory sites elicited by tick bites in genetically resistant, but not in susceptible bovines. Box whisker plots of cell counts of eosinophils (A) and basophils (B) present in skin biopsies of normal skin and of skin from a contra-lateral site containing a feeding tick at different developmental stages (nymph or adults) on susceptible (grey bars: Holstein breed; N = 4) and resistant (open bars: Nelore breed; N = 4) bovines artificially infested four times with 10,000 15-day-old larvae. After collection skin samples were immediately immersed in buffered formalin (pH 7.0). Samples were embedded in paraffin, 4 µm thick sections were made and stained with May Grünwald–Giemsa for total and differential cell counts; in infested skin they were limited to the area of the tick cement cone (objective 100×). The boxes show the 25th and 75th percentiles, the lines within the boxes mark the median, indicate the 10th and 90th percentiles and the dots indicate outliers. *Kruskal–Wallis One-Way Analysis of Variance on Ranks followed by multiple comparisons versus control group with Dunn’s test. Panels (C)–(F) show the conspicuous histological differences between the inflammatory infiltrates of lesions from resistant and susceptible bovines; nymph-infested skin from B. taurus and B. indicus (C and D, respectively); adult-infested skin from B. taurus and B. indicus (E and F, respectively). Note the morphological characteristics of bovine eosinophils (green arrows) and basophils (black arrows; objective 100×).
3.3. The genetic composition of the bovine host affects the number of transcripts for putative anti-haemostatic molecules expressed in salivary glands from R. microplus
The differences observed in clotting times of tick-infested skin from resistant and susceptible hosts could be caused by differences in the amounts of anti-haemostatic molecules secreted in the saliva from ticks feeding on these hosts. In order to examine this possibility, we determined if there were differences in the numbers of transcripts coding for putative anti-haemostatic molecules in cDNA libraries derived from salivary glands of different developmental stages of R. microplus feeding on resistant or susceptible hosts. In total, eight libraries were constructed: salivary glands from females fed on susceptible hosts, salivary glands from females fed on resistant hosts, salivary glands from males fed on susceptible hosts, salivary glands from males fed on resistant hosts, salivary glands from nymphs fed on susceptible hosts, salivary glands from nymphs fed on resistant hosts, unfed larvae derived from females fed on susceptible hosts, unfed larvae derived from females fed on resistant hosts. The following numbers of ESTs were obtained for each library: salivary glands from females fed on susceptible hosts: 1152; salivary glands from females fed on resistant hosts: 986; salivary glands from males fed on susceptible hosts: 1218; salivary glands from males fed on resistant hosts: 945; salivary glands from nymphs fed on susceptible hosts: 1134; salivary glands from nymphs fed on resistant hosts: 944; unfed larvae derived from females fed on susceptible hosts: 746; unfed larvae derived from females fed on resistant hosts: 798.
Transcripts were considered to encode putative anti-haemostatic molecules if they presented matches to metalloproteases, serine proteinase inhibitors or the BPTI/Kunitz family of serine protease inhibitors; only those hits with an E-value below 10−3 (cutoff value) and in positive frame (since the cDNA libraries were constructed in a uni-dirctional orientation and resulting clones were sequenced from the 5′ direction) were considered suitable for annotation as such. The data depicted in Table 1 show that the relevant transcripts were all overrepresented in particular cDNA libraries or in clusters of genes from these libraries derived from ticks fed on susceptible bovine hosts since their distribution differed from what is expected from random events of expression, as evaluated with the χ2 test.
Table 1.
Differentially expressed transcripts coding for putative anti-haemostatic proteins in libraries made from ticks feeding on susceptible and resistant bovines.
| Best match of overexpressed cluster to Acari protein database |
E-value | Libraries derived from: |
Number of ESTs in library from ticks fed on susceptible hosts |
Expected | Number of ESTs in library from ticks fed on resistant hosts |
Expected |
P-value χ2 test |
|---|---|---|---|---|---|---|---|
| Boophilin | 3e–016 | Male | 25 | 16 | 3 | 12 | <0.001 |
| [Boophilus microplus] gi|17529566 |
Salivary glands | ||||||
| Salivary gland metalloprotease | 8e–025 | Male | 12 | 7 | 0 | 5 | 0.006 |
| [Boophilus microplus] gi|71726984 |
Salivary glands | ||||||
| Salivary gland metalloprotease | 1e–025 | Male | 12 | 8 | 3 | 7 | 0.113 |
| [Boophilus plus] gi|71726984 |
Salivary glands | ||||||
| Putative secreted protease inhibitor | 8e–009 | Nymphal | 7 | 4 | 0 | 3 | 0.042a |
| [Ixodes scapularis] gi|67083711 |
Salivary glands |
Expected values for expression in both of the libraries from nymphs is less than 5, therefore the χ2 test is inaccurate.
4. Discussion
Reck et al. (2008) were the first to demonstrate that tick infestations are capable of causing systemic in vivo alterations of host haemostasis. The authors showed that these alterations were within normal limits, yet become more pronounced with the progression of the tick’s life cycle upon its bovine host. They proposed that this finding was proof of the role of tick-derived anti-haemostatics. However, the experimental design employed in that study is not entirely suitable to determine if the observed alterations were due to a direct inhibitory effect of tick saliva upon the components of the host’s haemostatic system or, rather, if the numerous bites caused by a parasitic cycle initiated with 20,000 larvae and ending with a mean of almost 1700 female ticks could be activating the vascular endothelium. Activated endothelium exposes tissue factor and depending on the extent of activation it can result in non-overt or overt disseminated intravascular coagulation (Francischetti, 2008b) with attendant alterations in haemostatic parameters (Toh and Hoots, 2007). Sphingomyelinase is component of venoms that can reduce the cell surface expression of thrombomodulin and Endothelial PC Receptor on endothelial cells, prolong the activated partial thromboplastin time and deplete clotting factors VIII, IX, XI, and XII (Peterson, 2006; van den Berg et al., 2007). Interestingly a sphingomyelinase was recently described in saliva of I. scapularis (Alarcon-Chaidez et al., 2009). Sphingomyelinase D is the primary venom dermonecrotic factor of another arachnid, the brown spider of the genus Loxosceles and, indeed, envenomation by this species frequently results in a coagulopathy with increased clotting time, activated partial thromboplastin time, fibrinogenomia and bleeding (McGlasson et al., 2007) and can progress to disseminated intravascular coagulation with bleeding (Berger et al., 1973) even though anti-haemostatic molecules have not been reported in its venom (Fernandes-Pedrosa et al., 2008). These events in Loxosceles envenomation are compatible with the observations by Reck Jr et al. of R. microplus-infested bovines. In other words, a systemic response to tick infestations with increased bleeding could be more likely caused by the large area of injured skin (Carvalho et al., 2008), rather than by a “pharmacological” response to tick anti-haemostatics. Furthermore, parasitic anti-haemostatic components could, in fact, act only locally in order to conserve this resource for the parasite. For these reasons, a more adequate approach to determine the in vivo mechanisms that result in a successful blood meal for the tick would be to examine the haemostatic parameters at the site where these parasites would actually need to counter haemostasis. Therefore, we examined the clotting time of blood from the skin in the immediate vicinity of a feeding tick. Our results support the hypothesis that tick anti-haemostatic molecules do indeed exert locally the same functions in vivo that are seen in vitro.
However, the useful study by Reck Jr. et al., it also did not compare haemostasis in contrasting host phenotypes of infestations. This aspect is important because within certain specific tick/host combinations some hosts are able to inhibit acquisition of the tick’s bloodmeal. Among these combinations, the bovine host presents the most striking example. The level of infestation by the cattle tick, R. microplus, varies according to the breed of the bovine hosts and, even after repeated infestations, Bos taurus breeds of hosts will still harbour significantly larger numbers of ticks than Bos indicus breeds (Mattioli and Cassama, 1995; Mattioli et al., 2000; Wambura et al., 1998). The mechanisms resulting in these distinct phenotypes are unknown and, among several possibilities, may be due to different amounts of inhibitors of haemostasis produced by tick salivary glands while feeding on the two types of host. Additionally, the effect of different development stages of this ectoparasite upon local haemostasis in vivo was not established even though it is known that the composition of tick saliva varies during its life cycle (Narasimhan et al., 2007). The present study shows for the first time that tick infestations can alter the clotting time of blood collected at the feeding site. We show that compared with normal skin the clotting time is increased in blood from tick-infested skin, but only in genetically susceptible B. taurus bovines. On the other hand, in genetically resistant bovines, clotting time is decreased when compared to normal skin of these hosts, possibly because haemostatic mechanisms are already activated in tick-bitten skin.
The finding that blood clots more rapidly in tick-bitten skin than in normal skin of resistant hosts has at least two explanations. Firstly, inflammation can affect haemostasis (Strukova, 2006; Francischetti et al., 2009) and, accordingly, we show that the inflammatory infiltrate at the site of the tick bite in resistant hosts contains significantly more eosinophils and basophils than tick-bitten skin of susceptible hosts (Fig. 3). Our findings are in accordance with those of Schleger et al. (1976) that show that the numbers of eosinophils present in lesions correlate with the degree of resistance of bovines to R. microplus. However, breeds of B. indicus, as well as basophils, were not studied in that work. Eosinophils are important in haemostasis because they express high levels of Tissue Factor (Moosbauer et al., 2007). Immunohistochemistry of skin of patients suffering from bullous pemphigoid, a disease characterized by infiltration of eosinophils, revealed strong reactivity for Tissue Factor, which co-localizes to eosinophils (Marzano et al., 2009). Basophils co-cultured with epithelial cells significantly increase their expression of Tissue Factor (Yamaguchi et al., 2008). In addition, Serum Amyloid A, the levels of which are increased in sera of genetically resistant, but not in susceptible bovine hosts that were exposed to ticks (Carvalho et al. 2008), induces marked expression of Tissue Factor and significantly inhibits expression of Tissue Factor Pathway Inhibitor by endothelial cells (Zhao et al. 2007). Therefore, the inflamed tick-bitten skin of the resistant host may contain a greater amount of Tissue Factor, a known activator of the coagulation cascade.
The second explanation for the finding that blood from resistant hosts clots more rapidly in tick-bitten skin than in normal skin is that the saliva from ticks feeding on resistant hosts may contain less anti-haemostatic molecules. This, in turn, could have two causes. It is possible that specific antibodies from resistant hosts neutralize the tick’s anti-haemostatic proteins. Tick-resistant animals are known to have higher levels of saliva-specific IgG1 and IgG2 (Kashino et al., 2005), supporting our hypothesis for the role of saliva-neutralizing antibodies. On the other hand, the homeostatic responses of resistant hosts could negatively affect the expression of genes encoding anti-haemostatic molecules and, thus, also decrease their availability. We show in the present study that, indeed, salivary glands obtained from ticks fed on resistant hosts present significantly lower levels of transcripts for genes that encode putative anti-haemostatic proteins, whereas ticks fed on susceptible bovine hosts express significantly larger numbers of these transcripts. These transcripts are significantly similar to previously described inhibitors of thrombin described in the Acari protein database (Tanaka et al., 1999; Lai et al., 2004; Macedo-Ribeiro et al. 2008) and of serine proteases (Ribeiro et al., 2006), and to a salivary metalloprotease shown to have fibrin(ogen)lytic activities in the saliva of the tick vector of Lyme disease (Francischetti et al., 2003) and to another from extracts of larvae of R. microplus (Untalan et al., unpublished, gi|71726984).
Salivary glands from females also expressed only small amounts of transcripts for proteins with Kunitz domains, trypsin inhibitors and for metalloproteases (data not shown). However, since the clotting time was increased in adult-infested skin of susceptible once and twice infested bovines, females must be assisted by the abundant anti-haemostatic molecules of the males, a phenomenon similar to what occurs with the inhibition of the antibody response by male ticks (Wang et al., 1998). Salivary glands of male Amblyomma variegatum and Ixodes ricinus contain higher anti-chemokine activity than extracts from female glands (Vančová et al., 2007, in press). If R. microplus males also secrete chemokine-binding molecules they too may assist the female by impairing recruitment of leukocytes to the feeding lesion. The finding that the clotting time of normal skin from bovines of the resistant breed was significantly longer than that of normal skin from bovines of the susceptible breed may be explained by the fact that cattle with high resistance to R. microplus have significantly more arteriovenous anastomoses in their skin than animals of low resistance (Schleger et al., 1981).
In summary, many molecules have been cited as inhibitors of tick-host homeostasis, but few studies correlate how different bovine phenotypes of infestation acts to neutralize the effect of tick saliva (Carvalho et al., 2008; Kashino et al., 2005; Kongsuwan et al., 2008; Bagnall et al., 2009). Our study of local responses to tick bites involving haemostasis and inflammation and our comparative study of gene expression profiles from ticks fed on susceptible or resistant bovine hosts point both to a bona fide role for the actions of tick anti-haemostatic salivary molecules in vivo and provides information to help explain the mechanisms of resistance against ticks seen in this useful bovine model.
Acknowledgments
This work received financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP and Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico – CNPq, and by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases. The authors thank Dr. Ivo M. Francischetti for reading the manuscript and his comments for its improvement and Dr. João S. Silva for generous and continuing support of this work performed in his laboratory. W.A.C., S.R.C.M., G.R.G. and D.D.M. received scholarships from FAPESP and ARRA and A.M.F. received scholarships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES.
References
- Alarcon-Chaidez FJ, Boppana VD, Hagymasi AT, Adler AJ, Wikel SK. A novel sphingomyelinase-like enzyme in Ixodes scapularis tick saliva drives host CD4 T cells to express IL-4. Parasite Immunology. 2009;31:210–219. doi: 10.1111/j.1365-3024.2009.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W. Local alignment statistics. Methods in Enzymology. 1996;266:460–480. doi: 10.1016/s0076-6879(96)66029-7. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall N, Gough J, Cadogan L, Burns B, Kongsuwan K. Expression of intracellular calcium signalling genes in cattle skin during tick infestation. Parasite Immunology. 2009;31:177–187. doi: 10.1111/j.1365-3024.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- Berger RS, Adelstein EH, Anderson PC. Intravascular coagulation: the cause of necrotic arachnidism. Journal of Investigative Dermatology. 1973;61:142–150. doi: 10.1111/1523-1747.ep12676202. [DOI] [PubMed] [Google Scholar]
- Carvalho WA, Bechara GH, More DD, Ferreira BR, da Silva JS, de Miranda Santos IK. Rhipicephalus (Boophilus) microplus: distinct acute phase proteins vary during infestations according to the genetic composition of the bovine hosts, Bos taurus and Bos indicus. Experimental Parasitology. 2008;118:587–591. doi: 10.1016/j.exppara.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Ciprandi A, de Oliveira SK, Masuda A, Horn F, Termignoni C. Boophilus microplus: its saliva contains microphilin, a small thrombin inhibitor. Experimental Parasitology. 2006;114:40–46. doi: 10.1016/j.exppara.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Fernandes-Pedrosa MF, Junqueira-de-Azevedo IL, Goncalves-de-Andrade RM, Kobashi LS, Almeida DD, Ho PL, Tambourgi DV. Transcriptome analysis of Loxosceles laeta (Araneae, Sicariidae) spider venomous gland using expressed sequence tags. BMC Genomics. 2008;9:279. doi: 10.1186/1471-2164-9-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM. Does activation of the blood coagulation cascade have a role in malaria pathogenesis? Trends in Parasitology. 2008b;24:258–263. doi: 10.1016/j.pt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Mather TN, Ribeiro JM. Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis. Biochemical and Biophysical Research Communications. 2003;305:869–875. doi: 10.1016/s0006-291x(03)00857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008a;15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, de Miranda Santos IKF, Ribeiro JMC. The role of saliva in tick feeding. Frontiers in Biosciences. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Manzano A, González-Llaven J, Lemini C, Rubio-Póo C. Standardization of rat blood clotting tests with reagents used for humans. Proceedings of the Western Pharmacology Society. 2001;44:153–155. [PubMed] [Google Scholar]
- Horn F, dos Santos PC, Termignoni C. Boophilus microplus anticoagulant protein: an antithrombin inhibitor isolated from the cattle tick saliva. Archives of Biochemistry and Biophysics. 2000;384:68–73. doi: 10.1006/abbi.2000.2076. [DOI] [PubMed] [Google Scholar]
- Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Research. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S, Da Silva IV, Jr, Sugino M, Ohashi K, Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine. 2005;23:1301–1311. doi: 10.1016/j.vaccine.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Kashino SS, Resende J, Sacco AM, Rocha C, Proenca L, Carvalho WA, Firmino AA, Queiroz R, Benavides M, Gershwin LJ, de Miranda Santos IK. Boophilus microplus: the pattern of bovine immunoglobulin isotype responses to high and low tick infestations. Experimental Parasitology. 2005;110:12–21. doi: 10.1016/j.exppara.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Koh CY, Kazimirova M, Trimnell A, Takac P, Labuda M, Nuttall PA, Kini RM. Variegin, a novel fast and tight binding thrombin inhibitor from the tropical bont tick. Journal of Biological Chemistry. 2007;282:29101–29113. doi: 10.1074/jbc.M705600200. [DOI] [PubMed] [Google Scholar]
- Kongsuwan K, Piper EK, Bagnall NH, Ryan K, Moolhuijzen P, Bellgard M, Lew A, Jackson L, Jonsson NN. Identification of genes involved with tick infestation in Bos taurus and Bos indicus. Developments in Biologicals (Basel) 2008;132:77–88. doi: 10.1159/000317146. [DOI] [PubMed] [Google Scholar]
- Lai R, Takeuchi H, Jonczy J, Rees HH, Turner PC. A thrombin inhibitor from the ixodid tick, Amblyomma hebraeum. Gene. 2004;342:243–249. doi: 10.1016/j.gene.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Letunic I, Goodstadt L, Dickens NJ, Doerks T, Schultz J, Mott R, Ciccarelli F, Copley RR, Ponting CP, Bork P. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Research. 2002;30:242–244. doi: 10.1093/nar/30.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo-Ribeiro S, Almeida C, Calisto BM, Friedrich T, Mentele R, Sturzebecher J, Fuentes-Prior P, Pereira PJ. Isolation, cloning and structural characterisation of boophilin, a multifunctional Kunitz-type proteinase inhibitor from the cattle tick. PLoS ONE. 2008;3:e1624. doi: 10.1371/journal.pone.0001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Neitz AW. Adaptation of ticks to a blood-feeding environment: evolution from a functional perspective. Insect Biochemistry and Molecular Biology. 2004;34:1–17. doi: 10.1016/j.ibmb.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Gaspar AR, Louw AI, Neitz AW. Apyrase activity and platelet aggregation inhibitors in the tick Ornithodoros savignyi (Acari: Argasidae) Experimental and Applied Acarology. 1998;22:353–366. doi: 10.1023/a:1024517209621. [DOI] [PubMed] [Google Scholar]
- Marzano AV, Tedeschi A, Fanoni D, Bonanni E, Venegoni L, Berti E, Cugno M. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. British Journal of Dermatology. 2009;160:266–272. doi: 10.1111/j.1365-2133.2008.08880.x. [DOI] [PubMed] [Google Scholar]
- Mattioli RC, Cassama M. Comparison of characteristics of life cycle in female ticks collected on N’Dama and zebu cattle. Tropical Animal Health and Production. 1995;27:150–154. doi: 10.1007/BF02248959. [DOI] [PubMed] [Google Scholar]
- Mattioli RC, Pandey VS, Murray M, Fitzpatrick JL. Immunogenetic influences on tick resistance in African cattle with particular reference to trypanotolerant N’Dama (Bos taurus) and trypanosusceptible Gobra zebu (Bos indicus) cattle. Acta Tropica. 2000;75:263–277. doi: 10.1016/s0001-706x(00)00063-2. [DOI] [PubMed] [Google Scholar]
- McGlasson DL, Harroff HH, Sutton J, Dick E, Elston DM. Cutaneous and systemic effects of varying doses of brown recluse spider venom in a rabbit model. Clinical Laboratory Science. 2007;20:99–105. [PubMed] [Google Scholar]
- Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, Lohse P, Patel KD, Engelmann B. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995–1002. doi: 10.1182/blood-2006-02-004945. [DOI] [PubMed] [Google Scholar]
- Nakajima C, Imamura S, Konnai S, Yamada S, Nishikado H, Ohashi K, Onuma M. A novel gene encoding a thrombin inhibitory protein in a cDNA library from Haemaphysalis longicornis salivary gland. Journal of Veterinary Medical Science. 2006;68:447–452. doi: 10.1292/jvms.68.447. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Sukumaran B, Bozdogan U, Thomas V, Liang X, DePonte K, Marcantonio N, Koski RA, Anderson JF, Kantor F, Fikrig E. A tick antioxidant facilitates the Lyme disease agent's successful migration from the mammalian host to the arthropod vector. Cell Host & Microbe. 2007;2:7–18. doi: 10.1016/j.chom.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth RA, Tomaz LS, Ortiz-Costa S, Atella GC, Ribeiro JMC, Francischetti IM, Monteiro RQ. Antithrombotic properties of Ixolaris, a potent inhibitor of the extrinsic pathway of the coagulation cascade. Thrombosis and Haemostasis. 2006;96:7–13. doi: 10.1160/TH06-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper MP, Waxman L, Smith DE, Schulman CA, Sardana M, Ellis RW, Schaffer LW, Siegl PK, Vlasuk GP. Characterization of recombinant tick anticoagulant peptide. A highly selective inhibitor of blood coagulation factor Xa. Journal of Biological Chemistry. 1990;265:17746–17752. [PubMed] [Google Scholar]
- Nienaber J, Gaspar AR, Neitz AW. Savignin, a potent thrombin inhibitor isolated from the salivary glands of the tick Ornithodoros savignyi (Acari: Argasidae) Experimental Parasitology. 1999;93:82–91. doi: 10.1006/expr.1999.4448. [DOI] [PubMed] [Google Scholar]
- Peterson ME. Brown spider envenomation. Clinical Techniques in Small Animal Practice. 2006;21:191–193. doi: 10.1053/j.ctsap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Pichotka J, Reichel H. Blood-clotting time in rabbits and its variations determined with a simple capillary method. American Journal of Physiology. 1950;162:632–638. doi: 10.1152/ajplegacy.1950.162.3.632. [DOI] [PubMed] [Google Scholar]
- Reck J, Jr, Berger M, Terra RM, Marks FS, da Silva VI, Jr, Guimaraes JA, Termignoni C. Systemic alterations of bovine hemostasis due to Rhipicephalus (Boophilus) microplus infestation. Research in Veterinary Science. 2008;86:56–62. doi: 10.1016/j.rvsc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM. How ticks make a living. Parasitology Today. 1995;11:91–93. doi: 10.1016/0169-4758(95)80162-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annual Review of Entomology. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochemical and Molecular Biology. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Schleger AV, Lincoln DT, McKenna RV, Kemp DH, Roberts JA. Boophilus microplus: cellular responses to larval attachment and their relationship to host resistance. Australian Journal of Biological Sciences. 1976;29:499–512. doi: 10.1071/bi9760499. [DOI] [PubMed] [Google Scholar]
- Schleger AV, Lincoln DT, Bourne AS. Arteriovenous anastomoses in the dermal vasculature of the skin of Bos taurus cattle, and their relationship with resistance to the tick, Boophilus microplus. Australian Journal of Biological Sciences. 1981;34:27–35. [PubMed] [Google Scholar]
- Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Frontiers in Biosciences. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- Tanaka AS, Andreotti R, Gomes A, Torquato RJ, Sampaio MU, Sampaio CA. A double headed serine proteinase inhibitor – human plasma kallikrein and elastase inhibitor – from Boophilus microplus larvae. Immunopharmacology. 1999;45:171–177. doi: 10.1016/s0162-3109(99)00074-0. [DOI] [PubMed] [Google Scholar]
- Toh CH, Hoots WK. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. Journal of Thrombosis and Haemostasis. 2007;5:604–606. doi: 10.1111/j.1538-7836.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Mather TN, Ribeiro JM. Exploring the sialome of the tick Ixodes scapularis. Journal of Experimental Biology. 2002;205:2843–2864. doi: 10.1242/jeb.205.18.2843. [DOI] [PubMed] [Google Scholar]
- van den Berg CW, Goncalves-de-Andrade RM, Magnoli FC, Tambourgi DV. Loxosceles spider venom induces the release of thrombomodulin and endothelial protein C receptor: implications for the pathogenesis of intravascular coagulation as observed in loxoscelism. Journal of Thrombosis and Haemostasis. 2007;5:989–995. doi: 10.1111/j.1538-7836.2007.02382.x. [DOI] [PubMed] [Google Scholar]
- Vančová I, Slovák M, Hajnická V, Labuda M, Simo L, Peterková K, Hails RS, Nuttall PA. Differential anti-chemokine activity of Amblyomma variegatum adult ticks during blood-feeding. Parasite Immunology. 2007;29:169–177. doi: 10.1111/j.1365-3024.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- Vančová I, Hajnická V, Slovák M, Nuttall PA. Anti-chemokine activities of ixodid ticks depend on tick species, developmental stage, and duration of feeding. Veterinary Parasitology. doi: 10.1016/j.vetpar.2009.09.029. in press. [DOI] [PubMed] [Google Scholar]
- Wambura PN, Gwakisa PS, Silayo RS, Rugaimukamu EA. Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus. Veterinary Parasitology. 1998;77:63–70. doi: 10.1016/s0304-4017(97)00229-x. [DOI] [PubMed] [Google Scholar]
- Wang H, Paesen GC, Nuttall PA, Barbour AG. Male ticks help their mates to feed. Nature. 1998;391:753–754. doi: 10.1038/35773. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Gabazza EC, Takei Y, Yano Y, Fujimoto H, D’Alessandro-Gabazza CN, Murakami E, Kobayashi T, Takagi T, Maruyama J, Suzuki K, Taguchi O. Role of thrombin in interleukin-5 expression from basophils. Biochemical and Biophysical Research Communications. 2008;368:116–120. doi: 10.1016/j.bbrc.2008.01.048. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhou S, Heng CK. Impact of serum amyloid A on tissue factor and tissue factor pathway inhibitor expression and activity in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:1645–1650. doi: 10.1161/ATVBAHA.106.137455. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bowman AS, Brigham DL, Essenberg RC, Dillwith JW, Sauer JR. Isolation and characterization of americanin, a specific inhibitor of thrombin, from the salivary glands of the lone star tick Amblyomma americanum (L.) Experimental Parasitology. 1997;87:30–38. doi: 10.1006/expr.1997.4175. [DOI] [PubMed] [Google Scholar]