Abstract
Objectives
The aim is to demonstrate significant polymerization shrinkage stress reduction in model resins through incorporation of addition-fragmentation chain-transfer moieties that promote network stress accommodation by molecular rearrangement. Monomers containing allyl sulfide linkages are incorporated to affect the shrinkage stress that arises during photopolymerization of model resins that contain an initiator and dimethacrylates. Radical-mediated allyl sulfide addition-fragmentation is enabled during polymerization. We hypothesize that allyl sulfide incorporation into methacrylate polymerizations promotes stress relaxation by enabling network adaptation.
Methods
A 1:2 mixture of tetrathiol and allyl sulfide-containing divinyl ethers is formulated with glass-forming dimethacrylates and compared to controls where the allyl sulfide is replaced with a propyl sulfide that is incapable of undergoing addition-fragmentation. Simultaneous shrinkage stress and functional group conversion measurements are performed. The Tg is determined by DMA.
Results
Increasing allyl sulfide concentration reduces the relative stress by up to 75% in the resins containing the maximum amount of allyl sulfide. In glassy systems, at much lower allyl sulfide concentrations, the stress is reduced by up to 20% as compared to propyl sulfide-containing systems incapable of undergoing addition-fragmentation chain transfer.
Significance
Shrinkage stress reduction, typically accompanying free-radical polymerization, is a primary focus in dental materials research and new product development. Allyl sulfide addition-fragmentation chain transfer is utilized as a novel approach to reduce stress in ternary thiol-ene-methacrylate polymerizations. The stress reduction effect depends directly on the allyl sulfide concentration in the given ternary systems, with stress reduction observed even in systems possessing super-ambient Tgs and low allyl sulfide concentrations.
Keywords: shrinkage stress, stress relaxation, polymer networks, allyl sulfide, addition-fragmentation chain transfer, photopolymerization
INTRODUCTION
Polymer-based dental restorative composites possess several advantageous properties such as rapid reaction, high strength, and durability. These composites are considered a favorable alternative to the more traditional amalgams as the clinical use of mercury is avoided. Moreover, the matching of aesthetically pleasing natural tooth colors is readily accomplished using polymer composite materials. Nevertheless, the shrinkage stress that accompanies the polymerization of these composite materials is a primary concern for dental clinicians since it can initiate microcracking of restorative materials, leading to bacterial microleakage and secondary caries [1]. While dimethacrylate-based composites are the most common material used as dental restoratives, they are accompanied by substantial polymerization-induced shrinkage stress [2–3].
Traditional dimethacrylate-based resins undergo a chain-growth polymerization mechanism, typically gelling at low conversion before vitrifying at higher conversions and thus developing shrinkage stress early on and throughout the polymerization [4]. Conversely, molecular weights and polymer structure build geometrically in a step-growth polymerization, leading to delayed gelation and reduced polymerization shrinkage stress. Thiol-ene photopolymerizations follow a step-growth mechanism and are proposed to reduce polymerization-induced shrinkage stress in dental materials [5–6]. Although organic thiols are frequently malodorous, high molecular weight thiols such as pentaerythritol tetra(3-mercaptopropionate) (PETMP) exhibit very low volatility, alleviating odor concerns. Additionally, thiol-ene resins have been examined in multiple studies as dental restorative materials since they exhibit rapid photopolymerization, necessary for clinical applicability. However, thiol-ene networks typically exhibit relatively low moduli and glass transition temperatures (Tg) when compared with methacrylate–based systems. Ternary mixtures of thiol-ene and methacrylate–based monomers yield a synergistic combination of attributes, blending enhanced mechanical properties with lowered shrinkage stress [7–8].
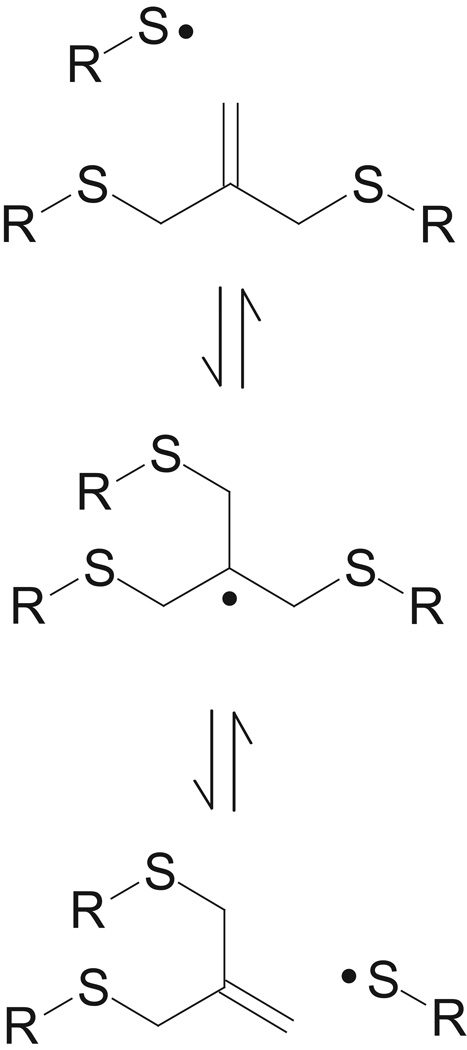
Recently, a novel mechanism for stress relaxation in a polymer network has been shown via photo-induced bond rearrangement of a polymeric network [9–11], i.e., the formation of a “covalent adaptable network” (CAN). The mechanism for this stress relaxation utilizes radical-mediated addition-fragmentation chain transfer events through allyl sulfide functionalities incorporated within the crosslinking strands of the polymer network, allowing crosslinking strand rearrangement in the presence of radicals (Figure 1). The addition-fragmentation reaction preserves the concentration of both the allyl sulfide and radical reactants, which subsequently participate in further chain transfer events [9, 11]. This cascading rearrangement of the network connectivity effects a global reduction in stress without any concomitant degradation of mechanical properties. When applied to thiol-ene networks, the allyl sulfide addition-fragmentation reduced shrinkage stress relative to an analogous network incapable of addition-fragmentation [9] ; however, the low Tg of that material (~ −20°C) precludes it from use as a dental restorative material. Here, we examine the effect of incorporating a dimethacrylate monomer into an allyl sulfide–containing thiol-ene system and determine its effects on the thermomechanical properties and polymerization-induced shrinkage stress. The effects of allyl sulfide concentration and polymer mobility are evaluated to ascertain under which conditions the addition-fragmentation mechanism is capable of reducing stress in model resins.
Figure 1.
Schematic of allyl sulfide addition-fragmentation chain transfer mechanism that promotes network relaxation and polymerization shrinkage stress.
MATERIALS & METHODS
Materials
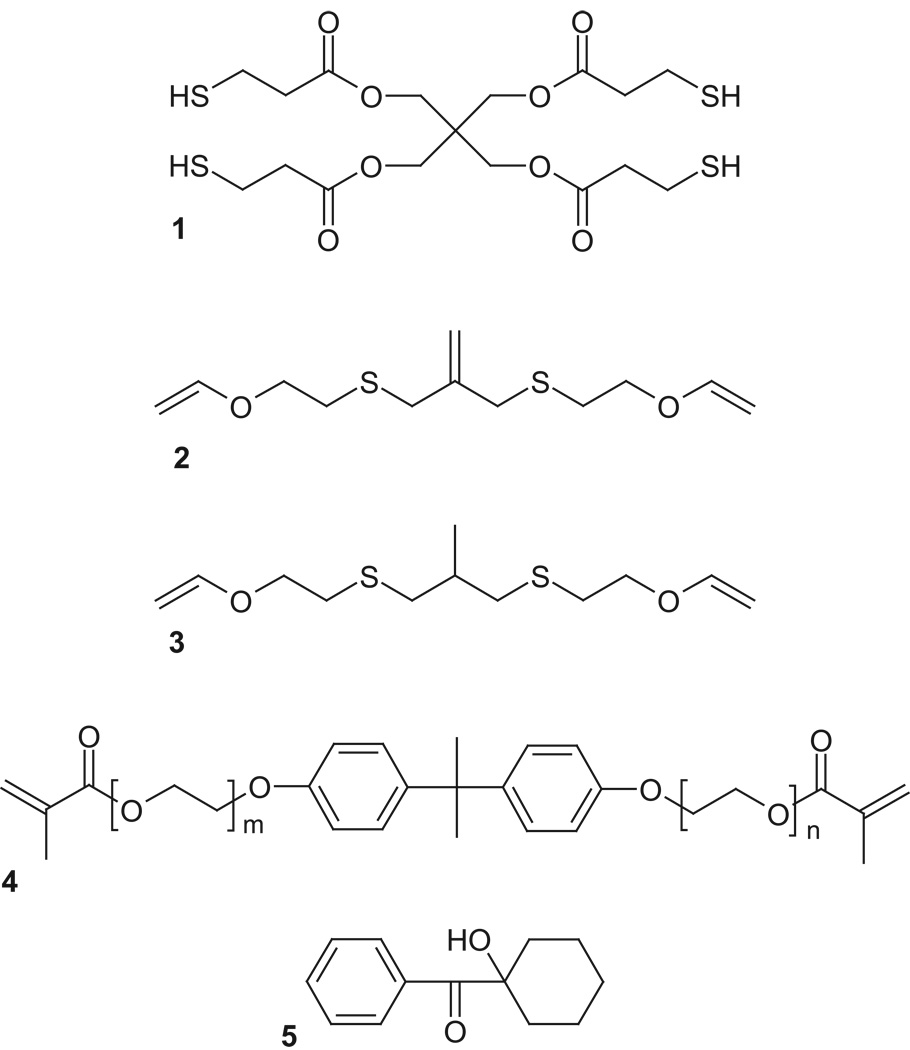
The monomers and photo-initiator used in this study is shown in Figure 2. Resins were comprised of a stoichiometric mixture of 2-methylene-propane-1,3-di(thioethyl vinyl ether) (MDTVE) and pentaerythritol tetrakis(3-mercaptopropionate) (PETMP, Evans Chemetics), which was formulated with ethoxylated bisphenol A dimethacrylate (EBPADMA, Esstech) in various ratios. 2-methyl-propane-1,3-di(thioethyl vinyl ether) (MeDTVE), a MDTVE analogue that replaces the allyl sulfide with a propyl sulfide moiety that is incapable of stress relaxation, was employed as a molecular control to isolate the effect of the allyl sulfide functionality on the stress evolution during polymerization. These monomers, with nearly identical molecular weight and structure, attempt to vary only the shrinkage stress while leaving all other properties of the resin the same. 1-Hydroxycyclohexylphenylketone (HCPK, Ciba Specialty Chemicals), an ultraviolet (UV)-active photoinitiator, was utilized at 1 wt%. Although visible light irradiation, in conjunction with camporquinone as photoinitiator, is the prevalent clinical approach to curing dental materials, HCPK is used in the current study as a model radical generator as it possesses low molar absorptivity. This approach ensures uniform irradiation intensity throughout the sample thickness and eliminates spurious effects owing to uneven cure rates. Although not the focus of this study, a potential advantage to UV irradiation is the prospect of initiatorless photopolymerization as achievable in thiol-ene based systems [12]. Since the goal of these studies is the demonstration of shrinkage stress behavior in model compounds rather than clinically relevant systems, the use of this combination of initiator and UV light is appropriate as it enables a more accurate assessment of the effects of allyl sulfide concentration on the stress development. MDTVE and MeDTVE were synthesized from 2-chloroethyl vinyl ether with 3-mercapto-2-(mercaptomethyl)-1-propene and 1,3-dimercapto-2-methylpropane, respectively, according to a method described in the literature [9].
Figure 2.
Materials used : (1) PETMP (2) MDTVE (3) MeDTVE (4) EBPADMA (5) HCPK
Methods
Simultaneous shrinkage stress and functional group conversion measurements were performed during polymerization using a cantilever-type tensometer (Paffenbarger Research Center, American Dental Association Health Foundation) coupled with a Nicolet 670 FTIR spectrometer via near-infrared transmitting optical fiber patch cables; a detailed description of this measurement technique is presented in the literature [6, 13].The infrared spectrometer was equipped with an indium gallium arsenide (InGaAs) detector and collected spectra at a rate of approximately 3 scans/sec and a resolution of 2 cm−1. Samples 6 mm in diameter and 1 mm thick were irradiated in situ for 20 minutes at an intensity of 3 mW/cm2 by a mercury arc lamp (Acticure, EXFO) equipped with an interference filter isolating the 365 nm Hg spectral line. The evolution of the methacrylate, vinyl ether and allyl sulfide double bond concentrations were determined by monitoring the infrared absorption peaks centered at 6166 cm−1 (C=C-H stretching, overtone), 6189 cm−1 (C=C-H stretching, overtone), and 6121 cm−1 (C=C-H stretching, overtone), respectively. As the methacrylate peak is overlapped with the vinyl ether and allyl sulfide peaks, Gaussian fitting was used to deconvolute the peak areas. This deconvolution technique was validated by comparing known model resin compositions with the spectrum deconvolution output and the error associated with deconvolution based on this validation was determined to be less than 4% conversion.
Dynamic mechanical analysis (DMA) was performed using a TA Instruments Q800 DMA to determine the elastic moduli (E′) and glass transition temperatures of polymerized samples. Samples were prepared by sandwiching the material between two glass slides separated by a 1 mm thick spacer and irradiating under condition identical to those employed in the tensometer. Experiments were performed at a strain and frequency of 0.1% and 1 Hz, respectively, scanning the temperature from −50°C to 140°C twice at 1°C/minute; the temperature scan was repeated to ensure the absence of dark polymerization at temperatures greater than the glass transition temperature (Tg). The Tg was assigned as the temperature at tan δ maximum of the second scan [14]. Analysis of variance (ANOVA), using a 95% confidence interval, was conducted to determine the significance in the differences between mean values.
RESULTS
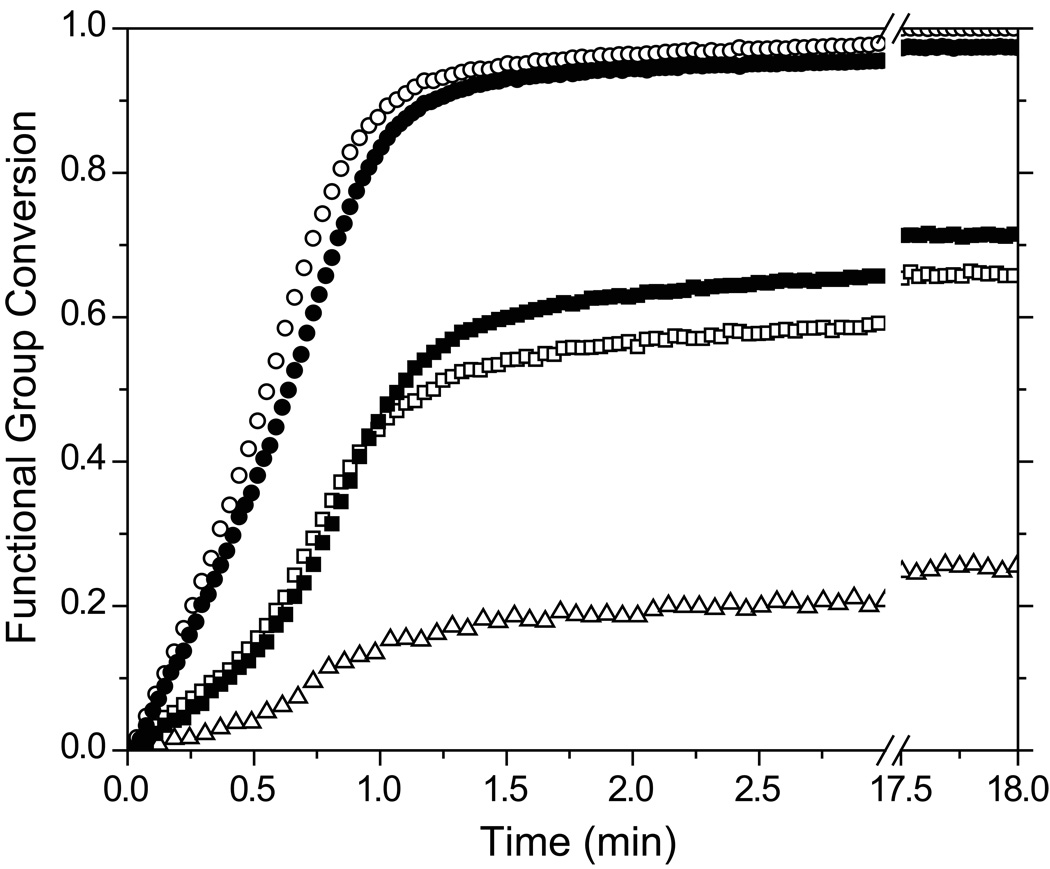
Formulations of PETMP-MDTVE-EBPADMA (allyl sulfide containing) and PETMP-MeDTVE-EBPADMA (identical but replacing the allyl sulfide with the propyl sulfide that is incapable of addition-fragmentation) were created and evaluated to ascertain differences in the polymerization kinetics, polymer network structure, and polymerization-induced shrinkage stress associated with the presence of the allyl sulfide functionalities that enable polymer network adaptation and the hypothesized stress reduction. Photopolymerization kinetics as measured by changes in the functional group conversions during the photopolymerization of PETMP-MDTVE-EBPADMA and PETMP-MeDTVE-EBPADMA ternary systems are shown in Figure 3. The ternary monomer systems are formulated with a 3:3:4 thiol:vinyl ether:methacrylate stoichiometric ratio for the results presented in Figure 3. While the methacrylate conversions of both systems develop similarly, both the polymerization rate and the final conversion of the vinyl ether are reduced in the MDTVE-based ternary system when compared with the equivalent MeDTVE-based system. The allyl sulfide moiety exhibited only 26% conversion, the lowest of the reactive functionalities.
Figure 3.
Methacrylate (circle), vinyl ether (square), and allyl sulfide (triangle) conversion versus time for allyl sulfide (open symbols) and propyl sulfide (closed symbols) containing materials in a 3:3:4 thiol:vinyl ether:methacrylate mol ratio. Samples contain 1 wt% HCPK, irradiated at 3 mW/cm2 using 365 nm light.
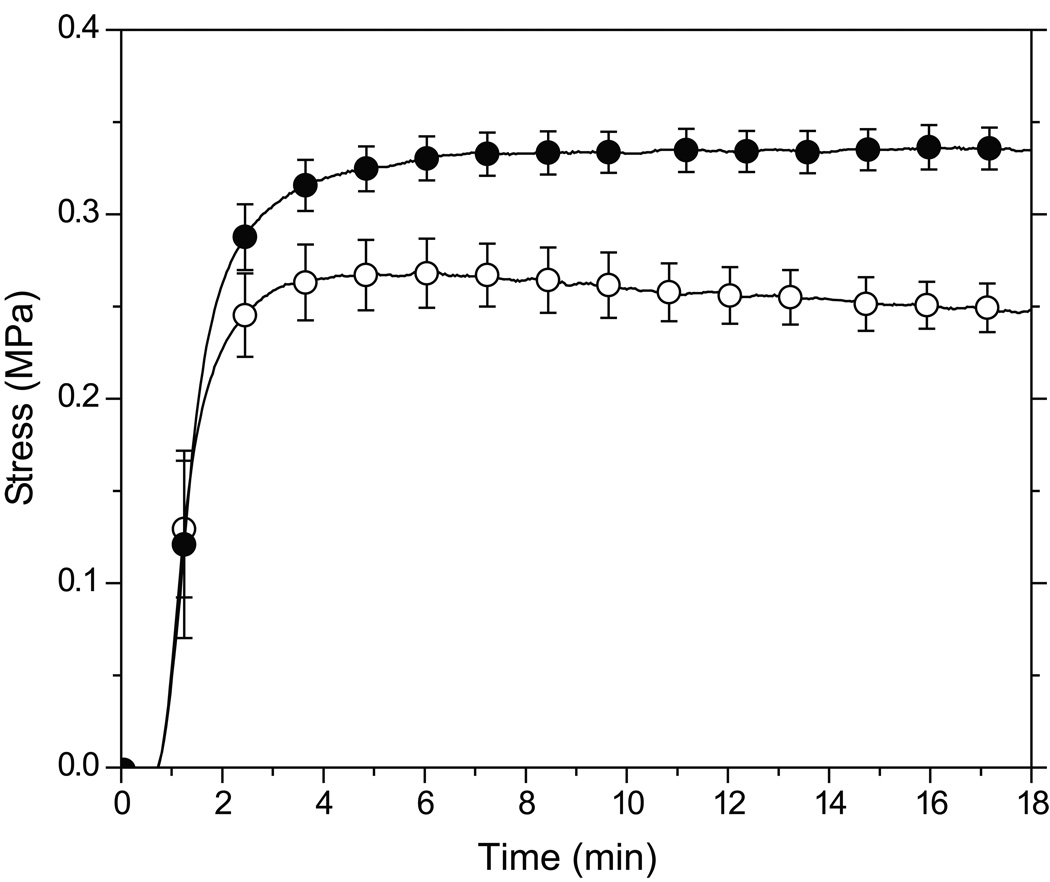
A comparison of the stress evolution during polymerization of allyl and propyl sulfide-containing resins with 3:3:2 thiol:vinyl ether:methacrylate stoichiometric ratios is shown in Figure 4. The allyl sulfide-containing ternary system exhibits lower final shrinkage stress than in the analogous propyl sulfide-containing system. Moreover, stress relaxation is observed in the latter stages of polymerization of the allyl sulfide-containing system.
Figure 4.
Polymerization shrinkage stress versus time of a 3:3:2 stoichiometric mixture of thiol:vinyl ether:methacrylate (PETMP-MDTVE-EBPADMA (○) and PETMP-MeDTVE- EBPADMA (●)). Samples contain 1 wt% HCPK, irradiated at 3 mW/cm2 using 365 nm light.
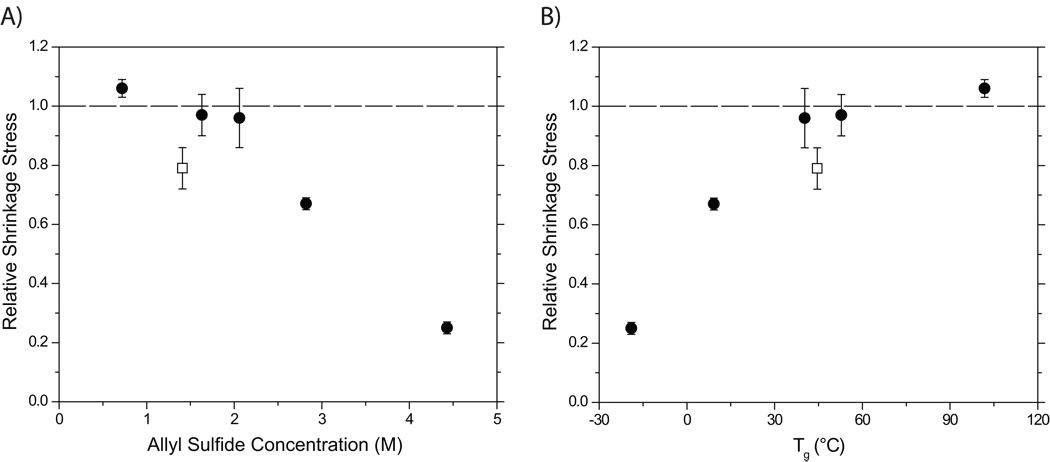
The observed final stresses for both the allyl and propyl sulfide-containing materials were non-dimensionalized via division by the modulus in the linear rubbery regime, which is directly related to the crosslink density through rubbery elasticity theory [15]. Here, we denote the ratio of dimensionless stresses in allyl to propyl sulfide-containing materials as the relative shrinkage stress (Figure 5). This approach further minimizes the difference in crosslink density between the allyl sulfide-containing networks and their propyl sulfide-containing analogues. The relative shrinkage stress decreases with increasing allyl sulfide concentration (Figure 5A) and decreasing Tg of the material (Figure 5B). The two resins containing the highest allyl sulfide concentrations demonstrated reduced shrinkage stress relative to the propyl sulfide-containing analogues at the 95% confidence interval. A ternary resin consisting of an off-stoichiometric thiol-ene mixture with an excess of thiol functional groups relative to the vinyl ether (2:1:2 thiol:vinyl ether:methacrylate) also exhibited a relative shrinkage stress less than one.
Figure 5.
(A) Relative polymerization shrinkage stress versus allyl sulfide concentration and (B) versus glass transition temperature. The experiments were conducted using stoichiometric thiol-ene mixture (●) and off-stoichiometric 2:1 thiol-ene mixture (□). Samples contain 1 wt% HCPK, irradiated at 3 mW/cm2 using 365 nm light.
The glass transition temperature, E′/RT (proportional to the crosslink density, as discussed later, R: gas constant and T: temperature at Tg+30°C), and E′ (at 25°C) all increase with increasing methacrylate and decreasing allyl sulfide proportion (Table 1). Additionally, while increasing the methacrylate molar ratio (from 3:3:2 to 1:1:6 – thiol:vinyl ether:methacrylate) yields only a slight decrease in the final methacrylate conversion (from approximately 100 to 84%), significant variation is observed in the final vinyl ether and allyl sulfide conversions (from approximately 99 to 54% and 8 to 62%, respectively).
Table 1.
Summary of final conversion, Tg, E′/RT, and elastic modulus.
| Vinyl ether monomer used in the ternary system (thiol:vinyl ether:methacrylate ratio) |
Allyl sulfide concentration (mol/L) |
Final functional group conversion [%] | Tg (°C) | E′/RT at T = Tg + 30°C (Mol/m3) |
Elastic modulus at T = 25°C (MPa) |
||
|---|---|---|---|---|---|---|---|
| Methacrylate | Vinyl ether | Allyl sulfide | |||||
| MDTVE (1:1:0) | 4.43 | - | 96 | 8 | −20.0 ± 0.8 | 5040 ± 40 | 12.5 ± 0.1 |
| MeDTVE (1:1:0) | - | - | 99 | - | −28.0 ± 0.2 | 4440 ± 40 | 11.0 ± 0.2 |
| MDTVE (3:3:2) | 2.80 | 100 | 85 | 14 | 9.2 ± 0.9 | 6500 ± 700 | 20 ± 2 |
| MeDTVE (3:3:2) | - | 98 | 88 | - | 5 ± 0 | 6100 ± 400 | 17 ± 1 |
| MDTVE (3:3:4) | 2.10 | 100 | 67 | 26 | 40 ± 2 | 9200 ± 700 | 460 ± 50 |
| MeDTVE (3:3:4) | - | 98 | 72 | - | 43.8 ± 0.6 | 9800 ± 100 | 540 ± 90 |
| MDTVE (1:1:2) | 1.60 | 99 | 62 | 33 | 52.8 ± 0.6 | 9700 ± 700 | 1040 ± 10 |
| MeDTVE (1:1:2) | - | 98 | 61 | - | 55 ± 1 | 10100 ± 700 | 1200 ± 100 |
| MDTVE (1:1:6) | 0.72 | 84 | 56 | 62 | 102 ± 2 | 17600 ± 900 | 2200 ± 200 |
| MeDTVE (1:1:6) | - | 87 | 54 | - | 108 ± 2 | 20000 ± 1000 | 2100 ± 100 |
| MDTVE (2:1:2) | 1.40 | 98 | 88 | 41 | 44.6 ± 0.3 | 7800 ± 300 | 1000 ± 100 |
| MeDTVE (2:1:2) | - | 97 | 95 | - | 39 ± 1 | 6880 ± 70 | 470 ± 20 |
DISCUSSION
Carbon-carbon double bond conversion profiles for analogous allyl and propyl sulfide-containing ternary systems (3:3:4 – thiol:vinyl ether:methacrylate) are shown in Figure 3. Two conversion regimes are apparent during the polymerization as indicated by the inflection point in the vinyl ether conversion profile occurring after approximately 40 seconds irradiation. The first regime is dominated by methacrylate homopolymerization as well as chain transfer to thiol, whereas after the inflection point the rate of vinyl ether incorporation into the polymer network, relative to the methacrylate, progressively increases. Similar trends have been observed previously in thiol-allyl-methacrylate ternary systems and have been attributed to the propensity of the methacrylate to homopolymerize, rather than copolymerize with other electron rich carbon-carbon double bonds such as the vinyl ether [7–8].
While both the vinyl ethers and allyl sulfides constitute enes that can engage in the overall thiol-ene polymerization process, the radical addition to the allyl sulfide, unlike the vinyl ether, regenerates both the allyl sulfide and thiyl radical species [11, 16]; thus, the allyl sulfide consumption during irradiation (Figure 3) indicates side reactions by hydrogen abstraction from thiol and/or copolymerization with methacrylate, leading to additional crosslinking [9]. A previous study has shown negligible copolymerization between the vinyl ether and allyl sulfide and is therefore discounted as a potential side reaction in the present work [9]. As the polymerization regime transitions towards thiol-ene incorporation, loss of the allyl sulfide is compounded by hydrogen abstraction. The effect of reduced thiol concentration through this side reaction is observed in the lower final vinyl ether conversion of the allyl sulfide-containing resin as compared to that of the propyl sulfide-containing resin (Figure 3). As the proportion of methacrylate is increased in the system, the allyl sulfide consumption is correspondingly raised, somewhat reducing the capacity for stress relaxation via the addition-fragmentation pathway (Table 1); however, this does not preclude the allyl sulfide from undergoing multiple addition-fragmentation chain transfer events prior to irreversible consumption.
Glassy polymer networks have been shown to be well suited as excellent dental restorative materials as they are capable of withstanding intraoral mechanical stresses. The Tg of a polymer network is dependent on its crosslink density and network strand flexibility [17–18]. According to the theory of rubbery elasticity, the crosslink density is proportional to E′/RT and increases as the proportion of methacrylate is raised (Table 1). Moreover, the bisphenol-A core of EBPADMA is a particularly inflexible functionality [19], leading to an increase in the overall chain stiffness. Thus, the increased Tg associated with higher methacrylate proportion is attributed to a combination of these two effects.
Polymerization shrinkage stress is the superposition of that stored by network formation and dissipated by stress relaxation. Typically, stress relaxation is achieved via the viscous flow of low molecular weight species in the early stages of polymerization. Incorporation of the allyl sulfide functionality provides an alternative avenue for stress relaxation by network rearrangement, both throughout and after the polymerization, as observed in the reduced polymerization shrinkage stress developed by the allyl sulfide-containing material versus the propyl sulfide analogue (Figure 4). The stress relaxation associated with the allyl sulfide incorporation into methacrylate-based polymerizations conclusively demonstrates the capability of addition-fragmentation chain transfer to reduce stress build up during polymerizations of multimethacrylates. Thus, this novel mechanism is actively occurring during conventional chain growth free-radical polymerizations.
The allyl sulfide-containing material demonstrates a maximum in the stress evolution and, at extended irradiation, undergoes stress relaxation whereas the propyl sulfide-containing material does not (Figure 4). The reduction in molecular mobility, associated with vitrification in highly crosslinked systems, effectively traps radical species [20] and increases their lifetime on the order of several months [21–22]. These species are able to react over extended time periods, suggesting the potential for further stress relaxation via addition-fragmentation chain transfer in allyl sulfide-containing systems subsequent to the polymerization reaction.
Polymerization shrinkage-induced stress is a consequence of the polymer network crosslinking, as the ultimate magnitude of the stress is related, in part, to the crosslink density. Thus, the relative shrinkage stress, which accounts for crosslink density via division by the elastic modulus, was used as the metric for comparison to compensate for the relatively small differences in the network crosslink density. The decrease in relative shrinkage stress as the allyl sulfide concentration in the active network is increased is attributed to a higher probability of addition-fragmentation chain transfer events (Figure 5A). As the polymerizations are carried out under ambient conditions, vitrification occurs at lower conversions for high methacrylate proportions. The rate of stress relaxation during the polymerization owing to addition-fragmentation in the vitrified regime appears slow, likely associated with the reduced molecular mobility; however, the ternary mixture with an off stoichiometric ratio of 2:1 thiol:vinyl ether (open square symbol in Figure 5) has a glass transition temperature greater than the cure temperature and exhibits more than 20% reduction in the relative stress during the polymerization. This behavior suggests that, despite vitrification, reduced polymerization stress is achievable and that the increased thiol promotes a relatively greater number of addition-fragmentation chain transfer events and ultimately a faster rate of network relaxation. Based upon these findings, we speculate that increasing the allyl sulfide content while increasing or maintaining the methacrylate concentration would produce a material exhibiting both low polymerization stress accompanied by a further increased in Tg. One approach to achieve this outcome would be to incorporate the allyl sulfide moiety directly into a methacrylate-functionalized monomer. Alternatively, one could synthesize allyl sulfide-based monomers capable of producing a similar crosslink density as methacrylate-based materials. For example, one could synthesize an alkyne-functionalized allyl sulfide monomer to be used in thiol-yne polymerizations [23].
CONCLUSIONS
In conclusion, allyl sulfide addition-fragmentation chain transfer reduces the final stress in ternary thiol-ene-methacrylate polymerizations by as much as 75% at high allyl sulfide concentrations. Although this effect is mitigated by lower allyl sulfide concentration as well as decreased mobility in the ternary systems presented here, stress reduction is observed in systems possessing super-ambient Tgs. This behavior indicates that the utilization of the allyl sulfide functionality and network adaptation in dental materials represents a new paradigm for polymerization stress reduction concurrent with and subsequent to the polymerization in these materials. Great opportunities exist for creating dental resins that simultaneously incorporate higher allyl sulfide concentrations into resins that achieve appropriate mechanical and thermal properties.
ACKNOWLEDGMENTS
This investigation was supported by NIDCR 2 R01 DE-010959-11 from the National Institute of Health.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burke FJT, Cheung SW, Mjor IA, Wilson NHF. Restoration longevity and analysis of reasons for the placement and replacement of restorations provided by vocational dental practitioners and their trainers in the United Kingdom. Quintessence International. 1999;30:234–242. [PubMed] [Google Scholar]
- 2.Labella R, Lambrechts P, Van Meerbeek B, Vanherle G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dental Materials. 1999;15:128–137. doi: 10.1016/s0109-5641(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 3.Walls AWG, McCabe JF, Murray JJ. The polymerization contraction of visible-light activated composite resin. Journal of Dentistry. 1988;16:177–181. doi: 10.1016/0300-5712(88)90032-2. [DOI] [PubMed] [Google Scholar]
- 4.Bowman CN, Kloxin CJ. Toward an enhanced understanding and implementation of photopolymerization reactions. AIChE Journal. 2008;54:2775–2795. [Google Scholar]
- 5.Carioscia JA, Lu H, Stanbury JW, Bowman CN. Thiol-ene oligomers as dental restorative materials. Dental Materials. 2005;21:1137–1143. doi: 10.1016/j.dental.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dental Materials. 2005;21:1129–1136. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Lee TY, Carioscia J, Smith Z, Bowman CN. Thiol-allyl ether-methacrylate ternary systems. Evolution mechanism of polymerization-induced shrinkage stress and mechanical properties. Macromolecules. 2007;40:1473–1479. [Google Scholar]
- 8.Lee TY, Smith Z, Reddy SK, Cramer NB, Bowman CN. Thiol-allyl ether-methacrylate ternary systems. Polymerization mechanism. Macromolecules. 2007;40:1466–1472. [Google Scholar]
- 9.Kloxin CJ, Scott TF, Bowman CN. Stress Relaxation via Addition-Fragmentation Chain Transfer in a Thiol-ene Photopolymerization. Macromolecules. 2009;42:2551–2556. doi: 10.1021/ma802771b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott TF, Draughon RB, Bowman CN. Actuation in crosslinked polymers via photoinduced stress relaxation. Advanced Materials. 2006;18:2128. [Google Scholar]
- 11.Scott TF, Schneider AD, Cook WD, Bowman CN. Photoinduced plasticity in cross-linked polymers. Science. 2005;308:1615–1617. doi: 10.1126/science.1110505. [DOI] [PubMed] [Google Scholar]
- 12.Cramer NB, Reddy SK, Cole M, Hoyle C, Bowman CN. Initiation and kinetics of thiol-ene photopolymerizations without photoinitiators. J Polym Sci Pol Chem. 2004;42:5817–5826. [Google Scholar]
- 13.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin-composites: I. Shrinkage stress characterization technique. Journal of Materials Science-Materials in Medicine. 2004;15:1097–1103. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Lee-Sullivan P, Thring RW. Determination of activation energy for glass transition of an epoxy adhesive using dynamic mechanical analysis. Journal of Thermal Analysis and Calorimetry. 2000;60:377–390. [Google Scholar]
- 15.Flory PJ. Principles of Polymer Chemistry. 1953 [Google Scholar]
- 16.Meijs GF, Rizzardo E, Thang SH. Preparation of Controlled Molecular-Weight, Olefin-Terminated Polymers by Free-Radical Methods - Chain Transfer Using Allylic Sulfides. Macromolecules. 1988;21:3122–3124. [Google Scholar]
- 17.Ueberreiter K, Kanig G. 2nd-Order Transitions and Mesh Distribution Function of Cross-Linked Polystyrenes. J Chem Phys. 1950;18:399–406. [Google Scholar]
- 18.Young RJ, Lovell PA. Introduction to Polymers. 1991 [Google Scholar]
- 19.Peutzfeldt A. Resin composites in dentistry: The monomer systems. European Journal of Oral Sciences. 1997;105:97–116. doi: 10.1111/j.1600-0722.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 20.Scott TF, Cook WD, Forsythe JS, Bowman CN, Berchtold KA. FTIR and ESR spectroscopic studies of the photopolymerization of vinyl ester resins. Macromolecules. 2003;36:6066–6074. [Google Scholar]
- 21.Zhu S, Tian Y, Hamielec AE, Eaton DR. Radical Trapping and Termination in Free-Radical Polymerization of Mma. Macromolecules. 1990;23:1144–1150. [Google Scholar]
- 22.Kloosterboer JG. Network Formation by Chain Crosslinking Photopolymerization and Its Applications in Electronics. Advances in Polymer Science. 1988;84:1–61. [Google Scholar]
- 23.Fairbanks BD, Scott TF, Kloxin CJ, Anseth KS, Bowman CN. Thiol-Yne Photopolymerizations: Novel Mechanism, Kinetics, and Step-Growth Formation of Highly Cross-Linked Networks. Macromolecules. 2009;42:211–217. doi: 10.1021/ma801903w. [DOI] [PMC free article] [PubMed] [Google Scholar]