Abstract
Pulmonary hypertension (PH) in patients with sickle cell disease (SCD) is linked to intravascular hemolysis, impaired nitric oxide bioavailability, renal dysfunction, and early mortality. Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthases (NOS), is associated with vascular disease in other populations. We determined the plasma concentrations for several key arginine metabolites and their relationships to clinical variables in 177 patients with SCD and 29 control subjects: ADMA, symmetric dimethylarginine (SDMA), NG-monomethyl L-arginine (L-NMMA), N-omega-hydroxy-L-arginine (NOHA), arginine and citrulline. The median ADMA was significantly higher in SCD than controls (0.94 vs. 0.31 μmol/L, p<0.001). Patients with homozygous SCD had a remarkably lower ratio of arginine to ADMA (50 vs. 237, p<0.001). ADMA correlated with markers of hemolysis, low oxygen saturation and soluble adhesion molecules. PH was associated with high levels of ADMA and related metabolites. Higher ADMA level was associated with early mortality, remaining significant in a multivariate analysis. Subjects with homozygous SCD have high systemic levels of ADMA, associated with PH and early death, implicating ADMA as a functional NOS inhibitor in these patients. These defects and others converge on the nitric oxide pathway in homozygous SCD with vasculopathy.
Keywords: Sickle Cell Disease, ADMA, SDMA, NOHA, Arginine, Pulmonary Hypertension
INTRODUCTION
Sickle cell disease (SCD), caused by homozygosity for a single amino acid substitution within the β-subunit of hemoglobin or by compound heterozygosity for the sickle and another β-globin mutation, is associated with a range of clinical complications. Intracellular polymerization of the abnormal hemoglobin results in rigid red cells that flow poorly through the microvasculature, promoting tissue ischemia and infarction. Pulmonary hypertension (PH), cutaneous leg ulceration, and priapism constitute a distinct constellation of sickle cell complications associated with particularly severe intravascular hemolysis (Kato, et al, 2007). This process releases cell-free hemoglobin and arginase into plasma, correlating with depletion of nitric oxide (NO) and arginine, the obligate substrate of the NO synthases (NOS) (Morris, et al, 2005, Reiter, et al, 2002). These pathophysiologically related complications have been called sickle vasculopathy or the hemolysis-endothelial dysfunction subphenotype, involving a decreased bioavailability of NO, a gas that normally maintains vascular homeostasis (Kato, et al, 2007). Additional factors that disrupt the normal production of NO may also contribute to vasculopathy in SCD.
Asymmetric dimethylarginine (ADMA) is a naturally occurring modified arginine found in plasma (Boger, 2004). It originates from hydrolysis of proteins bearing methylated arginine residues, a known post-translational modification (Teerlink, 2005). ADMA, normally metabolized by dimethylarginine dimethylaminohydrolases I and II (DDAH I and II), is an endogenous inhibitor of NOS isoforms. Elevated systemic levels of ADMA have been associated with atherosclerosis, particularly in patients with renal insufficiency (Boger and Zoccali, 2003). ADMA has been found at higher levels in idiopathic and chronic thromboembolic PH (Kielstein, et al, 2005, Skoro-Sajer, et al, 2007), PH related to congenital heart disease (Loukanov, et al, 2008), and in a rodent model of PH (Sasaki, et al, 2007), Elevation of ADMA in DDAH deficient mice is associated with endothelial dysfunction and elevated systemic and pulmonary blood pressure (Leiper, et al, 2007). ADMA has been found to be elevated in the plasma of patients with SCD, and associated with soluble vascular cell adhesion molecule-1 (sVCAM-1), a marker of endothelial activation normally suppressed by NO (Landburg, et al, 2008a, Schnog, et al, 2005). ADMA was also associated with lower hemoglobin, suggesting a correlation with more severe intravascular hemolysis. We hypothesized that elevated systemic levels of ADMA contribute to the hemolysis-vascular dysfunction subphenotype found in patients with SCD.
There are also other forms of modified arginine found in plasma. L-monomethylarginine (L-NMMA) is also a NOS inhibitor. Symmetric dimethylarginine (SDMA) is an isomer of ADMA, does not inhibit NOS (Leiper and Vallance, 1999), although it is not clear if it could inhibit arginine transport or other relevant aspects of the arginine-NO pathway. N-omega-hydroxyarginine (NOHA) is a metabolic intermediate of NOS activity (Morris, 2007). Herein we measured the plasma levels of these modified arginines in a large cohort of patients with SCD and examined their relationship with clinical characteristics and adverse outcomes in subjects with SCD. We find high plasma levels of ADMA in SCD patients are associated with the presence of PH, with its associated markers, and increased mortality. We also find a new, significant association of high NOHA levels with renal dysfunction.
MATERIALS AND METHODS
Patients
The patient and control populations in this study have been described previously (Gladwin, et al, 2004). The control group was approximately one quarter the size of the patient population and equivalent in ethnic, age and sex distribution. All patients provided informed consent under a protocol approved by the Institutional Review Board at the National Institutes of Health (clinicaltrials.gov identifier NCT00011648). Their baseline characteristics are summarized in Supplemental Table 1. They provided a medical history, and underwent physical examination, blood sampling, and Doppler echocardiography as previous described in detail (Gladwin, et al, 2004, Taylor, et al, 2008). Consistent with our previous validation data in this population (Gladwin, et al, 2004), PH was defined as a tricuspid regurgitant jet velocity (TRV) ≥2.5 m/sec, and moderate-to-severe PH was defined as TRV ≥3.0 m/sec. This is a research definition, and was used for individual patient diagnosis and management only in conjunction with clinically indicated right heart catheterization studies.
Laboratory measurements
Standard clinical laboratory assays were performed at the Department of Laboratory Medicine at the Clinical Center at the National Institutes of Health. Soluble vascular cell adhesion molecule-1 and soluble E-selectin levels were measured as previously described (Kato, et al, 2005), Arginase assays were performed as previously described (Morris, et al, 2005).
Amino acid assays
Plasma samples were stored at −80°C until analyses. After thawing and brief vortex mixing, aliquots (100 μL) were supplemented with [13C6]arginine internal standard, and subsequently quantified by liquid chromatography electrospray ionization tandem mass spectrometry using an ABI 365 triple quadrupole mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA) equipped with Ionics EP 10+ upgrade (Concord, Ontario, Canada) and Aria LX4 series multiplexed HPLC system with Flux pumps interface (Cohesive Technologies, Franklin, MA). Mass spectrometric analyses were performed in the positive ion mode with multiple reaction monitoring (MRM) using unique characteristic parent → daughter ion transitions for each analyte, as previously described (Nicholls, et al, 2007). Each analyte monitored demonstrated near quantitative recovery, good linearity over multiple orders of magnitude in concentration range, and intra- and inter-assay coefficients of variance of <10%.
Statistical analysis
Due to skewed distributions of the arginine metabolites, nonparametric analyses were used. Group comparisons were performed with the Mann-Whitney test, or the Kruskal-Wallis test where appropriate. Associations between continuous variables were assessed using the Spearman rank correlation coefficient. Survival analysis was performed using proportional hazards (Cox) regression. Survival data were current as of October 1, 2007. For the 124 homozygous SCD patients included in the survival analysis, median follow-up was 5.3 years for the 102 survivors and 3.3 years for the 22 patients who died. Two-sided p-values reported; p<0.05 was considered statistically significant. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Arginine metabolite levels in patients with SCD
Patients with SCD at steady state had significantly higher plasma levels of methylated arginines, including ADMA, SDMA and L-NMMA, compared to healthy African-American control subjects (p<0.001 for all analytes) (Table 1). In contrast, patients with SCD had lower levels of plasma L-arginine (p<0.001), NOHA (p=0.002), and citrulline (p=0.03)(Table 1). When these comparisons were repeated according to SCD genotype subgroup, homozygous sickle cell disease patients had the most dramatic and significant abnormalities in levels of arginine metabolites, and these overlapped the values seen in hemoglobin Sβ°-thalassemia patients, as expected parallel to the known clinical severity of each phenotype. Most prominently, the median ADMA level in patients with homozygous SCD was three times higher than in African American healthy control subjects (p<0.001), and median plasma L-arginine was 42% lower (p<0.001). As expected, differences from controls were smaller, but significant, in hemoglobin SC and hemoglobin Sβ+ thalassemia compound heterozygotes (Table 1). The ratio of arginine to ADMA, a marker of vascular disease risk in other disorders, was remarkably lower in SCD compared to controls (median 50 vs. 237, p<0.001). In order to exclude confounding effects due to different SCD genotypes, only patients with homozygous SCD were included for the remainder of the analysis.
Table 1.
Arginine Metabolites in SCD vs. controls. Values indicate median plasma levels at steady state in micromolar. Results are compared for control subjects compared to all SCD patients, and then the same SCD patients categorized by genotype.
| Arginine Metabolite |
Control (n=29) |
All SCD (n=177) |
SS (n=130) |
Sβ0-thal (n=3) |
SC (n=34) |
Sβ+-thal (n=8) |
|---|---|---|---|---|---|---|
| ADMA | 0.31 | 0.94 *** | 0.99*** | 1.48*** | 0.82*** | 0.78 |
| SDMA | 0.83 | 1.02 *** | 1.03** | 1.04 | 0.92 | 0.98 |
| L-NMMA | 0.13 | 0.18 *** | 0.18*** | 0.23 | 0.15 | 0.17 |
| L-Arginine | 78.3 | 46.4 *** | 45.5*** | 28.5* | 51.5** | 42.7** |
| NOHA | 2.50 | 1.93 ** | 1.80* | 2.15 | 2.23 | 2.11 |
| Citrulline | 20.1 | 17.7 * | 17.0* | 19.1 | 21.1 | 20.6 |
p<0.05 compared to control
p<0.01
p<0.001; Mann-Whitney test for all SCD compared to control subjects; Kruskal-Wallis test with Dunne’s post-test for comparisons of genotype categories to control subjects.
Relationship of methylated arginine levels to renal function
The methylated arginine levels showed significant relationships to markers of renal function. Homozygous SCD patients with moderate or severe renal insufficiency (serum creatinine >220 μmol/L, n=6) had levels of methylated arginines that were very significantly different from levels in patients without renal insufficiency (n=123), including lower levels of ADMA and higher levels of L-NMMA, SDMA and NOHA (Table 2). Serum citrulline levels also were much higher in patients with renal insufficiency. The inverse relationship of ADMA to creatinine is a consistent finding when all SCD patient data are analyzed as a continuous variable (linear regression r2 = 0.21, p < 0.001 using log-transformed values), so it is not due simply to outlier characteristics of the small renal failure cohort. Qualitatively similar correlations were seen with glomerular filtration rate as estimated by the Cockcroft-Gault formula (data not shown). Most strikingly, in homozygous SCD patients linear regression analysis demonstrated very strong links of serum creatinine to NOHA (r2=0.91, p<0.0001) and citrulline (r2=0.72, p<0.0001). The levels of the arginine-related amino acids in renal insufficiency patients were thus outliers in many respects, and were excluded from the remainder of the analysis.
Table 2. Arginine Metabolites in Renal Insufficiency.
Values indicate median plasma levels of arginine metabolites (in μmol/L) at steady state. The comparison includes patients with homozygous sickle cell anemia with serum creatinine < 220 μmol/l (n=123) or > 220 μmol/l (n=6)(Mann-Whitney test).
| Arginine Metabolite |
Creatinine < 220 μmol/L | Creatinine > 220 μmol/L | |||
|---|---|---|---|---|---|
| Median | Interquartile Range | Median | Interquartile Range | p | |
| ADMA | 1.02 | 0.82, 1.24 | 0.45 | 0.23, 0.71 | <0.001 |
| SDMA | 1.01 | 0.82, 1.28 | 3.64 | 3.09, 4.29 | <0.0001 |
| L-NMMA | 0.18 | 0.14, 0.22 | 0.27 | 0.23, 0.33 | <0.0001 |
| L-Arginine | 46 | 36, 57 | 50 | 45, 56 | NS |
| NOHA | 1.8 | 1.4, 2.4 | 34 | 16, 63 | <0.0001 |
| Citrulline | 16 | 12, 21 | 97 | 64, 113 | <0.0001 |
Clinical correlates of arginine metabolites in homozygous SCD
Among patients with homozygous SCD and relatively normal renal function, the levels of three arginine metabolites varied significantly with respect to degree of pulmonary hypertension. Levels of ADMA, SDMA and NOHA were significantly higher in patients with PH than those without (Table 3). ADMA and SDMA levels also correlated with the PH marker amino-terminal brain-type natriuretic propeptide (NT-proBNP)(Table 4), further supporting their link to PH. Several clinical indices of accelerated hemolysis (low hemoglobin levels, high reticulocyte counts, and high serum levels of lactate dehydrogenase or indirect bilirubin) showed significant correlations to ADMA, and to a lesser extent, with SDMA and L-NMMA (Table 4). In addition, ADMA was correlated with plasma arginase activity, which also has been linked to intravascular hemolysis. Low transcutaneous oxygen saturation correlated significantly with high levels of ADMA, SDMA, L-NMMA and the NOS intermediate NOHA (Table 4). Homozygous SCD patients taking hydroxyurea tended to have slightly lower levels of ADMA, but this was not significant.
Table 3. Arginine Metabolites in Pulmonary Hypertension.
Values indicate median plasma levels of arginine metabolites (in μmol/L) at steady state. Patients are categorized by Doppler echocardiographic measurement of tricuspid regurgitant jet velocity of < 2.5 m/sec (no PH, n=81), 2.5 – 2.9 m/sec (mild PH, n=26), or > 2.9 m/sec (moderate – severe PH, n=11). The comparison includes patients with homozygous sickle cell disease with serum creatinine levels < 220 μmol/L (Kruskal-Wallis test).
| Arginine Metabolite |
No PH | Mild PH | Mod-Severe PH | p |
|---|---|---|---|---|
| ADMA | 0.95 | 1.18 | 1.14 | 0.02 |
| SDMA | 0.96 | 1.11 | 1.17 | 0.008 |
| L-NMMA | 0.18 | 0.19 | 0.16 | NS |
| L-Arginine | 45 | 40 | 52 | NS |
| NOHA | 1.7 | 1.7 | 2.9 | 0.005 |
| Citrulline | 16 | 16 | 21 | NS |
Table 4. Correlations of arginine metabolites to clinical markers.
Values shown are the Spearman correlation coefficients of the respective arginine metabolites to the indicated clinical variables in patients with homozygous sickle disease without significant renal insufficiency. Blank cells indicate nonsignificant (p>0.05) correlations.
| Category | Variable | n | ADMA | SDMA | L-NMMA | NOHA |
|---|---|---|---|---|---|---|
| Pulmonary | NT-proBNP | 117 | 0.24** | 0.30** | - | 0.21* |
| Oxygen saturation | 69 | −0.48*** | −0.46*** | −0.25* | −0.42*** | |
| Blood pressure | Systolic blood pressure | 104 | - | - | - | 0.23* |
| Diastolic blood pressure | 104 | - | - | - | 0.24* | |
| Hematologic | Leukocyte count | 116 | 0.34*** | - | 0.25** | - |
| Hemoglobin | 116 | −0.38*** | −0.34*** | - | - | |
| Reticulocyte count | 109 | 0.39*** | - | - | - | |
| Fetal Hemoglobin | 117 | −0.40*** | −0.24** | −0.30* | - | |
| Lactate dehydrogenase | 102 | 0.30** | 0.29** | - | - | |
| Indirect bilirubin | 116 | 0.23* | - | - | - | |
| Hepatic | Alkaline phosphatase | 116 | 0.24** | 0.27** | 0.22* | - |
| Alanine aminotransferase | 116 | 0.20* | - | 0.22* | - | |
| Bilirubin, direct | 116 | 0.39*** | 0.32** | 0.20* | - | |
| Renal | Creatinine | 117 | - | 0.36*** | - | 0.61*** |
| Endothelial | Soluble VCAM-1 | 116 | 0.37*** | 0.35*** | 0.29* | 0.20* |
| Soluble E-selectin | 110 | 0.27** | 0.28** | - | 0.26** | |
| Plasma Arginase activity | 91 | 0.27* | 0.36*** | - | - |
p<0.05
p<0.01
p<0.001
NOS pathway
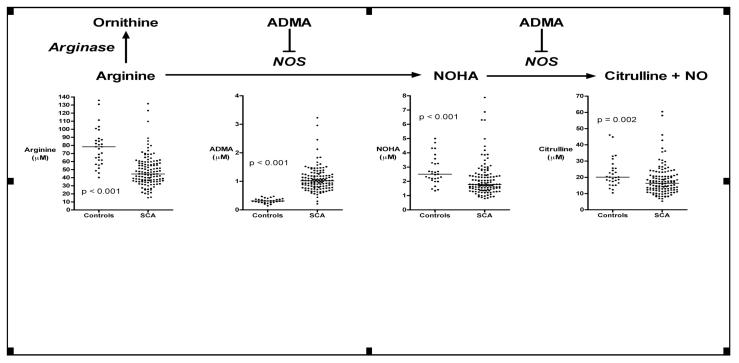
Patients with homozygous SCD and relatively normal renal function (n = 124) demonstrated significantly lower median plasma arginine levels than healthy African-American control subjects (n=29) (45 vs. 78 μmol/L, Mann-Whitney p<0.001)(Figure 1), presumably due in large part to plasma arginase, as indicated by a low ratio of arginine to ornithine as previously published (Morris, et al, 2005). Homozygous SCD patients have higher median ADMA (1.02 vs. 0.31 μmol/L, p<0.001), lower median NOHA (1.8 vs. 2.5 μmol/L, p<0.001), and lower median citrulline (16 vs. 20 μmol/L, p<0.01), potentially compatible with decreased flux through the NOS pathway.
Figure 1. Relationship of the NOS inhibitor ADMA to arginine metabolism in sickle cell disease.
Nitric oxide synthase (NOS) converts L-arginine to the intermediate NOHA, which is then converted to citrulline and nitric oxide. In patients with sickle cell disease with relatively normal renal function (serum creatinine < 220 μmol/L, n = 161), plasma arginine levels are lower than controls (n = 29), consistent with previously published effects of plasma arginase activity. Plasma levels of the NOS inhibitor ADMA are much higher than controls, and the downstream products of NOS activity are lower than controls, including both NOHA and citrulline. Horizontal bars indicate median values, and p value is calculated by the Mann-Whitney test.
While only a limited sample size (n = 6), a distinctly different profile emerged in the homozygous SCD patients with marked renal insufficiency, indicated by a serum creatinine >220 μmol/L. In these patients, median citrulline was 38-fold higher than in control subjects (n=29)(97 vs. 2.5 μmol/L, p<0.001), and median arginine was again low (50 vs. 78 μmol/L, p<0.01), consistent with a defect in conversion of citrulline to arginine, due to renal insufficiency (Supplemental Figure 1). Interestingly, in homozygous SCD patients with renal insufficiency, the NOS intermediate NOHA was elevated to very high levels compared to healthy control subjects (median 34 vs. 2.5 μmol/L, p<0.001).
Survival
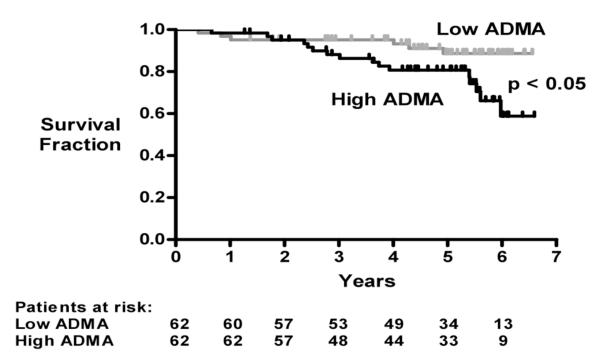
Follow up was available for 124 homozygous SCD patients with serum creatinine <220 μmol/L. Patients with ADMA above the group median value (>1.02 μmol/L, n = 62) had significantly shorter survival than patients with ADMA at or below the median value (≤1.02 μmol/L, n = 62, p = 0.02, Cox regression)(Figure 2). When analyzed as a continuous variable, higher ADMA was significantly associated with mortality (p=0.04), remaining significant (p=0.02) in a multivariable model with creatinine (p<0.001) as a covariate. However, ADMA lost significance (p = 0.08) when PH was added to the model, consistent with the association between ADMA and PH (Table 3).
Figure 2. Higher ADMA levels associated with early mortality.
Kaplan-Meier plot of survival fraction in 124 patients with homozygous sickle cell disease with serum creatinine < 220 μmol/L, divided into those with ADMA plasma levels above or below the median for this group (1.02 μmol/L). Patients with higher ADMA levels have earlier mortality (p = 0.02, Cox regression).
DISCUSSION
Our data from a large cohort indicate that patients with SCD have very high levels of the endogenous NOS inhibitor, ADMA - three times that of healthy African-American controls. This confirms and extends the preliminary observations of Schnog and colleagues on 12 patients with SCD (Schnog, et al, 2005). As might be predicted, the levels are the most abnormal in patients with homozygous SCD and hemoglobin Sβ° thalassemia, and elevated to an intermediate degree in hemoglobin SC and hemoglobin Sβ+ thalassemia. ADMA levels are linked to multiple markers of hemolytic severity, including low hemoglobin, and high levels of LDH, bilirubin, reticulocytes, fetal hemoglobin and arginase. ADMA is typically generated during proteolysis of arginine-methylated proteins (Teerlink, 2005), and it is tempting to speculate that in SCD, ADMA might be produced from the breakdown of proteins contained in sickle erythrocytes, which turn over at a rate up to twenty times normal (Crosby, 1955). Alternatively, there might be a hemolysis-linked inhibition of DDAH, the enzyme that hydrolyzes methylarginine (Ito, et al, 1999). In addition, endothelial shear stress induced protein arginine methyltransferase activity (Landburg, et al, 2008a), which reportedly is highest in patients with lowest hemoglobin levels (Osanai, et al, 2003), may contribute. Even though peripheral tissue oxygenation is often near normal due to the low affinity of HbS for oxygen, hypoxia induced down regulation of DDAH specifically in the pulmonary vasculature (10) could also contribute. However, it is not immediately apparent what factor might be contributing to the accumulation of plasma ADMA. In fact, this is the converse of what might have been expected, since DDAH activity is contained in red cells, and might be released during intravascular hemolysis (Kang, et al, 2001). Clearly hemolysis is not necessary for ADMA elevation in other vasculopathies without hemolysis, such as idiopathic PH (Kielstein, et al, 2004), but their epidemiologic association in SCD is found in our study and by Schnog and colleagues (Schnog, et al, 2005).
There is substantial evidence that intravascular hemolysis with SCD induces a multifactorial decrease in nitric oxide bioavailability. Intravascular hemolysis releases cell-free plasma hemoglobin that scavenges nitric oxide (Reiter, et al, 2002), and cell-free plasma arginase that is correlated with depletion of the NOS substrate arginine (Morris, et al, 2005). Markers of this process are linked to nitric oxide resistance in blood flow physiology assays and pulmonary vasoconstriction in humans and animals with SCD (Aslan, et al, 2001, Eberhardt, et al, 2003, Kaul, et al, 2000, Nath, et al, 2000, Reiter, et al, 2002). A similar pathophysiology is observed in other animal models of hemolysis and other human hemolytic diseases (Gramaglia, et al, 2006, Hsu, et al, 2007, Minneci, et al, 2005, Rother, et al, 2005). Thus, hemolysis-linked accumulation of the NOS inhibitor ADMA adds to the list of insults to NO bioavailability in patients with SCD.
Our data also confirm and extend that ADMA levels in patients with SCD are linked to a vasculopathic phenotype, specifically PH (as defined by TRV ≥ 2.5 m/sec, and also associated with a high NT-proBNP level), and corroborated by the endothelial activation markers soluble VCAM-1 and soluble E-selectin. These same patients also manifest lower oxygen saturation, which speculatively might be related to ventilation-perfusion mismatch due to pulmonary vascular tone dysregulation. Interestingly, the high ADMA level also is linked in our data with two additional markers previously correlated to PH in SCD, higher serum alkaline phosphatase and direct bilirubin level (De Castro, et al, 2008, Gladwin, et al, 2004, Onyekwere, et al, 2008). Our linkage of ADMA levels to increased mortality risk in SCD adds to the list of disease conditions with similar associations between ADMA and death (Meinitzer, et al, 2007, Nijveldt, et al, 2003, Ravani, et al, 2005, Schnabel, et al, 2005, Zoccali, et al, 2001).
One very unusual observation in our patients with SCD is the inverse relationship of ADMA levels to serum creatinine. In the general population, ADMA levels are positively correlated with serum creatinine levels, implying that ADMA is partly excreted by the kidney and accumulates during renal dysfunction (Xiao, et al, 2001). However, we find that in SCD patients, the relationship is inverted; ADMA levels are inversely related to serum creatinine levels, and fall as renal dysfunction progresses. Presumably, this paradoxical relationship must be induced by some aspect of SCD pathophysiology that impacts upon ADMA formation or clearance.
The other methylarginine derivatives provide a few interesting observations. First, the relationship of NOHA to serum creatinine level is very striking. This suggests that NOHA and creatinine are handled by a highly overlapping mechanism in the kidney. To our knowledge, such a relationship of plasma NOHA levels to renal function has not been previously reported in any patient cohort. NOHA is considered a transient intermediate of NOS activity, and its accumulation in renal insufficiency up to 20-fold was unexpected. More investigation is needed to understand the mechanism and physiologic implications of the close correlation of NOHA to creatinine. Second, the levels of the other endogenous NOS inhibitor, L-NMMA, are lower than ADMA, though it shares some of the epidemiologic correlations to ADMA, especially to cholestatic hepatic dysfunction and endothelial activation. Third, the pattern of SDMA correlations to markers of hemolysis, endothelial activation and PH overlaps that of ADMA, even though it has the converse relationship with renal function shown by ADMA. Lastly, the strong relationship of citrulline levels, renal dysfunction and PH in SCD invites speculation whether impaired renal regeneration of arginine from citrulline may influence the bioavailability of arginine to NOS for NO production, which is unclear from our limited data but merits further research. A recent study also supports the predictive value of elevated creatinine for poor survival in PH (Shah, et al, 2008). At the time of submission of this manuscript, another report of ADMA elevation in sickle cell pulmonary hypertension is in press (Landburg, et al, 2008b).
There are several limitations to this study. Our findings are limited by the purely correlative nature of this investigation, and they do not prove causation. However, other recently published data from a DDAH-deficient mouse convincingly suggests a causal relationship of ADMA with vasculopathy (Leiper, et al, 2007). A larger sample size might clarify some of the trends seen in our population, but our cohort already exceeds by over two-fold the previously published cohort for ADMA in SCD (Schnog, et al, 2005), and our analysis adds the other five arginine metabolite levels and their correlations with pulmonary hypertension and death, providing a more comprehensive picture. Due to the registry nature of this study, patients received a variety of clinically indicated or investigational treatments, and there is insufficient statistical power in the cohort to determine relationships to ADMA levels.
The correlation of elevated ADMA levels in PH in SCD suggests that this risk factor for atherosclerosis in the general population may also be a risk factor for the development of vasculopathy and PH in the SCD population. We propose that in sickle cell vasculopathy, the known prominent risk factor of hemolysis-associated reduction in nitric oxide bioavailability in SCD patients is modified by other established vascular disease risk factors, such as ADMA levels.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge intellectual support from Mark Gladwin, protocol management support by Mary K. Hall, and research nursing support from Wynona Coles, James Nichols, and Lori Hunter. This research was supported by intramural research funds from the National Institutes of Health (G.J.K., R.F.M., W.C.B. and J.G.T.) and by National Institutes of Health grants P01HL087018, P01 HL076491 and P01 HL077107 (S.L.H.).
Footnotes
CONFLICT OF INTEREST DISCLOSURE The authors declare no conflict of interests.
Clinical Trials Registration: http://clinicaltrials.gov/ct2/show/NCT00011648
REFERENCES
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc.Natl.Acad.Sci.U.S.A. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger RH. Asymmetric Dimethylarginine, an Endogenous Inhibitor of Nitric Oxide Synthase, Explains the “L-Arginine Paradox” and Acts as a Novel Cardiovascular Risk Factor. Journal of Nutrition. 2004;134:2842S–2847. doi: 10.1093/jn/134.10.2842S. [DOI] [PubMed] [Google Scholar]
- Boger RH, Zoccali C. ADMA: a novel risk factor that explains excess cardiovascular event rate in patients with end-stage renal disease. Atherosclerosis Supplements. 2003;4:23–28. doi: 10.1016/s1567-5688(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Crosby WH. The metabolism of hemoglobin and bile pigment in hemolytic disease. Am J Med. 1955;18:112–122. doi: 10.1016/0002-9343(55)90208-4. [DOI] [PubMed] [Google Scholar]
- De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83:19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- Eberhardt RT, McMahon L, Duffy SJ, Steinberg MH, Perrine SP, Loscalzo J, Coffman JD, Vita JA. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am J Hematol. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12:1417–1422. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- Kang ES, Cates TB, Harper DN, Chiang TM, Myers LK, Acchiardo SR, Kimoto M. An enzyme hydrolyzing methylated inhibitors of nitric oxide synthase is present in circulating human red blood cells. Free Radical Research. 2001;35:693–707. doi: 10.1080/10715760100301211. [DOI] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br.J Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. American Journal of Physiology - Heart and Circulatory Physiology. 2000;278:H1799–H1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Bode-Boger SM, Hesse G, Martens-Lobenhoffer J, Takacs A, Fliser D, Hoeper MM. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–1418. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Impraim B, Simmel S, Bode-Boger SM, Tsikas D, Frolich JC, Hoeper MM, Haller H, Fliser D. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–177. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- Landburg PP, Teerlink T, Muskiet FA, Duits AJ, Schnog JJ. Plasma concentrations of asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell patients but do not increase further during painful crisis. Am J Hematol. 2008a;83:577–579. doi: 10.1002/ajh.21184. [DOI] [PubMed] [Google Scholar]
- Landburg PP, Teerlink T, van Beers EJ, Muskiet FA, Kappers-Klunne MC, van Esser JW, Mac Gillavry MR, Biemond BJ, Brandjes DP, Duits AJ, Schnog JJ. Association of asymmetric dimethylarginine with sickle cell disease-related pulmonary hypertension. Haematologica. 2008b;93:1410–1412. doi: 10.3324/haematol.12928. [DOI] [PubMed] [Google Scholar]
- Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–548. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- Loukanov T, Arnold R, Gross J, Sebening C, Klimpel H, Eichhorn J, Hoss K, Ulmer HE, Kark M, Gorenflo M. Endothelin-1 and asymmetric dimethylarginine in children with left-to-right shunt after intracardiac repair. Clin Res Cardiol. 2008;97:383–388. doi: 10.1007/s00392-008-0645-x. [DOI] [PubMed] [Google Scholar]
- Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study) Clin Chem. 2007;53:273–283. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. Journal of Clinical Investigation. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr., Gladwin MT. Dysregulated Arginine Metabolism, Hemolysis-Associated Pulmonary Hypertension and Mortality in Sickle Cell Disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM., Jr. Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- Nath KA, Shah V, Haggard JJ, Croatt AJ, Smith LA, Hebbel RP, Katusic ZS. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. American Journal of Physiology - Regulatory, Integrative, and Comparative Physiology. 2000;279:R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Wang Z, Koeth R, Levison B, DelFraino B, Dzavik V, Griffith OW, Hathaway D, Panza JA, Nissen SE, Hochman JS, Hazen SL. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation. 2007;116:2315–2324. doi: 10.1161/CIRCULATIONAHA.107.693986. [DOI] [PubMed] [Google Scholar]
- Nijveldt RJ, Teerlink T, Van Der Hoven B, Siroen MP, Kuik DJ, Rauwerda JA, van Leeuwen PA. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22:23–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]
- Onyekwere OC, Campbell A, Teshome M, Onyeagoro S, Sylvan C, Akintilo A, Hutchinson S, Ensing G, Gaskin P, Kato G, Rana S, Kwagyan J, Gordeuk V, Williams J, Castro O. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008;29:309–312. doi: 10.1007/s00246-007-9018-x. [DOI] [PubMed] [Google Scholar]
- Osanai T, Saitoh M, Sasaki S, Tomita H, Matsunaga T, Okumura K. Effect of shear stress on asymmetric dimethylarginine release from vascular endothelial cells. Hypertension. 2003;42:985–990. doi: 10.1161/01.HYP.0000097805.05108.16. [DOI] [PubMed] [Google Scholar]
- Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol. 2005;16:2449–2455. doi: 10.1681/ASN.2005010076. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Doi S, Mizutani S, Azuma H. Roles of accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, and attenuated nitric oxide synthase activity in endothelial cells for pulmonary hypertension in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1480–1487. doi: 10.1152/ajplung.00360.2006. [DOI] [PubMed] [Google Scholar]
- Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C, Cambien F, Tiret L, Munzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97:e53–59. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- Schnog JB, Teerlink T, van der Dijs FP, Duits AJ, Muskiet FA. Plasma levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell disease. Annals of Hematology. 2005;84:282–286. doi: 10.1007/s00277-004-0983-3. [DOI] [PubMed] [Google Scholar]
- Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation. 2008;117:2475–2483. doi: 10.1161/CIRCULATIONAHA.107.719500. [DOI] [PubMed] [Google Scholar]
- Skoro-Sajer N, Mittermayer F, Panzenboeck A, Bonderman D, Sadushi R, Hitsch R, Jakowitsch J, Klepetko W, Kneussl MP, Wolzt M, Lang IM. Asymmetric dimethylarginine is increased in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2007;176:1154–1160. doi: 10.1164/rccm.200702-278OC. [DOI] [PubMed] [Google Scholar]
- Taylor J.G.t., Ackah D, Cobb C, Orr N, Percy MJ, Sachdev V, Machado R, Castro O, Kato GJ, Chanock SJ, Gladwin MT. Mutations and polymorphisms in hemoglobin genes and the risk of pulmonary hypertension and death in sickle cell disease. Am J Hematol. 2008;83:6–14. doi: 10.1002/ajh.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerlink T. ADMA metabolism and clearance. Vasc Med. 2005;10(Suppl 1):S73–81. doi: 10.1191/1358863x05vm597oa. [DOI] [PubMed] [Google Scholar]
- Xiao S, Wagner L, Schmidt RJ, Baylis C. Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney International. 2001;59:1466–1472. doi: 10.1046/j.1523-1755.2001.0590041466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccali C, Bode-Boger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frolich J, Boger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.