Abstract
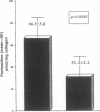
RATIONALE: Advanced glycosylation end products (AGEs) may play an important role in the development of diabetic vascular sequelae. An AGE cross-link, pentosidine, is a sensitive and specific marker for tissue levels of AGEs. OBJECTIVES: To evaluate the role of AGEs in the development of diabetic nephropathy and retinopathy, we studied pentosidine levels and the clinical characteristics of 48 subjects with insulin-dependent diabetes mellitus. Diabetic nephropathy was classified as normal, microalbuminuria, or gross proteinuria, and retinopathy was graded as none, background, or proliferative. NEWLY OBSERVED FINDINGS: Significant elevation of pentosidine (P = 0.025) was found in subjects with microalbuminuria or gross proteinuria (73.03 +/- 9.47 vs 76.46 +/- 6.37 pmol/mg col) when compared with normal (56.96 +/- 3.26 pmol/mg col). Multivariate analysis to correct for age, duration of diabetes, and gender did not modify the results. Elevated pentosidine levels were also found in those with proliferative when compared with those with background retinopathy (75.86 +/- 5.66 vs 60.42 +/- 5.98 pmol/mg col) (P < 0.05). CONCLUSIONS: Microalbuminuria is associated with elevated levels of pentosidine similar to those found in overt diabetic nephropathy suggesting that elevated AGE levels are already present during the earliest detectable phase of diabetic nephropathy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassiouny A. R., Rosenberg H., McDonald T. L. Glucosylated collagen is antigenic. Diabetes. 1983 Dec;32(12):1182–1184. doi: 10.2337/diab.32.12.1182. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Charonis A. S., Furcht L. T., Klein D. J., Mauer S. M., Steffes M. W., Tsilibary P. E. An overview of role of matrix components. Diabetes Care. 1991 Feb;14(2):157–159. doi: 10.2337/diacare.14.2.157. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Pongor S., Cerami A. Covalent attachment of soluble proteins by nonenzymatically glycosylated collagen. Role in the in situ formation of immune complexes. J Exp Med. 1983 Nov 1;158(5):1739–1744. doi: 10.1084/jem.158.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavers B. M., Bilous R. W., Ellis E. N., Steffes M. W., Mauer S. M. Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. N Engl J Med. 1989 Apr 13;320(15):966–970. doi: 10.1056/NEJM198904133201503. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Miyata S., Monnier V. Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J Clin Invest. 1992 Apr;89(4):1102–1112. doi: 10.1172/JCI115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. Prediction of clinical diabetic nephropathy in IDDM patients. Alternatives to microalbuminuria? Diabetes. 1990 Jul;39(7):761–767. doi: 10.2337/diab.39.7.761. [DOI] [PubMed] [Google Scholar]
- Molnar J. A., Alpert N. M., Wagner D. A., Miyatani S., Burke J. F., Young V. R. Synthesis and degradation of collagens in skin of healthy and protein-malnourished rats in vivo, studied by 18O2 labelling. Biochem J. 1988 Feb 15;250(1):71–76. doi: 10.1042/bj2500071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Elmets C. A., Frank K. E., Vishwanath V., Yamashita T. Age-related normalization of the browning rate of collagen in diabetic subjects without retinopathy. J Clin Invest. 1986 Sep;78(3):832–835. doi: 10.1172/JCI112648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Vishwanath V., Frank K. E., Elmets C. A., Dauchot P., Kohn R. R. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986 Feb 13;314(7):403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- Radoff S., Makita Z., Vlassara H. Radioreceptor assay for advanced glycosylation end products. Diabetes. 1991 Dec;40(12):1731–1738. doi: 10.2337/diab.40.12.1731. [DOI] [PubMed] [Google Scholar]
- Sell D. R., Lapolla A., Odetti P., Fogarty J., Monnier V. M. Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes. 1992 Oct;41(10):1286–1292. doi: 10.2337/diab.41.10.1286. [DOI] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J Clin Invest. 1990 Feb;85(2):380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19(1):77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Tsilibary E. C., Charonis A. S., Reger L. A., Wohlhueter R. M., Furcht L. T. The effect of nonenzymatic glucosylation on the binding of the main noncollagenous NC1 domain to type IV collagen. J Biol Chem. 1988 Mar 25;263(9):4302–4308. [PubMed] [Google Scholar]
- Viberti G. Recent advances in understanding mechanisms and natural history of diabetic renal disease. Diabetes Care. 1988 Nov-Dec;11 (Suppl 1):3–9. [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Ruben G. C. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol. 1987 Dec;105(6 Pt 1):2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]