Abstract
An 85-kDa breast tumor associated antigen (BTAA) has been identified and partially characterized from human breast tumors. As BTAA is poorly immunogenic, enhancement of the anti-tumor immunity induced by BTAA is required to obtain an objective clinical response. The potent immune activation by an aqueous preparation of neem (Azadirachta indica) leaf (NLP) suggests its possible utility for enhancing immune responses to tumor vaccines. Mice (Swiss and Balb/c) and rats (Sprague Dawley) immunized with BTAA and NLP have a higher IgG antibody response and a lower IgM response than mice immunized with BTAA alone. Antibody generated by immunization with BTAA and NLP can induce antibody-dependent cellular cytotoxicity (ADCC) and cytotoxic T cell (CTL) response towards BTAA-expressing MCF-7 cells. Antibody produced by vaccination with BTAA alone generated little cytotoxic response. The occurrence of ADCC and CTL response induced by BTAA plus NLP vaccination was possibly assisted by the induction of a Th1 response, as evidenced by the enhanced secretion of IFN-γ and decreased release of IL-10 from spleen cells and the greater production of IgG2a antibody in immunized mice. As NLP is nontoxic, abundantly available in the Indian subcontinent and can be extracted by a cost-effective method, this preparation may be considered a promising immune enhancer for BTAA vaccine.
Keywords: mice, vaccination, BTAA, neem leaf preparation, humoral immunity
Introduction
An 85-kDa breast tumor associated antigen (BTAA) has been identified and partially characterized from human breast tumors (1). BTAA was reported to be present in female breast tumors, but absent in malignant tumors of the uterine cervix, lung, stomach and liver or in breast tissues with benign diseases (1, 2). In our attempt to develop an immunogenic cancer vaccine, we have focused on BTAA, due to its distribution in high density on the surface of human breast tumor cells and on a breast cancer cell line, MCF-7 (unpublished observation). Although tumor antigens are found in cancer patients, immune responses protecting against tumor growth are rarely seen in tumor hosts (3). Enhancement of the antigenicity of the tumor antigen and anti-tumor immunity against the antigen is a prerequisite to develop a tumor vaccine.
A popular approach to enhance the antigenicity of a tumor antigen is its presentation along with adjuvant. An adjuvant can stimulate all wings of the immune system, individually or all together (4), by activating B cells, T cells, NK cells, monocytes/macrophages or dendritic cells (5, 6) and can induce the production of various cytokines (7, 8). Various preparations of the neem plant (Azadirachta indica) have such immunomodulatory properties (9, 10) and were shown to activate T cells (10), B cells (11) and macrophages (12). We have reported earlier the immunoprophylactic function of a nontoxic preparation (13) from neem leaf (NLP) to prevent the growth of murine Ehrlich's carcinoma and B16 melanoma (14). Studies utilizing adoptive cell transfer technology suggest that activation of various immunocompetent cells by neem leaf component(s) may be responsible for tumor growth restriction (15). This immune activation includes stimulation of NK/NK-T cells, and the secretion of TNF-α (15) and IFN-γ (16). Moreover, NLP mediated immune activation protects mice from leukopenia caused by cancer chemotherapy (17). Such immunostimulatory functions of NLP prompted us to investigate the adjuvant-like function of NLP to induce an effective anti-tumor immune response. In this context, the present investigation is designed to ascertain whether (i) NLP is efficient to augment the anti-BTAA immune response, (ii) NLP is able to induce T helper function, beneficial to the tumor host, and (iii) the anti-BTAA immune response has any anti-tumor function.
Results
NLP enhances the antibody response against BTAA
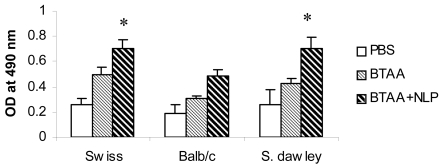
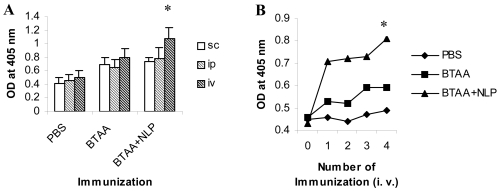
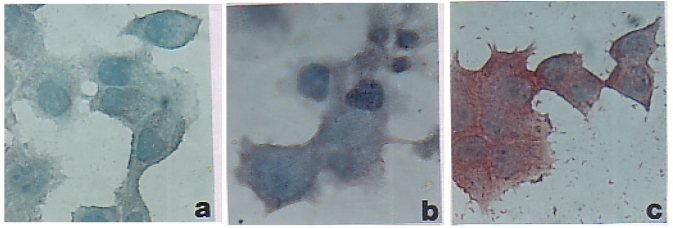
Three groups each of Swiss, Balb/c mice and Sprague Dawley rats were immunized with BTAA alone, BTAA in combination with NLP, or PBS using s.c., i.p. and i.v. routes and the generation of anti-BTAA antibody was measured by ELISA. It is evident from Figure 1 that immunization with BTAA alone generated anti-BTAA antibodies in mice and rats. Addition of NLP (1 unit) to BTAA significantly increased the anti-BTAA antibody response when immunization was given i.v., but not when given s.c. or i.p. (Figure 2A). Significant enhancement in the anti-BTAA antibody production by immunization with BTAA and NLP in comparison to that obtained with BTAA alone was observed following single or repeated i.v. immunizations (Figure 2B). The immune sera obtained from mice were also tested for their binding to BTAA positive MCF-7 cells. Reactivity of the sera of mice immunized with BTAA in combination with NLP with MCF-7 cells appeared stronger than that of the sera of the mice immunized with BTAA alone (Figure 3). Sera of the mice immunized with NLP alone had no anti-BTAA activity as tested by ELISA and immunocytochemistry (data not shown). Treatment with NLP was well tolerated by all mice and rats, which exhibited no apparent ruffling of fur, diarrhea, or other signs of toxicity.
Figure 1.
Generation of BTAA reactive antibodies in mice and rats. Groups of Swiss (n = 10), Balb/c (n = 6) mice and Sprague Dawley rats (n = 4) were immunized intravenously with PBS, BTAA alone (25 µg), or BTAA (25 µg) in combination with NLP (1 unit) weekly for 4 weeks. The presence of BTAA reactive antibodies in the sera of the immunized animals was assayed by ELISA. Immunization with NLP produces similar response as PBS. Each data point represents the mean ± 1 SD of observations from individual mice. *Significantly greater than the other values (P < 0.01).
Figure 2.
The route and number of immunizations influence the generation of BTAA reactive antibodies. (A) Effect of various routes of immunization on anti-BTAA antibody generation. Balb/c mice were immunized weekly for four weeks with PBS, BTAA alone (25 µg), or BTAA (25 µg) in combination with NLP (1 unit) by s.c., i.p. and i.v. routes. Seven days after the last immunization the mice were bled and anti-BTAA antibodies in the sera were measured by ELISA. Each data point represents the mean ± 1 SD of six observations. *Significantly higher than other values (P < 0.01). (B) Effect of multiple immunizations on anti-BTAA antibody generation. Groups of mice were immunized i.v. weekly for 1 to 4 weeks. Generation of anti-BTAA antibody was measured by ELISA after one week of immunization. Data from a representative mouse is presented for each group. *Values for the group immunized with BTAA and NLP are significantly higher than those of the other groups (P < 0.001).
Figure 3.
Reactivity of the sera of vaccinated Swiss mice with MCF-7 cells. MCF-7 cells were grown on tissue culture slides and incubated with sera (at 1:100 dilution) from mice immunized with PBS (a), BTAA alone (b) or BTAA in combination with NLP (c). Anti-BTAA antibody binding was determined by streptavidin-peroxidase method using aminoethylcarbazol as a substrate (Immunoperoxidase, magnification 200x).
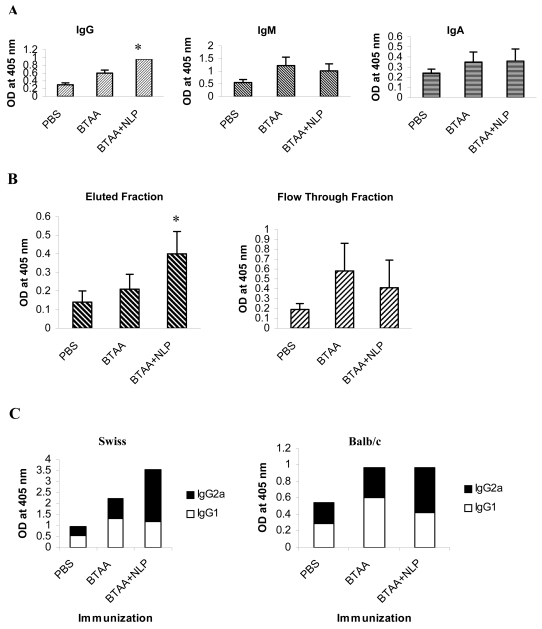
NLP increases the production of anti-BTAA IgG antibodies
Immunization with BTAA resulted in a 2-fold increase in IgG antibodies and a 2.2-fold increase in IgM response in Balb/c mice, in comparison to the IgG and IgM levels detected in control mice. Immunization with BTAA in combination with NLP resulted in a 3.2-fold increase in IgG response and a 1.8-fold increase in IgM response (Figure 4A). The increase in IgM response observed in the group vaccinated with BTAA and NLP was less than that treated with BTAA alone. The levels of IgA were similar in the mice vaccinated with BTAA alone and those vaccinated with BTAA in combination with NLP. These observations were confirmed by purification of IgG from pooled sera on a Protein A-Sepharose 4B column and examining the flow-through containing IgM. Samples with an equal optical density were tested by ELISA for IgG and IgM content. It may be observed in Figure 4B that the anti-BTAA IgG content increased following immunization with BTAA plus NLP, whereas the IgM response was lower in comparison to the levels obtained by immunization with BTAA alone. The anti-BTAA IgG subtypes generated by i.v. injection of mice with BTAA alone and BTAA in combination with NLP were analyzed by ELISA using commercially available anti-IgG1, -IgG2a, and -IgG2b tagged with alkaline phosphatase. As shown in Figure 4C, immunization with BTAA and NLP induced an IgG2a type antibody response, whereas injection with BTAA alone generated chiefly IgG1 antibody.
Figure 4.
Analysis of the immunoglobulin isotypes present in the sera of vaccinated mice. (A) Immunoglobulin profile of the sera of vaccinated mice. Three groups of mice were immunized with PBS, BTAA alone, or BTAA combined with NLP weekly for 4 weeks. Seven days after the last injection, the sera were assessed for IgG, IgA and IgM content by ELISA. The data correspond to mean ± SD of four observations. *Significantly higher as compared to the value of the BTAA immunized group (P < 0.01). (B) Isolation of IgG and IgM from the sera of vaccinated mice. Pooled sera from mice immunized with PBS, BTAA alone, or BTAA combined with NLP, were fractionated on a Protein A-Sepharose 4B column. Bound IgG was eluted and flow-through containing Igs (mainly IgM) other than IgG was collected. The OD of these two fractions was adjusted and the relative IgG and IgM content estimated by ELISA. *Significantly higher compared to the value of the BTAA immunized group (P < 0.01). (C) Three groups (n = 4 for each group) of mice (A: Swiss; B: Balb/c) were immunized (i.v.) with PBS, BTAA alone, or BTAA combined with NLP weekly for 4 weeks in total. Seven days after the last injection, sera were assessed for IgG1 and IgG2a anti-BTAA antibodies by ELISA after coating the plate with BTAA. Each bar represents the group mean of IgG2a/IgG1 response.
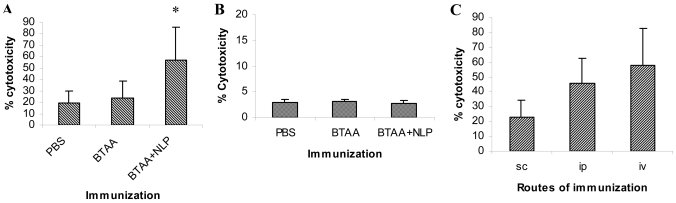
NLP-generated antibodies induce ADCC
Swiss mice were immunized with BTAA alone or BTAA in combination with NLP by s.c., i.p. and i.v. routes. Seven days after the last immunization, sera were collected from the mice, diluted 1:100 and used in an ADCC reaction. The peripheral blood mononuclear cells were co-cultured with the BTAA+ MCF-7 and BTAA- KB cells in the presence of immune sera at different effector:target ratios; data obtained at an E:T ratio of 10:1 are presented. As evident in Figure 5, panels A and B, immunization with BTAA alone generated sera that induced little ADCC. The immune sera of mice immunized with BTAA and NLP induced significant killing of the MCF-7 (Figure 5A), but not of the KB (Figure 5B), cells by effector splenic lymphocytes. The antibodies generated by immunization with BTAA and NLP administered by the i.v. route were more effective in inducing ADCC of MCF-7 cells as compared to those generated by immunization through s.c. and i.p. routes (Figure 5C). In Balb/c mice and Sprague Dawley rats, the generation of ADCC-inducing antibodies by immunization with BTAA and NLP was also observed (data not shown).
Figure 5.
Induction of ADCC by the sera of immunized mice. ADCC assay using MCF-7 (A) and KB (B) cells as target. Three groups of Swiss mice were immunized with PBS, BTAA alone, or BTAA combined with NLP weekly for 4 weeks. Seven days after the last injection, sera from individual mice were collected and tested in ADCC assays. Human peripheral blood mononuclear cells were used as effector cells and tumor cells (MCF-7 and KB) were used as target. (C) ADCC assay using MCF-7 as target and sera obtained by immunization through different routes. Swiss mice were immunized with BTAA and NLP through s.c., i.v. or i.p. routes weekly for 4 weeks. Seven days after the last injection, sera from individual mice were collected and tested in ADCC assays. Data obtained by using an E:T ratio of 10:1 and immune sera diluted 1:100 is presented. Each data represents the mean value ± 1 SD. *Significantly higher as compared to other values (P < 0.001).
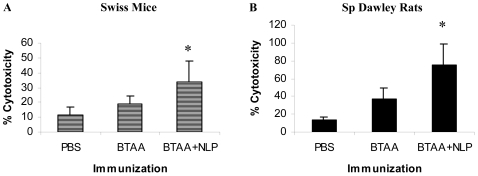
NLP enhances CTL activity
Swiss mice and Sprague Dawley rats were immunized weekly for 4 weeks with PBS, BTAA alone or BTAA in combination with NLP. Seven days after the last injection, the spleens were isolated and stimulated with BTAA. The CTL activity of the activated splenocytes against MCF-7 cells was determined in a CTL assay. The results revealed that immunization with BTAA alone induced little CTL activity in the splenocytes against MCF-7 cells (Figure 6). However, immunization of the mice with BTAA in combination with NLP generated significant CTL activity against MCF-7 cells in both Swiss mice (Figure 6A) and Sprague Dawley rats (Figure 6B). Generation of MCF-7 cell specific CTLs was also observed in Balb/c mice immunized with BTAA and NLP (data not shown). CTL activity detected in the case of antigen negative KB cells was significantly less than that observed in the case of MCF-7 cells (data not shown).
Figure 6.
CTL activity of the splenic cells of immunized mice and rats. Three groups of animals, either Swiss mice (A) or Sprague Dawley rats (B), were immunized i.v. with PBS, BTAA alone or BTAA in combination with NLP weekly for 4 weeks. Seven days after the last injection, splenic cells from individual mice were isolated, cultured in the presence of BTAA for 3-5 days and a CTL assay performed using these cells as effectors and MCF-7 cells as target at an E:T ratio 10:1. Each data represents the mean value ± 1 SD. *Significantly higher as compared to other values (P < 0.001).
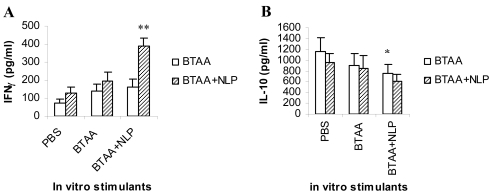
NLP enhances the BTAA-specific release of Th1 cytokine, IFN-γ
The splenic cells isolated from the Swiss mice immunized with PBS, BTAA alone, or BTAA in combination with NLP, were cultured in RPMI 1640 medium and in vitro stimulated with PBS, BTAA alone or BTAA in combination with NLP for 48 hours. The release of Th1 cytokine (IFN-γ) and Th2 cytokine (IL-10) in the spent media were measured by ELISA. It was observed that in vitro stimulation with BTAA alone or BTAA and NLP of spleen cells from mice immunized with BTAA and NLP induced a significant increase in the release of IFN-γ (Figure 7A). On the other hand, similar in vitro treatment resulted in a decrease in IL-10 secretion (Figure 7B). The observed changes were not detected when spleen cells from PBS injected mice were stimulated in vitro with either BTAA alone or BTAA and NLP, indicating BTAA-specific Th1 cytokine secretion.
Figure 7.
Release of IFN-γ and IL-10 from the splenocytes of immunized mice. Three groups of mice (n = 3 in each case) were immunized (i.v.) with PBS, BTAA alone, or BTAA in combination with NLP weekly for 4 weeks. Seven days after the last injection, spleen cells from individual mice were isolated and cultured in RPMI 1640 medium in the presence of either PBS, BTAA alone or BTAA and NLP for 48 hours. The IFN-γ (A) and IL-10 (B) content of the culture supernatants was analyzed by ELISA using a commercially available kit. Each data point corresponds to the mean ± 1 SD. **,*Statistically different from PBS controls (P < 0.001 and P < 0.05, respectively).
Discussion
The development of a tumor vaccine is dependent on the induction of the host's immune system, including the development of memory T cells and an antibody response. Most strategies for the development of tumor vaccines rely on the availability of tumor associated antigens and their derivatives (18, 19). In the present study, we have used BTAA as a tumor antigen (1, 2) with the objective of developing an immunotherapeutic tool for the treatment of malignant breast tumors. Immunization of mice and rats with BTAA could not activate tumor-specific cytotoxic effector functions. Such poorly immunogenic tumor antigens need help from immunostimulatory molecules or adjuvants that can assist in presenting the antigen properly to activate different immune cellular functions and induce the secretion of various cytokines (3, 7). Though a number of synthetic and natural adjuvants have been successfully used experimentally, alum (e. g., Al2O3) is still the only adjuvant approved for human use (20). Although safe, alum is a relatively weak adjuvant for antibody production and a poor inducer of cell mediated immune responses because of the induction of a Th2- rather than a Th1-type immune response (21).
The unique immunostimulatory property of the aqueous preparation of neem leaf (NLP) could be translated into the restriction of the murine tumor growth and an increased life span (13, 14). NLP is nontoxic, as systemic administration of NLP causes no hematotoxic, hepatotoxic and nephrotoxic effects (13). In the studies presented here, we have shown that NLP, when administered with BTAA, increases the anti-BTAA antibody response (Figure 1, Figure 2, and Figure 3). The almost identical enhancement in antibody response noted in Swiss, Balb/c mice and Sprague Dawley rats confirmed the adjuvant effect of NLP. Significant enhancement of the anti-BTAA antibody response following immunization with BTAA in combination with NLP by intravenous route, rather than intraperitoneal and subcutaneous routes, may be due to direct exposure of the immunogen to the lymphatic system, thereby resulting in better antigen presentation and cytokine secretion.
ADCC plays an important role in the host's defense against tumor. To test the efficacy of the immune sera in mediating ADCC against BTAA-expressing tumor cells, we have used the MCF-7 cell line. Generation of more IgM, rather than IgG, antibodies (Figure 4) by immunization with BTAA alone causes poor ADCC by anti-BTAA immune sera. The preponderance of an IgG response and the reduction in the level of IgM following immunization with BTAA in combination with NLP (Figure 4) explained the significant induction of ADCC against BTAA+ cells. IgG is an effective mediator of ADCC and complement-dependent cytotoxicity (22). Switching from an IgM response to an IgG response is known to help trigger a favorable cytokine network that regulates the cytotoxic function in either an antibody-dependent or independent fashion. Immunization with carbohydrate antigens, such as GD2, GM2, GD3, generally induces an IgM response (23). An anti-idiotypic antibody mimicking GD2 was reported to induce IgG rather than IgM, and thus proved to be effective as a better vaccine for GD2 expressing tumors (21). Generation of anti-BTAA antibody by using NLP as an adjuvant is comparable to the adjuvant effect obtained using Freund's adjuvant and the immunopotentiating effect of keyhole limpet hemocyanin (data not shown). It is important to note here that NLP has an excellent driving ability towards a Th1 type immune response (unpublished observation). This property of NLP is nicely adjoined during immunization with BTAA. Spleen cells from mice immunized with BTAA and NLP, when stimulated in vitro with either BTAA alone or BTAA and NLP, exhibited a significant increase in the secretion of Th1 cytokine (IFN-γ) and a decrease in the secretion of Th2 cytokine (IL-10) (Figure 7). IgG class switching to IgG2a following immunization with BTAA in combination with NLP (Figure 4C) also suggests NLP acts as an adjuvant with Th1-inducing capacity. Another promising adjuvant, CpGODN, has the ability to switch from a Th2 response to a Th1 response (24, 25). Th1 cytokines play a critical role in generating anti-tumor immune responses, by activating NK cells, CTLs and macrophages (26). The stimulation of the CTL activity of splenic cells from mice immunized with BTAA and NLP against MCF-7 cells (Figure 6) observed in the present study might be assisted by the IFN-γ released by the splenic cells after stimulation with NLP. Activation of antigen presenting cells by neem oil (10) and secretion of IFN-γ from NK cells and T cells by various products of neem (27) have been reported by others. Moreover, association of IFN-γ release with the predominance of IgG2a is established (28). Based on these evidences, it could be concluded that NLP is a potential adjuvant to enhance immunity against BTAA at the humoral and cellular levels.
The constituents present in neem leaf responsible for the adjuvant activity are not known. However, preliminary investigation suggests that 10% of the total NLP is protein (29). In addition, flavonoids may also be present in this preparation (29). Flavonoids are known inducers of Th1 response. Flavonoids present in grape seed were shown to stimulate production of IFN-γ by PBMCs, but had no effect on the level of the Th2-derived cytokine IL-6 (30). Nair et al. (31) reported that a flavonoid, quercetin, significantly induces gene expression, as well as the production of Th1-derived IFN-γ and the downregulation of Th2-derived IL-4 by normal PBMCs. Adjuvants inducing a Th1 response can manifest better antigen-specific anti-tumor immunity (26, 32).
From the dawn of civilization, various products of the neem plant have been consumed by human beings in normal health and disease (9, 33), and no toxicity has been reported. Based on our observations in the murine system, we have also reported that NLP is completely safe with no adverse effects on liver and kidney functions (13). Moreover, NLP has been shown to stimulate hematopoiesis. Our earlier reports (13-17) and the experimental data obtained from this study suggest that NLP is a safe, effective and economical adjuvant and can be used in tumor vaccine formulations, in animal and human studies.
Abbreviations
- BTAA
breast tumor associated antigen
- NLP
neem leaf preparation
Acknowledgements
The work was supported in part by the Department of Science and Technology, Govt. of India, through the grant with D. O. No. SR/SO/HS-24/2004. The first author (IM) gratefully acknowledges the financial assistance given by the Council of Scientific and Industrial Research (CSIR), India, through a CSIR-NET fellowship grant.
References
- 1.Pal S, Sanyal U, Chattopadhyay U. Purification and characterization of a new 85-kda glycoprotein antigen from human breast tumor. Int J Cancer. 1995;60:759–765. doi: 10.1002/ijc.2910600605. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay U, Pal S. A new 85 kDa breast tumor associated antigen - a potential diagnostic and prognostic agent. J Biosc. 1997;22:69–75. [Google Scholar]
- 3.Stevanovic S. Identification of tumor associated T-cell epitopes for vaccine development. Nat Rev Cancer. 2002;2:514–520. doi: 10.1038/nrc841. [DOI] [PubMed] [Google Scholar]
- 4.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 5.Kawarada Y, Ganss R, Garbi N, Sacher T, Arnold B, Hammerling GJ. NK- and CD8(+) T cell mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol. 2001;167:5247–5253. doi: 10.4049/jimmunol.167.9.5247. [DOI] [PubMed] [Google Scholar]
- 6.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen - a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 7.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas K, Chattopadhyay I, Banerjee RK, Bandopadhyay U. Biological activities and medicinal properties of neem (Azadirachta Indica). Curr Sci. 2002;82:1336–1345. [Google Scholar]
- 10.Upadhyay SN, Dhawan S, Garg S, Talwar GP. Immunomodulatory effects of neem (Azardirachta Indica) oil. Int J Immunopharmacol. 1992;14:1187–1193. doi: 10.1016/0192-0561(92)90054-o. [DOI] [PubMed] [Google Scholar]
- 11.Sadekar RD, Kolte AY, Barmase BS, Desai VF. Immunopotentiating effects of Azardirachta Indica (neem) dry leaves powder in broilers, naturally infected with IBD virus. Indian J Exp Biol. 1998;36:1151–1153. [PubMed] [Google Scholar]
- 12.Ray A, Banerjee BD, Sen P. Modulation of humoral and cell mediated immune responses by Azardirachta Indica (neem) in mice. Indian J Exp Biol. 1996;34:698–701. [PubMed] [Google Scholar]
- 13.Haque E, Mandal I, Pal S, Baral RN. Prophylactic dose of neem (Azadirachta indica) leaf preparation restricting murine tumor growth is nontoxic, hematostimulatory and immunostimulatory. Immunopharmacol Immunotoxicol. 2006;28:33–50. doi: 10.1080/08923970600623632. [DOI] [PubMed] [Google Scholar]
- 14.Baral RN, Chattopadhyay U. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int Immunopharmacol. 2004;4:355–366. doi: 10.1016/j.intimp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Haque E, Baral RN. Neem (Azadirachta indica) leaf preparation induces prophylactic growth inhibition of murine Ehrlich carcinoma in Swiss and C57BL/6 by activation of NK cells and NK-T cells. Immunobiology. 2006;211:721–731. doi: 10.1016/j.imbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Bose A, Ghosh D, Pal S, Chaudhuri S, Baral RN. Neem leaf preparation enhances in vitro cytotoxic efficacy of peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients by modulating cytokine signaling pathway [abstract]. Indian J Med Res (Suppl) 2005;121:60. [Google Scholar]
- 17.Ghosh D, Bose A, Haque E, Baral RN. Pretreatment with neem (Azadirachta indica) leaf preparation in Swiss mice diminishes leukopenia and enhances anti-tumor activity of cyclophosphamide. Phytother Res. 2006;20:814–818. doi: 10.1002/ptr.1948. [DOI] [PubMed] [Google Scholar]
- 18.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, Moynahan M, Houghton A, Norton L, Livingston PO. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 19.Foon KA, Lutzky J, Baral RN, Yannelli JR, Hutchins L, Teitelbaum A, Kashala OL, Das R, Garrison J, Reisfeld RA, Bhattacharya-Chatterjee M. Clinical and Immune responses in advanced melanoma patients immunized with an anti-idiotype (ID) antibody mimicking disialoganglioside GD2. J Clin Oncol. 2000;18:376–384. doi: 10.1200/JCO.2000.18.2.376. [DOI] [PubMed] [Google Scholar]
- 20.Audibert FM, Lise LD. Adjuvants: current status, clinical perspectives and future prospects. Immunol Today. 1993;14:281–284. doi: 10.1016/0167-5699(93)90046-N. [DOI] [PubMed] [Google Scholar]
- 21.Sen G, Chakraborty M, Foon KA, Reisfeld RA, Bhattacharya-Chatterjee M. Induction of IgG antibodies by an anti-idiotype antibody mimicking disialoganglioside GD2. J Immunother. 1998;21:75–83. doi: 10.1097/00002371-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Baral RN. Tumor vaccine: current trends in antigen specific immunotherapy. Indian J Exp Biol. 2005;43:389–406. [PubMed] [Google Scholar]
- 23.Livingston PO, Wong GYC, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Jones Calves M, Helling F, Ritter G, Oettgen HF, Old LJ. Improved survival in stage III melanoma patients with GM2 antibodies: A randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 24.Corral RS, Petray PB. CpG DNA as a Th1-promoting adjuvant in immunization against Trypanosoma cruzi. Vaccine. 2000;19:234–242. doi: 10.1016/s0264-410x(00)00172-9. [DOI] [PubMed] [Google Scholar]
- 25.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumor immunotherapy. Cancer Immunol Immunother. 2002;51:521–531. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thatte U, Dahanukar S. Rasayana Concept: clues from immunomodulatory therapy. In: Upadhyay SN, editor. Immunomodulation. New Delhi: Narosa Publising House; 1997. p. 141. [Google Scholar]
- 28.Collins JT, Dunnick WA. Germline transcripts of the murine immunoglobulin gamma 2a gene: structure and induction by IFN-gamma. Int Immunol. 1993;5:885–891. doi: 10.1093/intimm/5.8.885. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar K, Bose A, Laskar S, Chaudhuri SK, Dey S, Roychoudhuri PK, Baral RN. Antibody response against neem leaf preparation recognizes carcinoembryonic antigen. Int Immunopharmacol. 2007;7:306–312. doi: 10.1016/j.intimp.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Nair N, Mahajan S, Chawda R, Kandaswami C, Shanahan TC, Schwartz SA. Grape seed extract activates Th1 cells in vitro. Clin Diagn Lab Immunol. 2002;9:470–476. doi: 10.1128/CDLI.9.2.470-476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair MP, Kandaswami C, Mahajan S, Chadha KC, Chawda R, Nair H, Kumar N, Nair RE, Schwartz SA. The flavonoid, quercetin, differentially regulates Th-1 (IFNgamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim Biophys Acta. 2002;1593:29–36. doi: 10.1016/s0167-4889(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 32.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 33.Puri HS. Neem: the devine tree: Azadirachta indica. Amsterdam: Harwood Academic Publishers; 1999. [Google Scholar]
- 34.Baral RN, Sherrat A, Das R, Foon KA, Bhattacharya-Chatterjee M. Murine monoclonal anti-idiotypic antibody as a surrogate antigen for human HER-2/Neu. Int J Cancer. 2001;92:88–95. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1148>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
Materials and methods
Neem leaf preparation (NLP)
Mature neem leaves of same size and color (indicative of same age) taken from a standard source were shed-dried and pulverized. Extract obtained from 0.5 mg of neem dry powder is considered as one unit of NLP, generally used for the immunization of mice. Neem powder was soaked overnight in phosphate buffered saline (PBS), pH 7.4 one night before the experiment. Supernatant was collected by centrifugation at 300 x g for 7 minutes and was membrane-filtered (0.22 micron) for in vivo or in vitro use. The endotoxin content of the freshly prepared NLP was determined by the Limulus amebocyte lysate (LAL) test as per the manufacturer's (Salesworth India, Bangalore) instructions. The endotoxin content of all the batches of NLP was found to be less than 6 pg/ml.
Breast tumor associated antigen (BTAA) purification
BTAA was prepared by the method described earlier (1). In brief, tissue extract was prepared from surgically removed human malignant breast tumors by mincing and homogenizing the tissues in 0.15 M PBS, pH 7.2. Homogenates were centrifuged (Biofuge Stratos, Heraeus, Germany) successively at 6000 x g for 30 min and at 15,000 x g for 60 min at 0˚C. The supernatants were fractionated in a DEAE-cellulose column. The BTAA-containing gel eluate was further purified by reverse phase - high performance liquid chromatography using a protein PAK 300 SW column. Purification of BTAA was guided in every step by analysis by 12% SDS-polyacrylamide gel electrophoresis.
Animals
Female Swiss and Balb/c mice and Sprague Dawley rats (6 weeks old), obtained from the Animal Care and Maintenance Department of Chittaranjan National Cancer Institute were used for immunization with BTAA. The animals were maintained under standard laboratory conditions and according to the guidelines established by the Institutional Animal Care and Ethics Committee. Dry pellet diet and water were given ad libitum.
Cell lines
The human breast cancer (MCF-7) and oral cancer (KB) cell lines, originally obtained from the National Center for Cell Sciences, Pune, were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), penicillin (50 units/ml) and streptomycin (50 µg/ml) at 37˚C with the supply of 5% CO2.
Immunization of mice and rats
Groups of mice (10 Swiss and 6 Balb/c in each group) and rats (4 in each group) were immunized with BTAA (25 µg) alone, BTAA (25 µg) in combination with NLP (1 unit), or PBS in a total volume of 100 µl, once a week for 4 weeks. The dose of NLP was selected on the basis of results obtained earlier (13-17). All components of the injecting material were mixed before injection. Injection was given either subcutaneously (s.c.) on the right hind quarter, intraperitoneally (i.p.) or intravenously (i.v.) through the tail vein. Blood was collected (by retro-orbital puncture after anesthesia) 7 days after the last immunization, the serum was separated and stored at -20˚C until use. Spleens were collected from immunized mice and rats for the CTL assay.
ELISA
The presence of antibodies specific for BTAA in the serum samples was detected by ELISA (34). Briefly, the microtiter plates were coated overnight with 5 µg/ml of soluble BTAA and blocked with 1% gelatin. Serially diluted samples of control and test sera were added to the wells in triplicate and incubated for 2 hours. The plates were washed with PBS containing Tween-20 and goat anti-mouse Ig labeled with peroxidase (Sigma, St. Louis) was added at a dilution of 1:500. Color was developed using orthophenylenediaminedihydrochloride (OPD) and measured at 492 nm using a microtiter plate reader (Tecan Spectra, Grodig, Austria).
Immunocytochemistry (ICC)
Antibodies specific for BTAA were detected, localized and the intensity of expression assessed by ICC. In brief, MCF-7 cells were grown on chambered slides (Nunc, Roskilde, Denmark) to confluency and then fixed in 10% formal saline. After blocking for nonspecific binding with 10% normal rabbit serum, the cells were incubated with control and immune sera. Specific antibody binding was detected using biotinylated-rabbit anti-mouse IgG, streptavidin-peroxidase and aminoethylcarbazol (Vector Laboratories Inc, USA).
Determination of immunoglobulin isotypes and IgG subclasses by ELISA
For the determination of immunoglobulin isotypes and IgG subclasses in the test sera, goat anti-mouse IgG, IgM, IgA, IgG1, IgG2a and IgG2b antibodies labeled with alkaline phosphatase (Southern Biotechnology, Birmingham, AL) were used at a dilution of 1:1500 in PBS with 1% BSA. Immune reaction was detected using p-nitrophenylphosphate (pNPP) as a substrate dissolved in diethanolamine buffer, pH 9.8 (1 mg/ml). The optical density (OD) of the color reaction was measured at 405 nm.
Purification of anti-BTAA IgG and IgM antibodies
The serum samples from a group of mice were pooled, dialyzed against 0.15 M PBS, pH 7.4, and passed through a Protein A-Sepharose 4B (Sigma, USA) column. The flow-through was collected and column-bound antibodies were eluted with 0.1 M citrate buffer, pH 3.0. Fractions with an OD of more than 1.0 were pooled, dialyzed and concentrated using a Centricon membrane filter with a 10,000 molecular weight cut-off (Millipore Corp, Bedford, MA). The flow-through was also concentrated and the OD adjusted with PBS according to the eluted pooled fractions. Eluted fractions and flow-through were examined by ELISA for their IgG and IgM contents respectively.
Antibody-dependent cellular cytotoxicity (ADCC) assay
MCF-7 and KB cells (1 x 104) were cultured overnight in DMEM and used as target. The target cells were incubated with peripheral blood mononuclear cells as effector cells in the presence of different immune and non-immune sera (1:100 dilution) for 4 hours at 37˚C in a humidified atmosphere containing 5% CO2. The assay was performed using different effector:target (E:T) cell ratios. Cellular cytotoxicity was determined by measuring lactate dehydrogenase (LDH) released by the target cells using a commercially available kit (Roche Diagnostics, Mannheim, Germany). The amount of spontaneously released LDH was measured in wells that contained only target cells. Total released LDH was assessed by lysing the target cells with 2% SDS. The percentage of lysis was calculated according to the following formula: % specific lysis = [(experimental lysis - spontaneous lysis) / (maximum lysis - spontaneous lysis)] x 100. To calculate the lysis specifically attributable to ADCC, the percentage of lysis due to effector cells in the absence of the immune sera was subtracted from each value obtained. A percentage lysis of more than 12% was considered positive (34). Each assay was performed in triplicate.
CTL assay
Spleens were removed under sterile conditions from immunized and control mice (Balb/c) and rats (Sprague Dawley). Spleens were chopped into small fragments, gently minced on nylon mesh (BD-Falcon, USA) and washed with PBS. The splenic cells were suspended in RPMI 1640 tissue culture medium (Life Technologies, Grand Island, NY) supplemented with 10% FBS and penicillin-streptomycin solution. The effector spleen cells (2 x 105) were then cultured with antigen, BTAA (5 µg/ml). The cultures were maintained for 3-5 days in RPMI 1640 media. These effector spleen cells were serially diluted and cultured with target breast tumor cells at an E:T ratio of 10:1 at 37˚C in round bottom, 96-well microtiter plates. After 4 hours of incubation, 100 µl of supernatant was removed to estimate the release of LDH by the same method used for the ADCC assay. Each assay was performed in triplicate.
ELISA for cytokines
Cytokines secreted in the supernatants of murine spleen cell culture were measured by ELISA. Spleen cells isolated from immunized and control mice were cultured in RPMI 1640 medium for 48 hours and the supernatants were collected and stored at -20˚C until use. Culture supernatants (50 µl) were used to coat the wells of a microtiter plate overnight at 4˚C, followed by blocking with 5% BSA. The plates were then incubated with rat anti-mouse IFN-γ/IL-10 antibodies (e-Biosciences, San Diego, USA) overnight at 4˚C. After washing with PBS containing Tween 20, antibody binding was detected using goat anti-rat IgG peroxidase (Sigma, St. Louise). The intensity of the immune reaction was detected by the substrate para-nitrophenylphosphate in diethanolamine buffer.
Statistical analysis
All data shown represent the mean ± 1 standard deviation (SD). The significance of differences between the data obtained from different groups of mice was analyzed by the Student's t-test. A probability (P) less than 0.05 was considered to indicate statistical significance.