Abstract
The chimeric monoclonal antibody cG250 recognizes the CAIX/MN antigen. cG250 induces antibody-dependent cellular cytotoxicity (ADCC) responses in vitro that can be enhanced by IL-2. We studied the effects of adding daily low-dose subcutaneous IL-2 to cG250 for treatment of clear cell renal cell carcinoma (RCC). The primary endpoints of the trial were toxicity and immunological effects (human anti-chimeric antibodies [HACA], ADCC, natural killer [NK] and lymphokine-activated killer cell [LAK] activity); secondary endpoints were cG250 biodistribution and pharmacokinetics (PK) and tumour response rates. Eligible patients had unresectable metastatic or locally advanced clear cell RCC with measurable or evaluable disease. Nine patients were treated with six doses of cG250 (10 mg/m2/week, first and fifth doses trace-labelled with 131I), and 1.25 x 106 IU/m2/day IL-2 for six weeks. Treatment was generally well tolerated with no adverse events attributable to cG250. Two patients required a 50% dose reduction of IL-2 due to toxicity. No HACA was detected. 131I-labeled cG250 showed excellent targeting of tumour deposits. 131I cG250 PK: T½α 20.16 ± 6.59 h, T½β 126.21 ± 34.04 h, CL 39.67 ± 23.06 mL/h, Cmax 5.12 ± 0.86 µg/mL, V1 3.88 ± 1.05 L. IL-2 did not affect cG250 PK. A trend for increased percentage of circulating CD3-/CD16+CD56+ NK cells was observed. Some patients showed enhanced ADCC or LAK activity. No antitumour responses were observed. In conclusion, weekly cG250 with daily low-dose subcutaneous IL-2 is well tolerated. IL-2 does not influence cG250 biodistribution or increase HACA.
Keywords: clinical trial, renal cell carcinoma, human CA9 protein, cG250, chimeric antibody, IL-2
Introduction
In 2007, over 51,000 people in the USA are expected to develop renal cell carcinoma (RCC) or cancer of the renal pelvis, with more than 12,000 deaths (1). Patients with localized disease have 5-year survival rates of more than 90% (2); patients with metastatic disease have 5-year survival rates of around 30% (2) and the median survival is 10 months (3). RCC is therefore curable only if it is resectable.
To date chemotherapy has not demonstrated sufficient anti-tumour activity to prolong the survival of patients with metastatic RCC (4). Single agent or multiple agent chemotherapy has response rates less than 15-20%. Recent data indicate that inhibition of receptor tyrosine kinases (5, 6) or of mTOR leads to clinical benefit (7); however, these options are not available or suitable for many patients with RCC. The combination of these less than satisfactory responses to chemotherapy and surgery, together with indirect evidence that host immune mechanisms play a significant role in the natural history of RCC, supports continued exploration of the role of immunotherapy in this disease.
A recent Cochrane review concluded that interferon-α (IFN-α) provided a modest survival advantage in comparison to other therapies with an odds ratio for death at one year of 0.56 (95% confidence intervals 0.40 to 0.77) (8). In a recent phase III study, the response rate to high-dose interleukin-2 (IL-2) was 23.2% (22/95 patients), which was superior to low dose IL-2 plus interferon-α2b (9.9%, 9/91 patients) (9). Some long term remissions were described. In another meta-analysis involving 670 patients in 24 trials, patients receiving any cytokine therapy were compared against chemotherapy (10). Cytokine therapy was associated with an improved median survival for all levels of risk, with the benefit mainly seen in favourable and intermediate risk groups. However, only 4.5% of patients survived more than 5 years, although some long term survivors were rendered disease-free with surgery. These data suggest that cytokine-based immunotherapy may still have a role for carefully selected patients.
The G250 antigen (CAIX / MN) is a heat-sensitive transmembrane cell surface antigen homologous to carbonic anhydrase IX (11). G250 is present on more than 85% of RCCs, almost exclusively in the clear cell subtype, but expression in normal tissues is restricted to the gastric epithelium, biliary ducts, and some pancreatic acini (12, 13). Expression of this antigen is most common in clear cell RCC (14). Low CAIX staining is correlated with a worse prognosis (14-16). After treatment with high dose IL-2, survival of RCC patients is prolonged in the group in which G250/CAIX expression is observed, and prolonged survival beyond five years is only seen in the context of G250/CAIX expression (17). This correlation is independent of tumour grade and stage (17).
The murine monoclonal IgG1 antibody G250 recognizes the G250 antigen and has been used in clinical trials (13, 18, 19). The development of human anti-mouse antibody (HAMA) responses, however, precluded repeated administration of the murine antibody. A chimeric form of the antibody (mouse Fv and human Fc) was therefore constructed (cG250). cG250 is an IgG1 kappa chimeric antibody (20) and its specificity is identical to the murine antibody.
cG250 induces ADCC in vitro against G250 positive RCC lines (20), which is enhanced significantly by IL-2 at doses achievable in vivo (21). When IL-2 was added to the culture in concentrations above 10 IU/mL, significant enhancement of ADCC occurred and was maintained for seven days, with associated lymphokine-activated killer (LAK) cell activity observed after three days. Activity was seen at concentrations as low as 1 IU/mL (21).
cG250 has been studied in clinical trials as a single agent, either as cold antibody or labelled with various radioisotopes (19, 22-28). Treatment was well tolerated and human anti-chimeric antibody (HACA) responses were infrequent. cG250 targets clear cell RCC efficiently (22) and does not bind significantly to biliary tract cancers (29). In terms of clinical outcomes, some patients had stable disease and two partial remissions and one complete remission have been reported (23, 27). A phase I multiple dose study has been completed at our site in conjunction with the Mayo clinic (trial LUD 98-011) (30, 31). This trial showed that cG250 is safe, has a long half-life, targets RCC effectively and has some biologic activity (30).
The combination of cG250 with IL-2 has been previously studied. In a phase II trial of 35 patients with progressive RCC, a partial response was demonstrated at week 16 in one patient and 11 patients had stable disease. Clinical benefit, defined as the sum of patients with partial or complete responses and patients with stable disease of at least 24 weeks, was observed in 7 out of 30 evaluable patients (23%). The median survival was 22 months and the 2-year survival 45% (27). The effects of IL-2 on the biodistribution, tumour uptake or pharmacokinetics of cG250 were not assessed in this trial.
In another trial reported to date only in abstract form (32) and recently updated (27), 31 patients with metastatic RCC were treated with cG250 (20 mg weekly for three months) combined with interferon-α2a (3 MIU three times per week subcutaneously). Of the 31 patients, no grade 3 or 4 toxicities or HACA responses were observed. At week 16, 2/26 (8%) evaluable patients had a partial response and 14/26 (54%) had stable disease. Clinical benefit was observed in 11/26 patients (42%) (32). In this study the median survival was 30 months and the 2-year survival 57%.
We hypothesized that a combination of cG250 and low dose IL-2 would be safe, that IL-2 would have no effect on the pharmacokinetics or biodistribution of cG250, and that immunological effects of the combination would be observed.
Results
Patients
Patient characteristics are shown in Table 1. Nine patients (seven males, two females) participated and all were evaluable. The median age was 55 (range 39-71). Four patients had prior immunotherapy; no patient had prior chemotherapy. Three patients had not had prior nephrectomy. For those who had undergone nephrectomy, this had occurred between six weeks to four years prior to the first infusion of cG250. Median Karnofsky performance status was 90% (range 80-100%).
Table 1.
Patient characteristics.
In a retrospective analysis, the following prognostic indicators were determined for each patient where available: presence or absence of prior nephrectomy; time since diagnosis or nephrectomy; baseline haemoglobin; baseline lactate dehydrogenase (LDH). No correlations of these parameters with study outcome were found. The two patients who received an additional cycle of treatment did not have more favourable prognostic indicators than other patients.
Toxicity
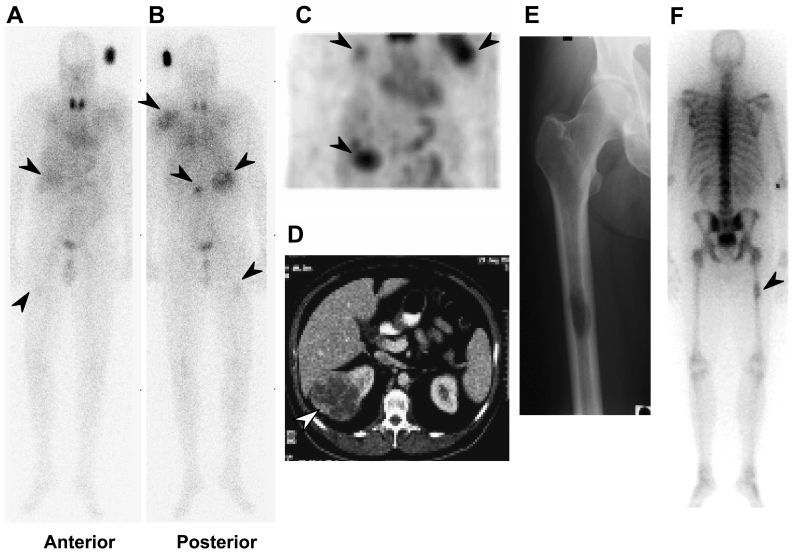
Toxicities are summarized in Table 2. All patients completed the study. Weekly cG250 with daily low-dose subcutaneous IL-2 was well tolerated and most adverse events were attributable to the underlying cancer. No adverse events were thought related to cG250. Adverse events attributable to IL-2 were as expected. IL-2 toxicities were mild to moderate in severity: one patient (patient 3) required two dose reductions over two cycles due to IL-2-related dyspnea. This patient successfully completed the second cycle of treatment after the dose reduction. Two serious adverse events (SAEs) occurred, both in patient 4 (hospitalization for bony metastatic disease); both events were unrelated to the study drugs. This patient was found to have previously undiagnosed metastatic disease in the right femur, which was discovered on gamma camera imaging after the first infusion of 131I-cG250 (Figure 1). The patient was admitted for prophylactic orthopedic fixation. The second hospitalization for this patient was for cancer-related back pain occurring one week after the final cG250 infusion.
Table 2.
Summary of toxicities related to IL-2.
Figure 1.
Biodistribution of 131I-cG250 in patient 4. (A) Anterior and (B) posterior whole body gamma camera image eight days after first infusion. Tumour uptake of 131I-cG250 (arrows) is seen in the right kidney (primary) and in the left scapula and L2 vertebra, as well as a previously undiagnosed right femur metastasis. (C) Single photon emission computed tomography (SPECT) 3D anterior coronal image of the abdomen and chest, showing uptake in tumour (arrows) in the right kidney, right upper rib and left scapula. (D) CT scan showing right kidney tumour (arrow). (E) Anteroposterior X-ray image of right femur showing lytic metastasis. (F) Whole body bone scan (posterior view) showing the previously undiagnosed right femur metastasis.
Haemoglobin fell slightly in all patients but this was not clinically significant. Mild eosinophilia was observed as expected for IL-2. No significant changes in patient blood chemistry were observed. Thyrotropin (TSH) fell to below normal in 3/9 patients, but there was no clinical or other biochemical evidence to suggest IL-2-induced hyperthyroidism. Subsequent assessment of thyroid function in these patients after the conclusion of the study showed normal TSH levels in two patients; no follow-up sample was available for the remaining patient.
Biodistribution of cG250
No differences in biodistribution and tumour uptake between the first and final 131I-cG250 infusion gamma camera images were observed in any patient. Targeting of tumour was excellent (Figure 1). The initial pattern of 131I-cG250 biodistribution in all patients was consistent with blood pool activity that cleared gradually with time (data not shown). Minor activity in the gut was also observed, similar to that seen in past cG250 protocols. Some visualization of thyroid and stomach was observed, related to dehalogenation of 131I-cG250 in vivo (Figure 1). Distinct uptake of 131I-cG250 in sites of primary and/or metastatic RCC where lesions were larger than 1.5 cm was evident in all patients, often as early as day 2 post-infusion. One occult metastatic lesion in the left femur of patient 4 was also detected (Figure 1). No other uptake of 131I-cG250 was evident in normal tissues.
Pharmacokinetics of cG250
Summarized data for infusion 1 are presented in Table 3. By measurement of blood 131I radioactivity, the initial (T½α) and terminal (T½β) half-lives of 131I-cG250 were 20.16 ± 6.59 h and 126.21 ± 34.04 h respectively (mean ± standard deviation). There was no significant difference in pharmacokinetic parameters of 131I-cG250 between infusions 1 and 5 (data not shown). Cmax and Cmin values measured by ELISA showed increasing levels with consecutive infusions. The Cmin value for infusion 1 as determined by ELISA was 1.41 ± 0.75 µg/mL. This indicates that the 10 mg/m2 dose of cG250 produced serum values greater than 1 µg/mL in most patients at one week post-infusion of cG250, a concentration that reproducibly produces in vitro ADCC of G250 target cells (21).
Table 3.
Pharmacokinetic parameters for cG250 first infusion.
Immunological assays
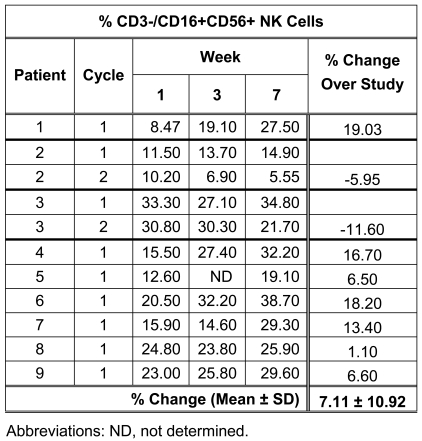
FACS analysis demonstrated a trend for an increase in the percentage of circulating CD3-/CD16+CD56+ NK cells during the study (Table 4). Small increases or decreases in total white blood cell counts and total lymphocyte counts, as well as CD3+/CD4+ and CD3+/CD8+ lymphocyte subsets, were observed in all patients during the study (data not shown).
Table 4.
Effects of IL-2 on CD3-/CD16+CD56+ NK cells during study.
The level of ADCC at the start of the study was patient dependent and did not correlate with clinical outcome. Specific ADCC of 5-15% was observed in 3/9 patients (patients 1, 5 and 8) and a low specific ADCC of <5% was observed in the other 6/9 patients.
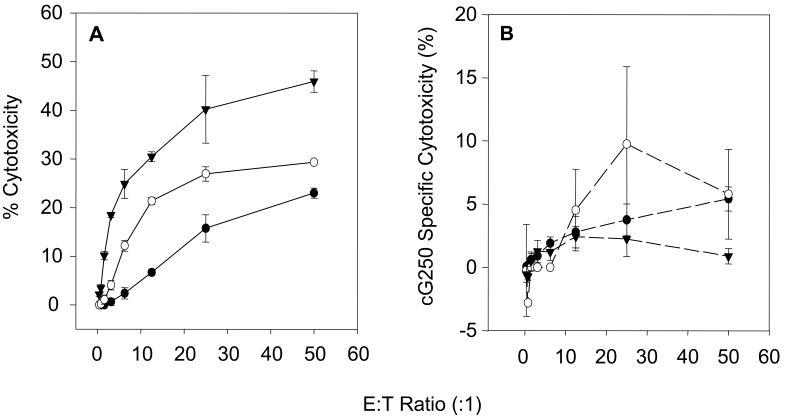
The observed level of ADCC and NK cell (LAK) activity varied greatly between individual patients and no consistent pattern was observed. Patient 3 demonstrated increasing ADCC activity throughout the study (Figure 2); however, no change to cG250 specific ADCC was observed for 7/9 patients on study, and patient 1 demonstrated a drop in activity over the course of treatment.
Figure 2.
ADCC and/or NK cell (LAK) activity of PBMCs isolated from patients 1 and 3. Week 1 (filled circles), week 3 (open circles) and week 7 (filled triangles). (A) LAK activity of patient 1, showing increasing LAK activity during the study. (B) ADCC activity of patient 3 (cycle 2 data). An increase in ADCC activity (E:T ratio 25:1) at week 3 was observed, which decreased to baseline level by week 7.
A marked increase in NK cell (LAK) activity was observed for patients 1, 2, 3 and 5 (Figure 2) while patients 4 and 8 demonstrated increased NK cell activity at week 3. The increased LAK activity correlated with increased NK cell (LAK) numbers for patients 1 and 5. In contrast, patients 2 and 3 demonstrated a decrease in NK cell numbers over the 2 cycles of therapy (Table 4). No change was observed in the NK cell (LAK) activity of the other patients, despite increased NK cell numbers being observed for these patients over the study period.
Human anti-chimeric antibody (HACA) assay
All sera tested were HACA negative. High background interference with the ELISA analysis was apparent in all samples from patient 4. However, a positive immune response was not observed and clinical manifestations of HACA were absent.
Clinical outcomes
No antitumour effects were observed. Seven patients had progressive disease at the end of cycle 1. At completion of cycle 1, two patients (patients 2 and 3) had stable disease. These patients were offered and successfully completed a second cycle of treatment. At restaging at the end of that cycle, one had continued stable disease and one had progressive disease. Neither patient was eligible for further treatment.
Statistics
The primary objectives of this pilot study were to assess the safety and immunogenicity of cG250 and IL-2 in patients with advanced renal cell carcinoma. Nine patients were planned to be accrued. For the safety component, if two or fewer of the planned nine patients experienced a dose-limiting toxicity, the study had approximately 90% power to show that the probability of dose-limiting toxicity was less than 0.49. With respect to immunological efficacy, the error rates of missing a promising agent or selecting a non-promising agent were set to be at most 10% and were computed employing an empirical Bayes formulation, using a large set of exploratory vaccine trials performed at Memorial Sloan-Kettering Cancer Center (33). Clinical responses and pharmacokinetic parameters were not primary endpoints and were to be reported descriptively.
Discussion
This trial confirms that treatment with weekly infusions of cG250 for six doses is safe. The only significant adverse events during the study were due either to the underlying disease or to concurrent IL-2, with no adverse events attributable to cG250. However, the addition of low-dose IL-2 to weekly cG250 was generally well tolerated.
The half-life of cG250 was found to be long, and blood trough levels of cG250 were easily detectable at the time of the subsequent dose. The calculated pharmacokinetic results (Table 3) showed no significant difference to the values determined for our previous study LUD98-011 (31). These values are longer that those observed for murine G250 (13, 18) and are highly comparable with values observed for other chimeric antibodies. Results obtained for clearance, volume of distribution and Cmax were also comparable to the reported human cG250 trials. It can be concluded that IL-2 as used in this study did not affect cG250 pharmacokinetics.
Targeting of cG250 to tumour was excellent. In at least one patient, a clinically significant but previously undiagnosed metastasis was detected by gamma camera imaging following the first infusion of 131I-cG250. Most sites of disease, particularly if greater than 1.5 cm in size, were easily detected by scanning after 131I-cG250 infusion. The pattern of biodistribution of 131I-cG250 was similar to our previous cG250 protocol LUD 98-011 (31) and an earlier cG250 study (17) and was consistent in all nine patients.
IL-2 did not increase the incidence of HACA; indeed, HACA was not detected during the study. Variable effects were observed with respect to white blood cell count, lymphocyte count, and lymphocyte subset (CD3+/CD4+ and CD3+/CD8+) analysis. A trend for increase in CD3-/CD16+CD56+ NK cell numbers was also observed. The increase in CD3-/CD16+CD56+ NK cell numbers in this study due to IL-2 treatment is consistent with previous reported trials (34, 35). Some patients showed enhanced ADCC or NK cell (LAK) activity, but this was not a consistent finding. Prior trials have also shown only modest increases in ADCC and NK cell (LAK) activity following cytokine treatment in cancer patients (34); this is most likely explained by the suppression of immune responses in patients with advanced or metastatic cancer.
Overall, the results from this study are comparable to those from our previous phase I study using cG250 alone (31). In that study, cG250 was well tolerated. Clinical effects were observed in that trial but not in the current trial; this discrepancy may be due to the small number of patients treated.
Another trial involved 35 patients with progressive clear cell RCC after nephrectomy (36). Patients received cG250 20 mg weekly (week 2 to 12) combined with daily low dose IL-2 (1.8 MIU daily), with a pulse of 5.4 MIU on days 1 and 3 of weeks 3, 5, 7, 9 and 11 for 12 weeks. In week 16, a partial response was demonstrated in one patient and stable disease in 11 patients. Seventeen patients with clinical benefit at week 16 received further treatment. The percentage of peripheral blood NK cells increased but lytic activity remained stable. The combination therapy was safe and well tolerated, although HACA was observed in 2 of 28 evaluable patients without apparent clinical sequelae. In agreement with our observations, an increase in NK cell populations and ADCC / NK cell activity following IL-2 treatment with cG250 were also reported. This trial, however, did not evaluate the biodistribution of cG250 following IL-2 treatment, nor the impact of IL-2 on cG250 pharmacokinetics, as was performed in our study.
Monoclonal antibody cG250 treatment has a potential role in the management of metastatic RCC; however, particular patient subgroups that may benefit from this treatment need to be delineated. Other investigators have used combinations of cG250 with cytotoxic drugs such as vinblastine (37). A large international phase III trial is underway to study the effects of adjuvant therapy with cG250 after resection of RCC (38). Future work will address other methods for enhancing the anti-tumour effect of cG250/CAIX-targeted therapies, such as radioimmunotherapy approaches with 131I-cG250 (39, 40), 90Y-cG250 and 177Lu-cG250, or combining cG250 with other therapeutics that have activity in RCC, such as sunitinib.
Abbreviations
- RCC
renal cell carcinoma
Acknowledgements
We gratefully acknowledge the staff of the Austin Hospital Department of Nuclear Medicine and of the Cancer Clinical Trials Centre. This study was sponsored by the Ludwig Institute for Cancer Research. Part of this study has been presented at the 2004 annual meeting of the American Society of Clinical Oncology (ASCO) in New Orleans, LA.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Tsui KH, Shvarts O, Smith RB, Figlin RA, deKernion JB, Belldegrun A. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090–1095. doi: 10.1016/s0022-5347(05)67699-9. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–417. [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. The Cochrane Database of Systematic Reviews. 2004. Immunotherapy for advanced renal cell cancer. Issue 3. Art. No.: CD001425. DOI: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- 9.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CP, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB. Randomized phase III trial of high-dose interleukin-2 versus subcutaneousinterleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 10.Pyrhönen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela T, Juusela H, Rintala E, Hietanen P, Kellokumpu-Lehtinen PL. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol. 1999;17:2859–2867. doi: 10.1200/JCO.1999.17.9.2859. [DOI] [PubMed] [Google Scholar]
- 11.Uemura H, Nakagawa Y, Yoshida K, Saga S, Yoshikawa K, Hirao Y, Oosterwijk E. MN/CA IX/G250 as a potential target for immunotherapy of renal cell carcinomas. Br J Cancer. 1999;81:741–746. doi: 10.1038/sj.bjc.6690757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, Pauwels EK, Jonas U, Zwartendijk J, Warnaar SO. Monoclonal antibody G250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38:489–494. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- 13.Oosterwijk E, Bender NH, Divgi CR, Welt S, Wakka JC, Finn RD, Carswell EA, Larson SM, Warnaar SO, Fleuren GJ, Oettgen HF, Old LJ. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol. 1993;11:738–750. doi: 10.1200/JCO.1993.11.4.738. [DOI] [PubMed] [Google Scholar]
- 14.Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 15.Bui MHT, Visapaa H, Seligson D, Kim H, Han KR, Huang Y, Horvath S, Stanbridge EJ, Palotie A, Figlin RA, Belldegrun AS. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461–2466. doi: 10.1097/01.ju.0000116444.08690.e2. [DOI] [PubMed] [Google Scholar]
- 16.Said J. Biomarker discovery in urogenital cancer. Biomarkers. 2005;10(Suppl 1):S83–S86. doi: 10.1080/13547500500215050. [DOI] [PubMed] [Google Scholar]
- 17.Atkins M, Regan M, McDermott D, Mier J, Stanbridge E, Youmans A, Febbo P, Upton M, Lechpammer M, Signoretti S. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 18.Divgi CR, Bander NH, Scott AM, O'Donoghue JA, Sgouros G, Welt S, Finn RD, Morrissey F, Capitelli P, Williams JM, Deland D, Nakhre A, Oosterwijk E, Gulec S, Graham MC, Larson SM, Old LJ. Phase I/II radioimmunotherapy trial with iodine-131-labeled mo-noclonal antibody G250 in metastatic renal cell carcinoma. Clin Cancer Res. 1998;4:2729–2739. [PubMed] [Google Scholar]
- 19.Oosterwijk E, Divgi CR, Brouwers A, Boerman OC, Larson SM, Mulders P, Old LJ. Monoclonal antibody-based therapy for renal cell carcinoma. Urol Clin North Am. 2003;30:623–631. doi: 10.1016/s0094-0143(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 20.Surfus JE, Hank JA, Oosterwijk E, Welt S, Lindstrom MJ, Albertini MR, Schiller JH, Sondel PM. Anti-renal-cell carcinoma chimeric antibody G250 facilitates antibody-dependent cellular cytotoxicity with in vitro and in vivo interleukin-2-activated effectors. J Immunother Emphasis Tumor Immunol. 1996;19:184–191. doi: 10.1097/00002371-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Smyth FE, Renner C, Lee FT, Oosterwijk E, Scott AM. Anti-renal cell carcinoma chimeric antibody G250: cytokine enhancement of in vitro antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother. 2002;51:171–177. doi: 10.1007/s00262-002-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steffens MG, Boerman OC, Oosterwijk-Wakka JC, Oosterhof GO, Witjes JA, Koenders EB, Oyen WJ, Buijs WC, Debruyne FM, Corstens FH, Oosterwijk E. Targeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250. J Clin Oncol. 1997;15:1529–1537. doi: 10.1200/JCO.1997.15.4.1529. [DOI] [PubMed] [Google Scholar]
- 23.Steffens MG, Boerman OC, de Mulder PH, Oyen WJ, Buijs WC, Witjes JA, van den Broek WJ, Oosterwijk-Wakka JC, Debruyne FM, Corstens FH, Oosterwijk E. Phase I radioimmunotherapy of metastatic renal cell carcinoma with 131I-labeled chimeric monoclonal antibody G250. Clin Cancer Res. 1999;5(10 Suppl):3268s–3274s. [PubMed] [Google Scholar]
- 24.Varga Z, de Mulder P, Kruit W, Hegele A, Hofmann R, Lamers C, Warnaar S, Mala C, Ullrich S, Mulders P. A prospective open-label single-arm phase II study of chimeric monoclonal antibody cG250 in advanced renal cell carcinoma patients. Folia Biol (Praha) 2003;49:74–77. [PubMed] [Google Scholar]
- 25.Divgi CR, O'Donoghue JA, Welt S, O'Neel J, Finn R, Motzer RJ, Jungbluth A, Hoffman E, Ritter G, Larson SM, Old LJ. Phase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancer. J Nucl Med. 2004;45:1412–1421. [PubMed] [Google Scholar]
- 26.Bleumer I, Knuth A, Oosterwijk E, Hofmann R, Varga Z, Lamers C, Kruit W, Melchior S, Mala C, Ullrich S, De Mulder P, Mulders PF, Beck J. A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer. 2004;90:985–990. doi: 10.1038/sj.bjc.6601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neville N, Bevan P, Klöpfer P, Mala C, Hofmann R, Kindler M, Siebels M, Oberneder R. Treatment with monoclonal antibody cG250 (Rencarex®) in combination with IFNα-2a significantly prolongs survival in patients with metastatic renal cell cancer patients.; 5th Kidney Cancer Symposium; 2006. September 22-23; Chicago (IL). [Google Scholar]
- 28.Divgi CR, Pandit-Taskar N, Jungbluth AA, Reuter VE, Gönen M, Ruan S, Pierre C, Nagel A, Pryma DA, Humm J, Larson SM, Old LJ, Russo P. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol. 2007;8:304–310. doi: 10.1016/S1470-2045(07)70044-X. [DOI] [PubMed] [Google Scholar]
- 29.Hendrickx BW, Punt CJ, Boerman OC, Postema EJ, Oosterwijk E, Mavridu A, Corstens FH, Oyen WJ. Targeting of biliary cancer with radiolabeled chimeric monoclonal antibody CG250. Cancer Biother Radiopharm. 2006;21:263–268. doi: 10.1089/cbr.2006.21.263. [DOI] [PubMed] [Google Scholar]
- 30.Wiseman GA, Scott AM, Lee FT, Gansen W, Hopkins W, Steinmetz S, Ingle JN, Croghan GA, Burch PA, Davis I, Moynihan TJ, Warnaar S, Ullrich S, Mason JV, Pugliese L, Divgi CR, Hoffman EW, Old LJ. Chimeric G250 (cG250) monoclonal antibody phase I dose escalation trial in patients with advanced renal cell carcinoma (RCC).; Proc Am Soc Clin Oncol; 2001. p. 257a. May 12-15; San Francisco (CA); Abstract 1027. [Google Scholar]
- 31.Davis ID, Wiseman GA, Lee FT, Gansen DN, Hopkins W, Papenfuss AT, Liu Z, Moynihan TJ, Croghan GA, Adjei AA, Hoffman EW, Ingle JN, Old LJ, Scott AM. A phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinoma. Cancer Immun. 2007;7:13. http://www.cancerimmunity.org/v7p13/070713.htm [PMC free article] [PubMed] [Google Scholar]
- 32.Bevan P, Mala C, Kindler M, Siebels M, Oberneder R, Beck HJ. Results of a phase I/II study with monoclonal antibody CG250 in combination with IFN α-2a in metastatic renal cell carcinoma patients.; J Clin Oncol; 2004. p. 4606. [Google Scholar]
- 33.Yao TJ, Begg CB, Livingston PO. Optimal sample size for a series of pilot trials of new agents. Biometrics. 1996;52:992–1001. [PubMed] [Google Scholar]
- 34.Meropol NJ, Barresi GM, Fehniger TA, Hitt J, Franklin M, Caligiuri MA. Evaluation of natural killer cell expansion and activation in vivo with daily subcutaneous low-dose interleukin-2 plus periodic intermediate-dose pulsing. Cancer Immunol Immunother. 1998;46:318–326. doi: 10.1007/s002620050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meropol NJ, Porter M, Blumenson LE, Lindemann MJ, Perez RP, Vaickus L, Loewen GM, Creaven PJ, Wilkes KA, Giedlin MA, Caligiuri MA. Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res. 1996;2:669–677. [PubMed] [Google Scholar]
- 36.Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC, Voller MC, Melchior S, Warnaar SO, Mala C, Beck J, Mulders PF. A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol. 2006;175:57–62. doi: 10.1016/S0022-5347(05)00040-6. [DOI] [PubMed] [Google Scholar]
- 37.Al-Batran SE, Neumann A, Atmaca A, Ruppert M, Karbach J, Ritter G, Hoffman E, Old L, Knuth A, Jaeger E. LUD01-014: Phase 1/2 study of chimeric monoclonal antibody cG250 in combination with vinblastine in patients with advanced renal cell carcinoma (ARCC).; J Clin Oncol; 2004. p. 2531. [Google Scholar]
- 38.WILEX AG - Pipeline. http://www.wilex.com/R&D/Pipeline.htm Accessed 18 July 2007.
- 39.Brouwers AH, Mulders PF, de Mulder PH, van den Broek WJ, Buijs WC, Mala C, Joosten FB, Oosterwijk E, Boerman OC, Corstens FH, Oyen WJ. Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. J Clin Oncol. 2005;23:6540–6548. doi: 10.1200/JCO.2005.07.732. [DOI] [PubMed] [Google Scholar]
- 40.Brouwers AH, Buijs WC, Mulders PF, de Mulder PH, van den Broek WJ, Mala C, Oosterwijk E, Boerman OC, Corstens FH, Oyen WJ. Radioimmunotherapy with [131I]cG250 in patients with metastasized renal cell cancer: dosimetric analysis and immunologic response. Clin Cancer Res. 2005;11:7178s–7186s. doi: 10.1158/1078-0432.CCR-1004-0010. [DOI] [PubMed] [Google Scholar]
- 41.Common Toxicity Criteria. http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf Version 2.0; dated April 30, 1999.
- 42.Uemura H, Okajima E, Debruyne FM, Oosterwijk E. Internal image anti-idiotype antibodies related to renal-cell carcinoma-associated antigen G250. Int J Cancer. 199456:609–614. doi: 10.1002/ijc.2910560424. [DOI] [PubMed] [Google Scholar]
Materials and methods
Trial design
Trial LUD00-010 was an open-label pilot study to investigate the effects of combining weekly infusions of cG250 with daily subcutaneous injections of low-dose IL-2 (Proleukin, Chiron). The primary objectives were (a) safety and (b) immunological effects of the combination. Safety was assessed by toxicity as defined by the National Cancer Institute Common Toxicity Criteria (CTC) Scale Version 2.0 (41). Immunological endpoints were serum HACA activity, peripheral blood natural killer cell (NK) phenotype as determined by flow cytometry, and peripheral blood ADCC activity. Secondary objectives of the study were to determine the blood pharmacokinetics, organ distribution and tumour distribution of cG250 given weekly in combination with daily low dose IL-2, and to evaluate the clinical efficacy of the combination.
Inclusion criteria were as follows: unresectable locally advanced or metastatic, histologically proven, clear cell carcinoma of the kidney; progressive disease prior to study entry, defined as an increase in the size of disease on two clinical or radiological assessments at least one month apart; measurable or evaluable disease; at least four weeks after chemotherapy, radiotherapy or immunotherapy; no other effective therapy available or appropriate; full recovery from surgery; expected survival of at least three months; Karnofsky performance status of at least 70%; adequate bone marrow, renal and hepatic function; age at least 18 and able to give written informed consent. Exclusion criteria: prior exposure to monoclonal antibody unless there was no evidence of circulating human anti-mouse, anti-chimeric, or anti-human antibody; active central nervous system metastases (unless adequately treated and no evidence of progression for at least three months); positive HIV test; other clinically significant medical illness; other coexisting or previous malignancy within five years (except treated cervical carcinoma in situ or treated non-melanoma skin cancer; concomitant treatment with systemic glucocorticoids, anti-histaminic or non-steroidal anti-inflammatory drugs (unless used in low doses for prevention of an acute cardiovascular event); participation in a clinical trial involving another investigational agent within 4 weeks; pregnancy or lactation; women of childbearing potential not using medically acceptable means of contraception; psychiatric or addictive disorders that may compromise the ability to give informed consent; lack of availability of a patient for immunological and clinical follow-up assessment. All patients provided written informed consent prior to any study-specific procedures. The trial protocol was approved by the Human Research Ethics Committee of Austin Hospital and was performed under the Australian Therapeutic Goods Administration Clinical Trials Notification (CTN) scheme and FDA IND BB-8023. All patients were treated at the Austin Hospital in Heidelberg, Melbourne, Australia.
The treatment regimen was as follows. A total of 6 doses of cG250 (Biological Production Facility, Ludwig Institute for Cancer Research, Melbourne, Australia) were given at 10 mg/m2/dose, infused over 30 minutes once weekly; the first and fifth weekly doses were trace-radiolabeled with 8-10 mCi (296-370 MBq) iodine-131 (131I) to facilitate the evaluation of cG250 biodistribution and pharmacokinetics. IL-2 (1.25 x 106 IU/m2/day) was administered by subcutaneous injection, commencing on the day of the first cG250 infusion. Patients received potassium iodide solution, 10 drops orally twice a day for seven days, beginning on the morning of the 131I-cG250 infusions (day 1 and 29). Dose modifications of cG250 (reduction in infusion rate or cessation of infusion) were permitted for adverse events occurring during the infusion. If dose-limiting toxicity attributable to IL-2 occurred, IL-2 treatment was to be withheld until the toxicity had resolved to grade 2 or less. Subsequent doses were to be reduced by 50%, with a further 50% reduction (to 25% of the initial dose) permitted if necessary. If further dose reductions were required then the patient was to be removed from the study.
Patients with stable or responding disease were permitted to receive a second six-week cycle of treatment, to commence between four and eight weeks after completion of the first cycle. Only patients with responding disease and with no evidence of HACA were eligible to receive a third or subsequent treatment. Patients who demonstrated an increased clearance of 131I-cG250 during week 5 of cycle 1 were not eligible to receive further treatment cycles. Increased clearance was defined as more than 50% decrease in the half-life of whole body clearance of 131I-cG250, in conjunction with reduced tumour uptake compared to that observed for week 1 imaging.
Blood samples were collected before treatment and at various time points during the study as described below. Tumour evaluations were performed prior to treatment and two weeks after the sixth dose.
131I-cG250 biodistribution
Infusions of cG250 trace-labelled with 8-10 mCi (296-370 MBq) of 131I were administered in weeks 1 and 5 for determination of tumour targeting, normal organ biodistribution, and pharmacokinetics. Gamma camera imaging with anterior and posterior whole body scans using conjugate view methodology were performed on five occasions after each trace-labelled infusion: 1-4 hours after 131I-cG250 infusion (day 1) and on day 2, days 3 or 4, 5 or 6 and 7 or 8. A standard was included in the field of view at each imaging time point. Single-photon emission computed tomographic (SPECT) imaging of relevant areas of disease were performed on at least one occasion following 131I-cG250 infusion.
Pharmacokinetic studies
Pharmacokinetics of cG250 were determined by measurement of radioactivity in blood samples after 131I-cG250 infusion (weeks 1 and 5), and by measurement of cG250 protein in blood by ELISA. Blood samples for these assays were drawn in weeks 1 and 5 on the day of infusion (immediately prior to the infusion, 10 minutes, 1 hour, and 4 hours after completion of the infusion), day 2, days 3 or 4, 5 or 6 and 7 or 8, and were stored at -70˚C to -80˚C for later ELISA analysis. Blood samples (10 mL) were collected for cG250 trough and peak serum levels immediately prior to each cG250 infusion and one hour after the completion of the infusion at infusions 2, 3, 4 and 6.
Serum obtained after infusion of 131I-cG250 was counted in a gamma counter (Packard Instruments, Canberra, Australia). Duplicate standards prepared from the injected material were counted at each time point with serum samples to enable calculations to be corrected for the isotope’s physical decay. The results were expressed as percent injected dose per liter serum (%ID/L) and converted to µg/mL for pharmacokinetic calculations.
Chimeric G250 antibody levels in serum samples, standards or relevant control antibody samples were assessed by a sandwich ELISA. Briefly, wells of microtiter plates were coated with anti-G250 idiotype (NUH-82) monoclonal antibody (42). Biotinylated NUH-82 was used as the tracer antibody. Biotinylation of NUH-82 was performed according to the manufacturer's instructions (Pierce, Australia). The range of measurement was 3.15 ng/mL to 10.0 µg/mL after half-log serial dilutions of serum. All samples were assayed in triplicate and were diluted by a factor of at least 1:2.
Pharmacokinetic parameters were estimated from individual patient 131I and pooled ELISA serum clearance data using a curve fitting program (WinNonlin, Pharsight Co., Mountain View, CA). A two-compartment model with macro-parameters, no lag and a bolus i.v. was used to calculate pharmacokinetic parameters of Cmax (maximum serum concentration); AUC (area under the serum concentration curve extrapolated to infinite time); CL (total serum clearance); T½α and T½β (half lives of the initial and terminal phases of disposition); and V1 (volume of central compartment).
Immunological assays
Flow cytometry
Flow cytometry was performed on peripheral blood mononuclear cells (PBMCs) to determine lymphocyte numbers, T cell subsets, NK numbers and phenotype. The following markers were examined in the following combinations: CD3+/CD4+ (CD4+ T cells), CD3+/CD8+ (CD8+ T cells), CD3-/CD16+CD56+ (NK cells), CD20+ (B cells), and CD14+ (monocytes). Blood samples (10 mL) for flow cytometry were collected prior to the infusion on day 1, prior to the third infusion, and at day 43 after completion of the treatment cycle. Direct immunofluorescence was performed on the day of sample collection using standard techniques on a Coulter Epics Elite ESP flow cytometer (Coulter Corporation, Miami, Florida, USA). Lymphocyte population subsets in the peripheral blood of patients were expressed as the percentage of lymphocytes and total number in 1 mL blood. CD14+ macrophages were expressed as a percentage of all white blood cells and the total number in 1 mL blood. Total number of each subclass of lymphocytes from 1 mL of fresh blood was calculated according to the FACS and blood count data.
ADCC and NK activity
Patient blood samples were collected before treatment (week 1) and at weeks 3 and 7 for assessment of immune function and white blood cell profiles. Freshly isolated PBMCs were tested in triplicate with G250-positive SK-RC-52 target cells at an effector:target (E:T) ratio range of 50:1 to 0.39:1 in the presence of a predetermined optimal concentration of cG250 (1 µg/mL) (21) or IgG1 isotype control (huA33; Ludwig Institute for Cancer Research, Melbourne, Australia) to evaluate ADCC (24). To evaluate the nonspecific cellular lytic activity of patient PBMCs, K562 cells were used as targets at E:T ratios of 50:1 to 0.39:1, in the absence of cG250 antibody. After incubation at 37˚C for four hours, cells were pelleted by centrifugation and 50 µL of supernatant harvested for quantification of 51Cr release. Controls included in the assay corrected for spontaneous release (medium alone) and total release (10% Tween20/PBS). The percentage cell lysis (cytotoxicity) was calculated according to the following formula: Percentage cytotoxicity = [(sample counts - spontaneous release) x 100]/(total release - spontaneous release). Specific cytotoxicity mediated by cG250 was determined by subtracting any activity observed with isotype control antibody at each of the E:T ratios examined. Cytotoxicity assessments were performed in triplicate and the mean values reported with standard deviations. The data were expressed as specific cG250 cytotoxic activity (ADCC) on target SK-RC-52 cells, and NK cell (or LAK) activity on target K562 cells at different E:T ratios.
Human anti-chimeric antibody assays
Patient serum samples for HACA assessment were collected prior to each infusion, at day 43 and, where possible, three months after patients completed treatment. The assay of anti-cG250 antibodies in human serum was performed by ELISA based upon previously published methods (22). Serum samples (450 µL) were diluted 1:2 and then serially diluted for analysis in duplicate. The cG250 anti-idiotype monoclonal antibody NUH-82 was included as a positive control. The limit of quantitation (in samples at minimum dilution) was 16 ng/mL for the NUH-82 anti-idiotype Ab and 150 ng/mL for anti-IgG Abs. Samples with values greater than the anti-idiotype NUH-82 limit of quantitation (16 ng/mL) were considered HACA positive. Results were reported as "negative" (<16 ng/mL) or "positive" (with the titer of anti-cG250 antibodies provided in ng/mL).