Abstract
Recent results have shown a correlation between survival and frequency of tumour infiltrating T lymphocytes in colorectal cancer patients. However, it remains unclear whether the frequency of regulatory T cells is higher in colorectal cancer as compared to normal colon. To address this question we analysed the frequency and function of regulatory T cells in the peripheral blood and tumour infiltrating lymphocytes of colorectal cancer patients. The proportion of regulatory T cells in the peripheral blood of colorectal cancer patients (mean 8%) was significantly higher than that in normal controls (mean 2.2%). There were significantly more regulatory T cells in tumour infiltrating lymphocytes (mean 19.2%) compared to lymphocytes from an autologous non-malignant portion of the colon (mean 9%). Regulatory T cells from colorectal cancer patients were FOXP3 positive and suppressed the proliferation of autologous CD4+ CD25- cells. A higher density of tumour infiltrating regulatory T cells was found in patients with advanced as compared to early disease. These results support the hypothesis that increased numbers of regulatory T cells in the blood and tumours of colorectal cancer patients may influence the immune response against cancer and suggest that strategies to overcome regulatory T cell activity may be beneficial in the treatment of human colorectal cancer.
Keywords: human, colorectal cancer, regulatory T cells, FACS, immunohistochemistry
Introduction
There has been renewed interest in regulatory T (Treg) cells over the last 10 years and in the role they play in modulating immune responses to self and foreign antigens. Regulatory T cells are a heterogeneous group of cells, including CD4+ CD25+ (Treg) T cells, Tr1 and Th3 regulatory T cells (1, 2) and CD8+ regulatory T cells (3). The CD4+ CD25+ Treg cells are currently the best defined and characterised among the various subgroups of regulatory T cells (4). They account for approximately 1-3% of CD4+ T cells in human peripheral blood lymphocytes (PBLs) and about 5-10% of CD4+ T cells in the mouse spleen. These cells are characteristically CD4+ CD25+ CTLA4+ GITR+ in both mice and humans (5-8). Unless these markers are used in combination, it is difficult to differentiate Treg cells from non-Treg cells, as none of the markers are Treg cell specific on their own. FOXP3, a transcription factor whose expression confers the regulatory phenotype on both murine and human T cells, is now thought to represent the most specific Treg cell marker (9-12).
Pages et al. (13) reported a link between metastatic colorectal cancer and a weaker immune response to the tumour, as defined by the frequency of tumour infiltrating CD3+ T cells. This work has recently been extended by assessing the density of T cells in and around colorectal tumours in 415 patient samples (14). They concluded that the density of CD3+ T cells around the tumour proved to be a more efficient prognostic marker than the UICC TNM classification. Quantitative real-time PCR was used to identify a cluster of genes for Th1 adaptive immunity with an inverse correlation to tumour recurrence. The presence of Treg cells in the human gastrointestinal tract (GIT) has recently been demonstrated and their function characterised. It had been previously assumed that Treg cells might be present as the gut is considered a tolerogenic organ and because Treg cells can prevent and treat inflammatory bowel disease (IBD) in mice. Treg cells have been identified in the human gastric and the colonic mucosa (15-17) and have been shown to be important in suppressing tumour-specific immunity in mouse models. Depletion of CD4+ CD25+ T cells in vivo using an anti-CD25 antibody before tumour challenge protected multiple strains of mice from developing tumours (18-21). Patients with ovarian, breast, lung and pancreatic cancer have increased proportions of Treg cells in the peripheral blood and in tumour infiltrating lymphocytes (TILs) (22, 23). However, the frequency, distribution and function of naturally occurring Treg cells in the peripheral blood and the TILs of colorectal cancer patients prior to this study were not well documented.
Results
Increased frequency of CD4+ CD25+ cells in the peripheral blood of colorectal patients
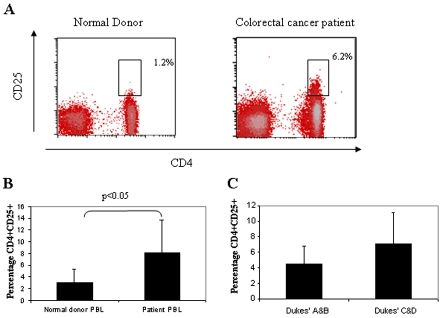
We analysed the frequency of CD4+ CD25+ T cells in the PBLs of 35 patients with colorectal cancer and 10 healthy donors by flow cytometry. Only CD4+ T cells which were CD25hi were considered to represent the Treg cell population, as it has been shown that CD25lo cells contain a heterogeneous pool of activated cells and Treg cells (24). We found that CD4+ CD25hi cells accounted for 1.8% (range 0.5-4%) of all CD4+ T cells in healthy donors (24, 25). In contrast, CD4+ CD25hi cells accounted for 6.2% (range 3.5%-12%) of CD4+ T cells in the PBLs of colorectal cancer patients, P < 0.05 (Figure 1, panels A and B). Although there were fewer Treg cells in the PBLs of patients with Dukes' A and B (4.5%) cancer compared to those with Dukes' C and D (7.1%), this was not a statistically significant difference (Figure 1C). Phenotypic analysis of CD4+ CD25hi T cells demonstrated the expression of CD45RO, CTLA4 and the FOXP3 protein (Figure 2).
Figure 1.
Colorectal cancer patients have more Treg cells in the PBLs than normal donors. (A) Ex vivo FACS staining of peripheral blood lymphocytes (PBLs) from a normal donor and a colorectal cancer patient. The percentage shown is that of CD25+hi lymphocytes in the CD4+ population. (B) Percentage of Treg cells in the PBLs of colorectal cancer (n = 35) and normal patients (n = 10). (C) Percentage of CD4+ CD25+ T cells in the PBLs of patients with Dukes' A and B disease (n = 21) versus Dukes' C and D disease (n = 14).
Figure 2.
CD4+ CD25+ Treg cells express FOXP3, CTLA4 and CD45RO. Shown are the results of a FACS analysis of peripheral blood Treg cells. PBLs from colorectal cancer patients were separated into CD4+ CD25- and CD4+ CD25+ populations and stained for FOXP3, CTLA 4 and CD45RO.
Ability of CD4+ CD25+ Treg cells from colorectal cancer patients' PBLs to suppress T cell proliferation
The functionality of peripheral blood Treg cells from colorectal cancer patients was assessed using 3H thymidine proliferation assays. CD4+ CD25+ T cells from the peripheral blood were isolated by magnetic beads ex vivo. Peripheral blood Treg cells were placed in culture with autologous peripheral blood CD4+ CD25- T cells. Treg cells were capable of suppressing the proliferation of CD4+ CD25- T cells in a dose-dependent manner during a 5-day suppression assay (Figure 3). These results demonstrate that Treg cells isolated from the peripheral blood of colorectal cancer patients are functional and capable of suppressing lymphocyte proliferation.
Figure 3.
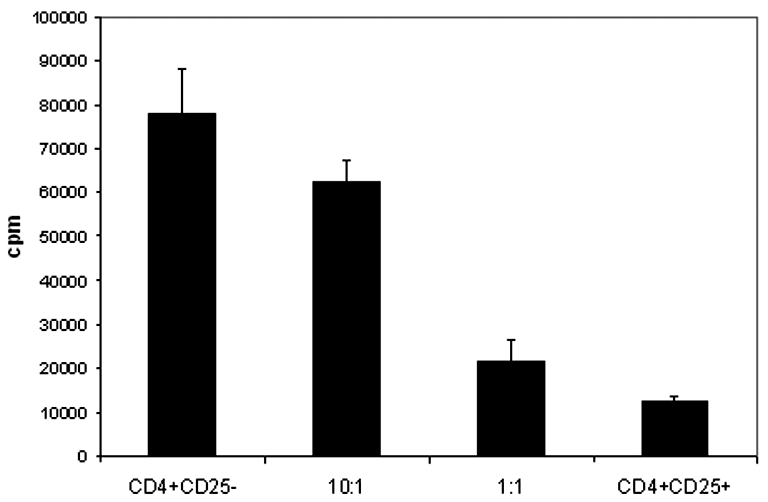
Peripheral blood Treg cells in colorectal cancer patients are functional and suppress proliferation of autologous CD4+ CD25- lymphocytes. Stimulated Treg cells from colorectal cancer patients suppress the proliferation of autologous PBL CD4+ CD25- T cells (n = 3) in a dose-dependent manner. Responder CD4+ CD25- T cells were cultured either alone (left bar) or with different ratios of autologous CD4+ CD25+ Treg cells in the presence of anti-CD3 and anti-CD28 antibodies. Proliferation was assessed by 3H incorporation after 5 days of culture.
Increased frequency of Treg cells in TILs
We then examined the frequency Treg cells infiltrating the colon of colorectal cancer patients. Lymphocytes infiltrating colorectal tumour and normal mucosa were isolated and the frequency of CD4+ CD25+ Treg cells was determined by flow cytometry ex vivo (Figure 4). We observed a significantly higher frequency of Treg cells in the tumour (mean 15.2%, range 8-28%), as compared to the autologous normal colonic mucosa (mean 4.8%, range 3-15%), P < 0.05 (Figure 4, panels A and B) reflecting the findings in peripheral blood. Although there were fewer Treg cells in the tumours of patients with Dukes' A and B (17%) cancer compared to those with Dukes' C and D (21%), this difference was not statistically significant possibly reflecting the low numbers studied (Figure 4C).
Figure 4.
More Treg cells are found in colon cancer stroma compared with normal colonic mucosa. (A) Ex vivo FACS staining of lymphocytes from normal colonic mucosa and from colon tumours. The percentage shown is that of CD25+hi lymphocytes in the CD4+ population. (B) Percentage of CD4+ CD25+ T cells in the tumour and the normal colonic mucosa of colorectal cancer patients (n = 33). (C) Percentage of CD4+ CD25+ T cells in the tumour of patients with Dukes' A and B disease (n = 19) versus Dukes' C and D disease (n = 14).
Immunohistochemical staining of the tumour and normal colon for FOXP3 showed the location of Treg cells in the tissues (Figure 5). In normal colonic mucosa, FOXP3+ cells were found mainly in isolated lymphoid follicles and very few were found in the colonic lamina propria. In contrast, in colorectal cancers, significant numbers of FOXP3+ cells were found in the tumour stroma ,as well as in the lymphoid follicles. No FOXP3+ cells were seen in the normal or malignant colonic epithelium.
Figure 5.
FOXP3-positive cells are found in normal colon, primary and metastatic colon cancer tissue. Immunohistochemical FOXP3 staining in paraffin sections of (A) normal colonic mucosa, (B) normal colon with a lymphoid follicle, (C) primary colorectal cancer and (D) metastatic colorectal cancer.
Suppressive function of tumour infiltrating Treg cells
The suppressive function of Treg cells within the tumour microenvironment was assessed using a 3H thymidine proliferation assay. TILs were sorted ex vivo into CD25+ and CD25- fractions using magnetic beads, and cultured either individually or in the ratios shown in Figure 6. The tumour infiltrating Treg cells suppressed the proliferation of CD25- T cells in a 5-day suppression assay.
Figure 6.

Treg cells from colorectal cancer tissues are functional and suppress autologous CD25- lymphocytes. Tumour infiltrating Treg cells suppress the proliferation of autologous tumour infiltrating CD4+ CD25- T cells (n = 3). Responder CD4+ CD25- T cells were cultured either alone (left bar) or with different ratios of autologous CD4+ CD25+ Treg cells isolated from the tumour, in the presence of anti-CD3 and anti-CD28 antibodies. Proliferation was assessed by 3H incorporation after 5 days of culture.
Tumour infiltrating Treg cell numbers and tumour stage
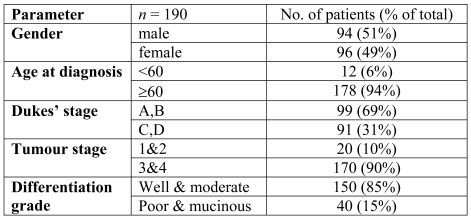
The trend observed towards increased Treg cell numbers in Dukes' stage C and D was further investigated in a larger series of patients. To facilitate the screening of a large number of specimens, tissue arrays of routinely fixed tissues from colorectal tumours and the corresponding normal colon from patients with colorectal cancer were constructed. The characteristics of the colorectal cancer patients whose tumours were arrayed are shown in the Table 1. There was a mean of 33 (range 4-80) FOXP3+ cells per hpf (high power field) in tumours and a mean of 12 (range 0-35) FOXP3+ cells per hpf (P < 0.01) in adjacent normal colon, consistent with the flow cytometry Treg cell data.
Table 1.
Clinical characteristics of patients whose tumours were used in the tissue array.
To determine if the frequency of tumour infiltrating Treg cells correlated with the tumour stage, the number of Treg cells found in the tumours of the patients described in Table 1 was analysed in groups defined by Dukes' stage. There was a lower number of Tregs in the tumours of patients with Dukes' A and B disease, i.e. those with early disease, n = 39 (range 1-122), compared with those with Dukes' C and D disease, n = 45 (range 6-134), but this did not reach statistical significance.
Discussion
Patients with high frequencies of infiltrating CD3+ T cells within their colorectal tumours have been shown to have a better 5-year survival rate (73%) than those with low levels of CD3+ T cells (30%) (14). The adaptive immune response to colorectal cancers therefore appears to have an important role in the progression of this disease. However the role of Treg cells in this process has not yet been defined.
The GIT is considered a 'tolerogenic' organ that responds in a regulated manner to antigens derived from food and microbes. Consistent with this chronic exposure to foreign antigens, the GIT is one of the few normal non-lymphoid tissues where FOXP3+ T cells have been identified in expression screening using a normal tissue array (9).
The frequency of Treg cells within colorectal cancers is probably controlled by more than one factor. Treg cell numbers may be influenced by tumour-associated factors that have been reported in other tumours (26). For example, Ghiringhelli et al. (26) showed in animal models that soluble factors secreted by tumour cells injected into mice and rats converted dendritic cells (DCs) into TGF-β secreting cells which were capable of inducing Treg cell proliferation. These tumour-secreted factors have not yet been identified. While this has not yet been shown with human DCs and tumour lines, a similar mechanism could explain the increased numbers of Treg cells found in the peripheral blood, lymph nodes and tumours of cancer patients.
This model would provide an explanation for Treg cell proliferation in the absence of inflammation and bacteria, as most cancers are sterile.
Bacterial translocation across the mucosal surface is increased in colorectal cancer, possibly due to increased permeability of tight junctions and by necrosis and ulceration of the tumour surface (27, 28). Bacterial translocation also occurs in both inflammatory bowel disease (29) and infective causes of intestinal inflammation and is thought to be associated with the proliferation of Treg cells. Makita et al. (15) and Maul et al. (16) isolated Treg cells from normal and inflamed colon and showed that they were capable of suppressing the proliferation and cytokine production of autologous CD4+ CD25- T cells. Treg cell numbers were increased in the intestinal mucosa of patients with active inflammatory bowel disease (IBD). However, this increase was relatively lower than the increase seen in patients with infective causes of intestinal inflammation, e.g. diverticulitis (16). This suggests that the number of Treg cells in the intestine could be controlled by inflammation. Maul et al. (16) suggested that bacterial products such as LPS or flagellin, working through TLR4 and TLR5 respectively, may be responsible for the increased numbers of Treg cells seen in these inflammatory gastrointestinal conditions. TLR4 and TLR5 signalling have been described in murine Treg cells and LPS shown to stimulate proliferation of Treg cells in vitro (30).
Although a higher number of Treg cells was observed in patients with advanced versus early disease, this was not statistically significant. One reason for this may be that the number of patient samples was too low to reach statistical significance. Alternatively, we cannot rule out the possibility that the frequency of Treg cells in colorectal cancer does not vary with the stage of disease, perhaps due to stimuli from translocated bacteria.
Treg cells were observed in the mucosal lymphoid follicles of both the normal and malignant colon (Figure 5). However, compared with the normal colon where there were fewer Treg cells in the lamina propria, there was an increase in the number of Treg cells in the tumour stroma. It is not known whether Treg cells in vivo exert their function mainly in lymph nodes or in the intestinal lamina propria. However, it would appear that they are present in both the mucosal lymphoid follicles and in the stroma of colon cancers (Figure 5). Treg cells could therefore suppress both the induction of an immune response in the lymphoid tissue, as well as the proliferation and cytotoxic function of effector cells in the tumour stroma.
In humans it has been shown that denileukin diftitox, a diphtheria toxin linked IL2 molecule which binds to CD25 and daclizumab, an anti-CD25 antibody, may be beneficial in some patients with cancer (31). They are believed to act through the elimination of Treg cells, which would then permit the emergence of an effective anti-tumour immune response. It would be interesting to see if daclizumab or denileukin diftitox are beneficial to all cancer patients who have increased numbers of Treg cells or if they are only beneficial in certain types of cancer.
The results presented here extend the published data on Treg cells in other malignancies by demonstrating a significant infiltration of Treg cells into colon carcinomas. This data may prove useful in the future in targeting patients at high risk of relapse for more aggressive adjuvant therapy.
Acknowledgements
This work was funded by CRUK (C399/A6199), and the MRC and the US Cancer Research Institute. SE Pratap was funded by the MRC (G84/6376). We would like to thank Andrea Tarlton for her technical assistance with the work.
References
- 1.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 2.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MGA. CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 3.Filaci G, Suciu-Foca N. CD8+ T suppressor cells are back to the game: are they players in autoimmunity? Autoimmun Rev. 2002;1:279–283. doi: 10.1016/s1568-9972(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 5.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 8.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 9.Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of FOXP3 protein expression in human CD4(+)CD25(+) regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 12.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 16.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Lundgren A, Stromberg E, Sjoling A, Lindholm C, Enarsson K, Edebo A, Johnsson E, Suri-Payer E, Larsson P, Rudin A, Svennerholm AM, Lundin BS. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–531. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. http://www.cancerimmunity.org/v2p1/020201.htm [PubMed] [Google Scholar]
- 19.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 20.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 21.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 23.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 24.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 25.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 28.Lescut D, Colombel JF, Vincent P, Cortot A, Fournier L, Quandalle P, Vankemmel M, Triboulet JP, Wurtz A, Paris JC. Bacterial translocation in colorectal cancers. Gastroenterol Clin Biol. 1990;14:811–814. [PubMed] [Google Scholar]
- 29.Ambrose NS, Johnson M, Burdon DW, Keighley MR. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn's disease surgery. Br J Surg. 1984;71:623–625. doi: 10.1002/bjs.1800710821. [DOI] [PubMed] [Google Scholar]
- 30.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden O. Remission of a refractory, anaplastic large-cell lymphoma after treatment with daclizumab. N Engl J Med. 2004;351:1466–1467. doi: 10.1056/NEJM200409303511424. [DOI] [PubMed] [Google Scholar]
Materials and methods
Patients and samples
The study was approved by the Oxfordshire Clinical Research Ethics Committee. Informed consent was obtained from each patient. Fresh blood, tumour and normal colonic tissues were obtained from patients undergoing surgery for primary colorectal carcinoma (n = 35, mean age 70.3 years, range 50-76). Blood was collected from patients before surgery. Normal colonic tissue was obtained from a macroscopically normal part of the excised colon, at least 10 cm from the tumour margin. None of the patients had previously received radiotherapy or chemotherapy. Blood from 10 normal donors were used as controls. Tissue arrays of colorectal cancer and normal colonic mucosa were generated from the paraffin donor blocks of a separate cohort of 100 patients who had undergone surgery for colorectal cancer.
Lymphocyte extraction
Peripheral blood lymphocytes were isolated from blood by Ficoll density gradient centrifugation.
Tumour infiltrating lymphocytes were isolated from colonic tissues. Freshly isolated tissue was collected in wash medium (RPMI 1640 supplemented with penicillin and streptomycin). The tissue was washed 4 times with wash medium, before being cut into small pieces using blades. The tissue was digested in RPMI-1640 containing 2% FCS and 1mg/ml type IV collagenase (Worthington, Freehold, NJ) for 2 hours at room temperature. A single cell suspension was obtained by passing the digested tissue through a cell strainer. A fraction of the single cell suspension was used for ex vivo FACS staining and the remainder was sorted into a CD4+ CD25+ and a CD4+ CD25- population. The lymphocyte yield was 0.4-0.8 x 106 cells and 0.75-3.5 x 106 cells per gram tissue from normal colon and from colon cancer respectively.
Isolation of CD4+ CD25+ Treg cells
Lymphocytes were isolated from peripheral blood, colorectal cancer and normal colonic mucosa as described above. The CD4+ cells were obtained by negatively sorting the mononuclear cells with CD8 and CD14 magnetic beads (Miltenyi Biotec, GmbH, Germany). These were then positively sorted for CD4+ CD25+ cells using CD25 magnetic beads (Miltenyi Biotec).
Paraffin tissue arrays
To facilitate the screening of a large number of specimens, tissue arrays of routinely fixed tissues from colorectal tumours and the corresponding normal colon from patients with colorectal cancer were constructed. A total of 4 cores of 1 mm diameter were taken from each paraffin block in order to make 4 separate arrays of colorectal cancers and 4 matched arrays of normal colon. Sections cut from these arrays were stained for FOXP3.
Immunohistochemistry
Frozen sections were fixed in acetone, washed in PBS and blocked with goat serum before 50 µl of primary antibody was added for 30 minutes at room temperature. Sections were then washed in PBS and incubated with peroxidase-conjugated goat-anti mouse IgG (Sigma-Aldrich, St. Louis, MO) for 30 minutes. Bound peroxidase activity was developed using AEC kit (Vector, Peterborough, United Kingdom). The sections were counter-stained with hematoxylin.
For staining paraffin sections, slides were first placed in a 60˚C oven for 20 min prior to dewaxing in Citroclear twice for 5 min and rehydration through graded alcohols. Sections were subjected to antigen retrieval by microwaving for 10 minutes in 50 mM Tris pH 9.0 and 2 mM EDTA pH 8.0. Endogenous peroxidase was quenched with the Dako peroxidase block (Dako, Cambridgeshire, UK). The primary FOXP3 antibody was applied for 30 min and subsequent immunostaining was carried out using the DAKO Envision system. The substrate used was DAB (DAKO). The stained sections were counterstained with hematoxylin (Gill's No. 3; Sigma Chemical Co.) and mounted in Aquamount (Merck/BDH). The number of FOXP3 positive lymphocytes present in a high power field (hpf) was counted for each 1 mm core by 2 independent observers and the number of FOXP3+ cells for each core was determined by taking the average of both counts. The number of FOXP3+ cells per hpf for each tumour or normal colon was determined by taking the average of the 4 arrays. In total 98 tumours and 100 normal colon specimens were counted.
Antibodies
The following antibodies were used to stain ex vivo single cell suspensions and in immunohistochemistry staining: CD4-FITC (RPA-T4), CD25-PE (M-A251), CD45RO-APC (UCHL1), CTLA4-APC (BNI3) and HLA-DR APC (L243), all produced by Pharmingen (Oxford, United-Kingdom). FOXP3 murine monoclonal antibodies were as previously described (9). Clone 236A/E7 was used for IHC and clone 150D/E4 was used for FACS staining.
FACS
Cells analysed for FOXP3 by flow cytometric analysis were processed as described previously (9). Flow cytometric analyses were carried out on a FACS Calibur (Becton Dickinson, Oxford, United Kingdom) and data were analysed using the CellQuest software.
3H thymidine suppression assay
This assay was carried out as previously described (9). The assay was performed in 96 well plates (Nalge Nunc, Rochester, NY, USA) coated with anti-CD3 (clone UCHT1, at 5 µg/ml, BD Biosciences Pharmingen). Cells were cultured in RPMI-1640 medium supplemented with 5% human AB serum, 2 mM L-glutamine, 100 U/µg/ml penicillin/streptomycin, 0.5 mM sodium pyruvate, 0.05 mM nonessential amino acids (Gibco/Invitrogen) and soluble anti-CD28 (clone 28.2, at 5 µg/ml, BD Biosciences Pharmingen). The CD4+ CD25- responder cells were cultured at 5 x 104/well and a variable number of CD4+ CD25+ regulatory cells were added. 3H thymidine at 0.5 µCi per well was added for the final 16 h of a 5-day assay.
Statistical analysis
Statistical analysis was carried out using the Student's t-test for continuous variables. Significance was set at 0.05.