Abstract
The idea of generating cytotoxic T-lymphocytes that have anti-tumor activity has been the focus of many clinical trials aimed at delivering effective immunotherapy to cancer patients. We have gained insight into the human immune system in cancer patients as a result of these numerous clinical investigations. It is now clear that although various vaccination methods are capable of inducing tumor antigen-specific T-cells in the circulating blood, these immunological responses are infrequently correlated with clinical responses. Therefore, it appears that priming of a T-cell response is not sufficient for tumor regression and other avenues, downstream of the priming phase, need to be investigated. Mechanisms of immune evasion at the effector phase of the anti-tumor phase are currently under investigation, with an increasing focus on the tumor microenvironment. There is evidence indicating that multiple variables may contribute to immune escape, including: regulatory cells; inhibitory ligands on tumor cells, such as PD-L1 and B7x; soluble factors such as TGF-β and IL-10; and nutrient-catabolizing enzymes, such as indoleamine-2,3-dioxygenase (IDO). In addition, there are ongoing efforts to assess the presence and function of effector cells within the tumor microenvironment. Tumor infiltrating lymphocytes (TILs) have been observed in patients with melanoma, colon cancer, and ovarian cancer. TILs in these patients have been associated with favorable clinical outcomes. In the clinical setting of bladder cancer, as compared to melanoma, there is limited data regarding TILs. This review will focus on immunological responses to bladder cancer and ongoing studies to identify factors that are amenable to therapeutic manipulation.
Keywords: human, bladder cancer, tumor infiltrating lymphocytes, prognosis
Bladder cancer
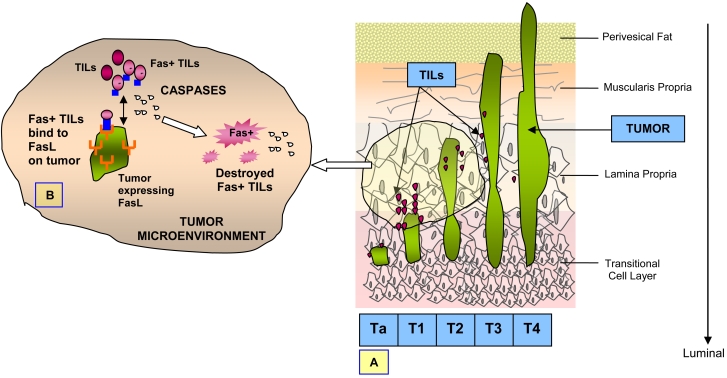
Bladder cancer, the majority of which are diagnosed pathologically as transitional cell carcinoma (TCC) or urothelial carcinoma (UC), is the only solid tumor other than melanoma for which immunotherapy confers a survival benefit. The tumors of the bladder can be classified depending on their depth of invasion. The bladder lining consists of a mucous layer of surface cells, called transitional epithelial cells or the urothelium that expand and deflate depending on the volume of urine present inside the bladder. Disease that affects only the urothelial lining can be papillary and designated as pTa disease. Underneath the urothelium is a thin layer of connective tissue, also called the lamina propria, and tumor in this area is designated as pT1 disease. The next layer is a wider zone of muscle tissue called the muscularis propria, with disease involvement designated as pT2. Beyond this muscular zone, another layer of fatty connective tissue separates the bladder from other nearby organs and disease extending to this perivesical fat is designated as pT3. Disease beyond the bladder and extending to nearby organs or structures is designated as pT4 (Figure 1A). Disease that has spread to distant sites from the bladder is labeled as metastatic.
Figure 1.
Bladder cancer and TILs. (A) Urothelial carcinomas can be classified as pTa (papillary), pT1 (lamina propria invasion), pT2 (muscle invasive), pT3 (invasion to perivesical fat), and pT4 (locally advanced). (B) Interaction of FasL on tumor cells and Fas-positive T-lymphocytes leads to elimination of Fas-positive TILs, which appears to be a caspase-mediated event.
In the setting of non-muscle invasive disease, intravesical bacillus Calmette-Guérin (BCG) is pivotal as an immunological agent in the treatment of early TCC and leads to a cure rate of approximately 90% in select patients, such as those with unifocal and pathological T1 lesions. Intravesical BCG has been used to treat superficial TCC for more than 20 years; randomized trials have shown that BCG results in lower recurrence rates and significantly superior survival rates (1). Although the mechanism of action of BCG is poorly understood, scientific evidence continues to implicate an immunological mechanism. BCG plays a significant role in the maturation of dendritic cells (DCs) by signaling through different Toll-like receptors, as indicated by its upregulation of the DC maturation marker CD83 (2) and secretion of inflammatory cytokines such as IL-12, IFN-γ, and TNF-α. A variety of cytokines has been detected in the urine of patients treated with intravesical BCG, and BCG causes an influx of granulocytes and mononuclear cells into the bladder wall (3-10). Although the significance of these infiltrating cells remains controversial, there are ongoing studies to characterize these cells and assess their functional status.
Immunological alterations that occur with the development of bladder cancer
Apart from the physiological changes in and around the bladder as a result of tumor development, there are several notable immunological changes that occur simultaneously. Changes may be phenotypic in nature, such as the loss or addition of certain molecules on the cell surface of the urothelial cells, or changes may be related to the functionality of certain molecules.
Blood group antigens, major histocompatibility complex (MHC) antigens, and other cellular adhesion molecules which are expressed on the cell surface of normal cells tend to have variable expression on bladder cancer cells. For example, decreased expression and incomplete biosynthesis of certain proteins in the family of blood group antigens have been noted (11-12). It has also been observed that the loss of blood group antigens increases irreversibly with tumor progression. The class I human leukocyte antigen (HLA) in humans, otherwise known as MHC class I antigen, is normally expressed on the urothelial cell surface; however, in urothelial malignancies, it has been observed that there is a loss of HLA class A, B and C expression (13-16). Such a loss of MHC class I antigen expression also directly correlates with tumor progression. Some other molecules that play a role in immuno-oncology and cellular adhesion events also appear to be diminished in their expression in the initial stages of bladder malignancies. Molecules such as CD44 and ICAM-1, which normally promote the adhesion and activation of T-lymphocytes, seem to have decreased expression on bladder cancer cells (17-18).
Furthermore, certain subpopulations of circulating immune cells appear to be associated with clinical outcome in UC patients. Innate immune cells, known as natural killer (NK) cells, obtained from circulating peripheral blood mononuclear cells of bladder cancer patients were studied in 67 patients and 29 healthy donors. A decreased NK cell activity in TCC patients was observed as compared to healthy controls and furthermore, it was noted that disease recurrence was associated with decreased NK cell activity (19).
Another factor which appears to impact the immunological response to bladder cancer is cellular architecture. In one study (20), the impact of physical changes in cellular architecture on autologous T-lymphocyte activation was examined. Two autologous bladder carcinoma cell lines were used as targets for T-lymphocytes. In an effort to compare the effect of cell architecture of these targets on the overall cytokine production levels, the cell lines were grown with varying cellular geometry including a two-dimensional monolayer and a three-dimensional spheroid. Interestingly, it was observed that there was a reduction in cytokine production by T-cells after interaction with the three-dimensional structure of tumor cells as compared to the two-dimensional structure of tumor cells. The data implied that information obtained from a two-dimensional structure of the cells in an in vitro study may not be comparable to in vivo occurrences as mimicked by the three-dimensional tumor microenvironment. Cellular architecture also appears to play a role in the immune response generated in the tumor microenvironment.
Tumor infiltrating lymphocytes in the tumor microenvironment
Tumor infiltrating lymphocytes (TILs) are lymphocytes of the host immune system that have been observed within tumor sites; presumably they migrate to the tumor site in order to combat the growing malignant cells. They are normally activated T cells, natural killer cells and non-T or non-B lymphocytes. These lymphocytes can be physically characterized by cluster differentiation and certain surface-antigen groupings. However, the phenotypic characteristics and the T-cell populations in cultured TILs can be affected by the components of culturing media as demonstrated by Haas et al. (21) and Housseau et al. (22). In the study by Haas et al. (21), the isolation and expansion of human TILs from urological malignancies, including testicular, bladder, and prostate cancer, were examined. The initial TIL population was mainly comprised of CD3+ T-cells, with subpopulations of CD4+ and CD8+ T-cells. The authors observed that over time, the ratio of CD4+ and CD8+ subpopulations varied as a consequence of culture conditions. In the study by Housseau et al. (22), human TILs from primary urothelial carcinomas were characterized. The cytolytic properties of these TILs were examined by the establishment of short-term autologous tumor cell lines for obtaining neoplastic targets with minimal phenotype modifications in comparison with fresh tumor cells; five pairs of autologous TIL/tumor cell pairs were examined. Four of the five TIL cultures manifested lysis against their autologous counterparts and three of these four cytotoxic TIL lines demonstrated MHC class I-dependent cytotoxicity, as established by blocking experiments with anti-HLA class I mAb W6.32. One of these three TIL lines, which demonstrated high levels of cytotoxicity, was further examined and was found to have numerous CD8+ T-cells. The depletion of CD4+ cells from this culture indicated that CD8+ MHC class I CTLs were the predominant effectors.
Even though these studies demonstrated that there are effector cells in urothelial carcinomas that are capable of specific cytotoxic activity against tumor cells, the fact remains that these effector cells are incapable of suppressing tumor growth in vivo. Some studies have proposed activation-induced cell death (AICD) to be a major cause of death of TILs, thus explaining their inability to control tumor growth (23-25). Other studies have proposed that cancers can become "immune privileged" sites by expressing Fas-ligand (26-28). Fas-ligand (FasL) is a cell surface protein of the tumor necrosis factor family that induces apoptosis on Fas-bearing cells when FasL binds to Fas. Fas-mediated apoptosis is dependent on the activation of different members of a family of cysteinylaspartate proteases called caspases, including caspase-8, -9, and -3, which are responsible for both the initiation of the apoptotic cascade and the execution of the cell damage. Interaction of FasL with Fas+ TILs has been demonstrated to lead to cell death (29). Therefore, expression of FasL by tumor cells may provide a mechanism by which cancer cells escape eradication by TILs (Figure 1B).
In a recent study by Chopin et al. (30), FasL expression was observed in 45% (n = 45) of TCC samples, while expression of FasL was not observed in normal urothelium (n = 20). A correlation existed between FasL expression and high tumor grade and stage (13% in superficial Ta-T1 versus 81% in invasive T2-T4; P < 0.0001). Two primary bladder TCC cell lines, established from two FasL-positive invasive bladder TCC tissues, were shown to specifically mediate FasL cell death in two conventional Fas-sensitive T lymphocyte targets. Further analysis of one of these cultures demonstrated its ability to induce FAS-mediated killing of autologous T-lymphocytes in vitro. Fas-mediated apoptosis of IFN-γ-producing CD8+ TILs was evident by the detection of activated caspases -8, -9, and -3 expression on these cells, which were found in close association with FasL-expressing TCC cells in situ. In short, these results suggest that TCC-expressed FasL may induce apoptosis of anti-tumor T-lymphocytes in vivo and may provide new insights into the mechanisms that allow for the suppression of TIL immunological function.
In addition, signaling defects in TILs have also been implicated in the dysfunction of lymphocytes at the tumor site. These include loss of signal-transducing zeta (ζ) chain (31) or reduction in the epsilon chain of p56, Zap70 and p59 expression (32-33). In some experiments, the lost ζ chain was seen to be reversible after treatment with IL-2 (34). Another signaling mechanism that emerged quite recently and which could potentiate the down regulation of TIL proliferation and activation is an alteration in the signaling pathways of IL-2/IL2R (35). It has also been observed that cancer-derived matrix metalloproteinases (MMP) play an important role in cleaving IL2Rα chain, thus resulting in host immunosuppression, tumor metastases, and lymphovascular invasion by interfering with the signaling pathways of IL-2 (36).
Other studies indicate that the enzyme indoleamine-2,3-dioxygenase (IDO) is responsible for creating an immunosuppressive environment. IDO, an enzyme classically known for its role in the tryptophan degradation pathway, has recently emerged as an important immunomodulator of T-cell function and inducer of tolerance. The induced expression of IDO by dendritic cells may suppress T-cell responses and promote tolerance either through direct effects on T cells (mediated by tryptophan depletion or tryptophan metabolites) or through effects of IDO on the dendritic cell. Friberg et al. (37) observed that the enzyme IDO is responsible for metabolizing tryptophan found on antigen presenting cells, thus compromising their efforts in anti-tumor cytotoxicity. Furthermore, it has been found that the enzyme IDO is produced by tumor cells in order to resist attacks by the host immune system (38) and thus weaken or even nullify the effects of potential immunosurveillance mechanisms.
Furthermore, it appears that a mechanism of "cancer immunoediting" may be responsible for the development of tumors that are resistant to immune-mediated events. Shankaran et al. (39) demonstrated that the immune system may promote the emergence of primary tumors with reduced immunogenicity that are capable of escaping immune recognition and destruction. The hypothesis of cancer immunosurveillance, which states that sentinel thymus-dependent cells of the body constantly survey host tissues for nascently transformed cells, has been demonstrated in several studies (40-42). However, since immunosurveillance represents only one dimension of the complex relationship between the immune system and cancer, the hypothesis of cancer immunoediting was proposed to encompass the various interactions, including elimination, equilibrium, and escape, that occur between the host immune system and developing cancer cells. In the elimination phase, the immune system is able to eradicate developing tumor cells; however, as the immune system continues to exert pressure on the tumor cells, escape variants occur and are able to persist in a state of equilibrium, whereby the immune system keeps further development of these cells at a minimum until finally, the escape phase occurs when the cancer cells have accrued sufficient mutations that enable evasion of the immune system (42).
Therefore, there are many mechanisms which appear to contribute to the inefficiency of TILs in their ability to eradicate tumor cells. Deeths et al. (43) proposed that certain tumor cell mechanisms, such as activation-induced non-responsiveness (AINR), may possibly induce CD8+ TILs death, thus jeopardizing the cytotoxic abilities and functions of the host immune system. Other mechanisms, such as down regulation of HLA class I molecules, loss of co-stimulatory molecules, and removal or blocking of tumor antigens by tumor cells also result in immune escape by tumor cells. Contrastingly, some investigators have also explored the possible inherent reasons for the inhibition or the inability of CD8+ TILs to counter the antagonistic tumor cells. Two mechanisms have been proposed: a defect in the cytolytic pathways or an overexpression of inhibitory molecules. Recent studies by Sheu et al. (44) illustrate that the down-regulation of perforin completely blocks the cytotoxic mechanisms of TILs. Additionally, they also found that inhibitory NK receptors such as CD94/NKG2A can be up-regulated on TILs by cytokines such as IL-15 and TGF-β which are derived from cancer cells. This can be a further contributing factor for the inertness of cytotoxic TILs in their response towards tumor cells. All of these mechanisms can be seen as potential targets for novel immunotherapy agents that are aimed at overcoming the suppressive and regulatory factors within the tumor microenvironment.
TILs as a prognostic factor in bladder cancer
An early study by Mostofi et al. (45) reported a favorable prognosis with higher number of TILs present in TCC. Later, a prolonged recurrence-free survival rate was correlated with an increased number of T-zone histiocytes in TCC indicating a competent host immune system as a favorable factor (46). According to another study (47, 48), denser TILs were associated with invasive (pT3-T4) TCC tumors as opposed to superficial papillary tumors. It appears that a higher prevalence of TILs is associated with a favorable response, even in the setting of a more invasive disease.
In another study by Tsujihashi et al. (49), a comparison of the functional activities of TILs and peripheral blood lymphocytes (PBLs) from 22 bladder cancer patients led to the conclusion that a higher number of TILs was associated with better clinical outcome and fewer occurrences of tumor metastases. TILs and PBLs were isolated from the patients and used against a natural killer (NK) myeloid leukemic line, myeloid K562, a fresh bladder tumor, and a cultured bladder tumor, HT1197, as their targets in vitro. The amount of lymphocytes in TILs and PBLs was measured by flow cytometry and showed a predominantly higher cell population of T-cells present in both PBLs and TILs. A 51Cr release cytotoxicity assay revealed that there was a spontaneous response to the NK target cells by the PBLs which was of 23.4%, compared to the response by the TILs which was of 3.5%. But when the TILs and PBLs were cultured with an immunomodulating cytokine, IL-2, it was noted that immune responses by both the PBLs and TILs were enhanced. However, interestingly, there was a tumor-specific response by TILs which was not observed for the PBLs. The cytotoxic response of IL-2-treated TILs was higher against autologous bladder cancer tumors than against the artificially cultured tumor tissue, HT1197. Therefore the presence of IL-2, a lymphokine known to be released from helper T-cells for the generation of cytotoxic T-cells, boosted the TIL cytotoxic response to the tumor tissues in vitro (49-50). These data continue to support the concept that TILs are capable of effective immunity against tumor cells when the appropriate immunomodulatory agents are present.
In another study by Lipponen et al. (51), tumor tissues from 514 patients with TCC were analyzed for a correlation between TILs, recurrence-free survival and metastases. Biopsy specimens from tumor tissues were analyzed by histological staining in order to categorize the tumors as papillary or nodular. The quantification of TILs was categorized as: weak or absent; moderately populated; or densely populated. In pTa-pT1 staged, papillary grade tumors, a higher density of TILs indicated an unfavorable prognosis, and these patients were noted to have a lower survival rate as compared to similarly staged patients with a lower density of TILs. In pT3-pT4 staged nodular tumors, a higher rate of survival was observed with a higher density of TILs around the tumor site, predicting a favorable prognosis. Recently, we analyzed the presence of intratumoral CD8 T cells, the expression of MHC class I antigen and the expression of the NY-ESO-1 tumor antigen in UC samples and correlated our findings with clinical outcome. Immunohistochemical staining for intratumoral CD8 T cells in tissue samples from 69 patients with UC showed that patients with advanced UC (pT2, pT3, or pT4) and higher numbers of CD8 TILs within the tumor (8 or more) had better disease-free survival (P < 0.001) and overall survival (P = 0.018) than did patients with similar-staged UC and fewer intratumoral CD8 TILs (52).
Conclusions and future directions
The presence of tumor infiltrating lymphocytes in bladder cancer is associated with a favorable prognosis but is not sufficient to overcome inhibitory changes within the tumor microenvironment over a prolonged period of time. Migration of lymphocytes from the circulating immune system to the tumor site implies that the host immune system is capable of initiating an anti-tumor response. Unfortunately, changes that occur as a result of mutations within tumor cells eventually create an immunosuppressive tumor microenvironment that prevents tumor eradication by TILs. Nevertheless, new findings and research on TILs and the tumor microenvironment are ongoing in efforts to reverse the multitude of immunosuppressive mechanisms.
The rational development of immunotherapy applicable for patients with solid tumors, analogous to that used for molecularly targeted therapy, is possible. Improvements in the understanding of immune regulation in the context of cancer, as well as novel agents that are able to target specific immune regulatory pathways, and evolving methodology to characterize human cancer at the cellular and molecular level will result in more informative clinical studies. The knowledge gained through the proposed integration of tumor biology with immunology will be the foundation for the effective application of immunotherapy to the treatment of solid tumors. Our ongoing studies in bladder cancer include utilization of a novel anti-CTLA4 monoclonal antibody aimed at releasing the inhibitory mechanisms on T-cells so as to enhance T-cell activity against tumor cells. We are currently enrolling bladder cancer patients onto a phase I neoadjuvant clinical trial with anti-CTLA4 and will conduct in-depth immunological studies on the obtained tumor tissues to characterize the various TIL populations and their function as compared to TILs obtained from untreated tissues. These types of studies will continue to provide valuable information regarding the significance of various subpopulations of TILs and therapeutic agents that will enhance anti-tumor responses by TILs in bladder cancer patients.
Abbreviations
- BCG
bacillus Calmette-Guérin
- IDO
indoleamine-2,3-dioxygenase
- TCC
transitional cell carcinoma
- UC
urothelial carcinoma
References
- 1.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–1694. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 2.Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of toll-like receptors. Infect Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer EC, Somogyi L, de Ruiter GJ, de Reijke TM, Kurth KH, Schamhart DH. Role of interleukin-8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res. 1997;25:31–34. doi: 10.1007/BF00941903. [DOI] [PubMed] [Google Scholar]
- 4.Thalmann GN, Sermier A, Rentsch C, Möhrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol. 2000;164:2129–2133. [PubMed] [Google Scholar]
- 5.Jackson AM, Alexandroff AB, Kelly RW, Skibinska A, Esuvaranathan K, Prescott S, Chisholm GD, James K. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guérin (BCG) immunotherapy. Clin Exp Immunol. 1995;99:369–375. doi: 10.1111/j.1365-2249.1995.tb05560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Radio-immunoassay detection of interferon-gamma in urine after intravesical Evans BCG therapy. J Urol. 1990;144:1248–1251. doi: 10.1016/s0022-5347(17)39713-6. [DOI] [PubMed] [Google Scholar]
- 7.Haaff EO, Catalona WJ, Ratliff TL. Detection of interleukin 2 in the urine of patients with superficial bladder tumors after treatment with intravesical BCG. J Urol. 1986;136:970–974. doi: 10.1016/s0022-5347(17)45142-1. [DOI] [PubMed] [Google Scholar]
- 8.De Boer EC, De Jong WH, Steerenberg PA, Aarden LA, Tetteroo E, De Groot ER, Van der Meijden AP, Vegt PD, Debruyne FM, Ruitenberg EJ. Induction of urinary interleukin-1 (IL-1), IL-2, IL-6, and tumour necrosis factor during intravesical immunotherapy with bacillus Calmette-Guérin in superficial bladder cancer. Cancer Immunol Immunother. 1992;34:306–312. doi: 10.1007/BF01741551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Böhle A, Gerdes J, Ulmer AJ, Hofstetter AG, Flad HD. Effects of local bacillus Calmette-Guerin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol. 1990;144:53–58. doi: 10.1016/s0022-5347(17)39365-5. [DOI] [PubMed] [Google Scholar]
- 10.de Boer EC, de Jong WH, van der Meijden AP, Steerenberg PA, Witjes F, Vegt PD, Debruyne FM, Ruitenberg EJ. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urol Res. 1991;19:45–50. doi: 10.1007/BF00294021. [DOI] [PubMed] [Google Scholar]
- 11.Coon JS, Weinstein RS, Summers JL. Blood group precursor T-antigen expression in human urinary bladder carcinoma. Am J Clin Pathol. 1982;77:692–699. doi: 10.1093/ajcp/77.6.692. [DOI] [PubMed] [Google Scholar]
- 12.Hirohashi S, Clausen H, Yamada T, Shimosato Y, Hakomori S. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and -81 expressed in cancer of blood group O or B individuals: its identification as Tn antigen. Proc Natl Acad Sci USA. 1985;82:7039–7043. doi: 10.1073/pnas.82.20.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korthals HR, Van Vinninghe AL, Neumann HAM. The presence of β 2 microglobulin on the membrane of the keratinocyte in premalignant skin disorders. Br J Dermatol. 1981;104:515–519. doi: 10.1111/j.1365-2133.1981.tb08165.x. [DOI] [PubMed] [Google Scholar]
- 14.Mauduit G, Turbitt M, MacKie RM. Dissociation of HLA heavy chain and light chain (β2 microglobulin) expression on the cell surface of cutaneous malignancies. Br J Dermatol. 1983;109:377–381. doi: 10.1111/j.1365-2133.1983.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson K, Evrin PE, Welsh KI. Production of β2-microglobulin by normal and malignant human cell lines and peripheral lymphocytes. Transplant Rev. 1974;21:53–84. doi: 10.1111/j.1600-065x.1974.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 16.Turbitt ML, Mackie RM. Loss of β2 microglobulin from the cell surface of cutaneous malignant and premalignant lesions. Br J Dermatol. 1981;104:507–513. doi: 10.1111/j.1365-2133.1981.tb08164.x. [DOI] [PubMed] [Google Scholar]
- 17.Graham SD. Immunology of the bladder. Urol Clin North Am. 1992;19:541–548. [PubMed] [Google Scholar]
- 18.Hart IR, Birch M, Marshall JF. Cell adhesion receptor expression during melanoma progression and metastasis. Cancer Metastasis Rev. 1991;10:115–128. doi: 10.1007/BF00049409. [DOI] [PubMed] [Google Scholar]
- 19.Carballido J, Alvarez-Mon M, Solovera OJ, Menéndez-Ondina L, Durántez A. Clinical significance of natural killer activity in patients with transitional cell carcinoma of the bladder. J Urol. 1990;143:29–33. doi: 10.1016/s0022-5347(17)39854-3. [DOI] [PubMed] [Google Scholar]
- 20.Dangles V, Validire P, Wertheimer M, Richon S, Bovin C, Zeliszewski D, Vallancien G, Bellet D. Impact of human bladder cancer cell architecture on autologous T-lymphocyte activation. Int J Cancer. 2002;98:51–56. doi: 10.1002/ijc.10140. [DOI] [PubMed] [Google Scholar]
- 21.Haas GP, Solomon D, Rosenberg SA. Tumor infiltrating lymphocytes from nonrenal urological malignancies. Cancer Immunol Immunother. 1990;30:342–350. doi: 10.1007/BF01786883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Housseau F, Zeliszewski D, Roy M, Paradis V, Richon S, Ricour A, Bougaran J, Prapotnich D, Vallancien G, Benoit G, Desportes L, Bedossa P, Hercend T, Bidart JM, Bellet D. MHC-dependent cytolysis of autologous tumor cells by lymphocytes infiltrating urothelial carcinomas. Int J Cancer. 1997;71:585–594. doi: 10.1002/(sici)1097-0215(19970516)71:4<585::aid-ijc13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Chappell DB, Zaks TZ, Rosenberg SA, Restifo NP. Human melanoma cells do not express Fas (Apo-1/CD95) ligand. Cancer Res. 1999;59:59–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Restifo NP. Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med. 2000;6:493–495. doi: 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radoja S, Saio M, Frey AB. CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ. J Immunol. 2001;166:6074–6083. doi: 10.4049/jimmunol.166.10.6074. [DOI] [PubMed] [Google Scholar]
- 26.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 27.O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell J, Bennett MW, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: cancer as a site of immune privilege. Immunol Today. 1999;20:46–52. doi: 10.1016/s0167-5699(98)01382-6. [DOI] [PubMed] [Google Scholar]
- 29.Yagita H, Seino K, Kayagaki N, Okumura K. CD95 ligand in graft rejection. Nature. 1996;379:682. doi: 10.1038/379682a0. [DOI] [PubMed] [Google Scholar]
- 30.Chopin D, Barie-Moniri R, Maillé P, Le Frère-Belda M, Muscatelli-Groux B, Merendino N, Lecerf L, Stoppacciaro A, Velotti F. Human urinary bladder transitional cell carcinomas acquire the functional Fas ligand during tumor progression. Am J Pathol. 2003;162:1139–1149. doi: 10.1016/S0002-9440(10)63910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, Taupin JL, Vivier E, Anderson P, Kiessling R. Decreased expression of the signal-transducing ζ chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 32.Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transducing molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 33.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–3145. [PubMed] [Google Scholar]
- 34.Rabinowich H, Banks M, Reichert TE, Logan TF, Kirkwood JM, Whiteside TL. Expression and activity of signaling molecules in T lymphocytes obtained from patients with metastatic melanoma before and after interleukin 2 therapy. Clin Cancer Res. 1996;2:1263–1274. [PubMed] [Google Scholar]
- 35.Lopez CB, Rao TD, Feiner H, Shapiro R, Marks JR, Frey AB. Repression of interleukin-2 mRNA translation in primary human breast carcinoma tumor-infiltrating lymphocytes. Cell Immunol. 1998;190:141–155. doi: 10.1006/cimm.1998.1390. [DOI] [PubMed] [Google Scholar]
- 36.Sheu BC, Lien HC, Ho HN, Lin HH, Chow SN, Huang SC, Hsu SM. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003;63:6537–6542. [PubMed] [Google Scholar]
- 37.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 38.Chiou SH, Sheu BC, Chang WC, Huang SC, Hong-Nerng H. Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol. 2005;67:35–50. doi: 10.1016/j.jri.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 40.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 41.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 42.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Ann Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 43.Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J Immunol. 1999;163:102–110. [PubMed] [Google Scholar]
- 44.Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167:2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 45.Mostofi FK, Sesterhenn I. Lymphocytic infiltration in relationship to urological tumors. Natl Cancer Inst Monogr. 1978;49:133–141. [PubMed] [Google Scholar]
- 46.Lopez-Beltran A, Morales C, Reymundo C, Toro M. T-zone histiocytes and recurrence of papillary urothelial bladder carcinoma. Urol Int. 1989;44:205–209. doi: 10.1159/000281505. [DOI] [PubMed] [Google Scholar]
- 47.Morita T, Tokue A, Minato N. Analysis of natural killer activity and natural killer cell subsets in patients with bladder cancer. Cancer Immunol Immunother. 1990;32:191–194. doi: 10.1007/BF01771456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikemoto S, Kishimoto T, Wada S, Nishio S, Maekawa M. Clinical studies on cell-mediated immunity in patients with urinary bladder carcinoma: blastogenic response, interleukin-2 production and interferon-gamma production of lymphocytes. Br J Urol. 1990;65:333–338. doi: 10.1111/j.1464-410x.1990.tb14751.x. [DOI] [PubMed] [Google Scholar]
- 49.Tsujihashi H, Matsuda H, Uejima S, Akiyama T, Kurita T. Immunocompetence of tissue infiltrating lymphocytes in bladder tumors. J Urol. 1988;140:890–894. doi: 10.1016/s0022-5347(17)41851-9. [DOI] [PubMed] [Google Scholar]
- 50.Itoh K, Tilden AB, Balch CM. Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating into human metastatic melanomas. Cancer Res. 1986;46:3011–3017. [PubMed] [Google Scholar]
- 51.Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1993;29:69–75. doi: 10.1016/0959-8049(93)90579-5. [DOI] [PubMed] [Google Scholar]
- 52.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]