Abstract
XAGE-1 is a cancer/testis (CT) antigen and has been shown to be immunogenic in lung cancer patients. Among 4 alternative splicing variants, XAGE-1a, b, c and d, XAGE-1b mRNA was dominantly expressed in cancer. In this study, we generated a XAGE-1b mAb, USO9-13. The B cell epitope recognized by the USO9-13 mAb was in the C-terminal region of the XAGE-1b protein and is also recognized by sera from patients with lung adenocarcinoma. Using USO9-13 and an anti-Flag mAb, we examined the translation products of the 4 transcripts. The XAGE-1a and b transcripts translated to the XAGE-1b protein. The XAGE-1c transcript possibly translated to 9- and 17-aa polypeptides. The XAGE-1d transcript translated to a protein consisting of 69 amino acids. Immunofluorescence analysis using USO9-13 mAb showed that the XAGE-1b protein is located in the nuclei of cells. Immunohistochemically, nuclear staining was heterogeneously observed in 25/47 lung adenocarcinomas, 1/12 hepatocellular carcinomas and 1/11 gastric cancers, but not in adjacent normal tissues. These findings suggested that XAGE-1b is a promising target molecule for a cancer vaccine against lung cancer.
Keywords: human, lung cancer, XAGE-1, alternative splicing, monoclonal antibody, immunohistochemistry
Introduction
Forty-four CT antigen genes or gene families have been identified by immunological or genetic approaches (1). Several CT antigens have been shown to elicit spontaneous humoral and cellular immune responses in cancer patients simultaneously (2, 3). Based on their restricted expression in normal tissues and high immunogenicity, CT antigens are attractive targets for cancer vaccines. Initial expression studies of CT antigens were mostly done at the level of mRNA expression by RT-PCR. Studies of the expression of CT antigens at the protein level provide crucial information regarding their distribution in tumor samples, as shown in studies of the MAGE, NY-ESO-1 and SSX families (4).
The XAGE-1 gene was originally identified as a PAGE/GAGE-related gene on the X chromosome by EST analysis (5). The expression profile of XAGE-1 suggested that it has the characteristics of a CT antigen (6-12). Transcription of the XAGE-1 gene is regulated by methylation of the CpG island in the promoter, and 4 alternative RNA splicing variants, XAGE-1a, b, c and d, have been identified (11, 13). By serological analysis of antigens by recombinant expression cloning (SEREX), we previously identified XAGE-1b as a dominant antigen recognized by serum from a lung adenocarcinoma patient using an autologous tumor cell line and showed that XAGE-1b is immunogenic in patients with lung adenocarcinoma (14).
In this study, we generated a mAb specific to XAGE-1b protein and investigated the translation products of 4 XAGE-1 transcripts using this mAb and an anti-Flag mAb. We also investigated XAGE-1b protein expression in tumor tissues by immunohistochemistry (IHC).
Results
Generation of a mAb against XAGE-1b protein
We previously showed dominant expression of XAGE-1b mRNA compared to the other variant XAGE-1 mRNAs in normal testis and tumors. XAGE-1a, b, c and d mRNA expression was observed in 0, 15, 0 and 6 of 49 lung cancers (15) and 1, 14, 1 and 3 of 54 prostate cancers (16), respectively.
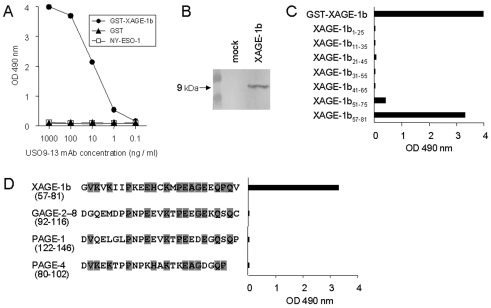
To analyze XAGE-1b protein expression, we produced a mAb by immunizing mice with XAGE-1b DNA and protein. Five BALB/c mice were administered a XAGE-1b expression vector, pcDNA3.1/XAGE-1b, using in vivo electroporation. The serum IgG antibody was examined by ELISA using recombinant GST-XAGE-1b protein. The mouse with the highest titer was boosted with the protein and the spleen cells were fused with NS-1 cells. Seven positive hybridoma clones were obtained. As shown in Figure 1A, USO9-13 mAb was reactive with recombinant GST-XAGE-1b protein at a concentration as low as 10 ng/ml, but not with GST (glutathione S-transferase) alone or irrelevant NY-ESO-1 protein. The mAb recognized both native and denatured recombinant XAGE-1b protein. In immunoblot analysis, USO9-13 mAb detected a 9-kDa molecule from 293T cells transiently transfected with pcDNA3.1/XAGE-1b (Figure 1B).
Figure 1.
Generation of a mAb against XAGE-1b. (A) Reactivity of USO9-13 mAb with recombinant proteins determined by ELISA. (B) Immunoblotting of lysate of 293T cells transiently transfected with pcDNA3.1/XAGE-1b using USO9-13 mAb as probe. Mock, empty vector. (C) Reactivity of USO9-13 mAb (1 µg/ml) with 25-mer overlapping peptides from XAGE-1b determined by ELISA. (D) Reactivity of USO9-13 mAb (10 µg/ml) against C-terminal peptides from XAGE-1b-related molecules. Shaded, conserved residues.
Identification of XAGE-1b B cell epitopes recognized by USO9-13 mAb and sera from patients with lung adenocarcinoma
A XAGE-1b B cell epitope recognized by USO9-13 mAb was analyzed by ELISA using 7 25-mer peptides overlapping by 15 amino acids. As shown in Figure 1C, USO9-13 mAb recognized the XAGE-1b57-81 peptide located at the C-terminus of XAGE-1b protein.
XAGE-1b protein shows partial homology with an amino acid sequence at the C-terminus of GAGE-2-8 (17, 18), PAGE-1 (19) (renamed GAGE-B1), and PAGE-4 (20) (renamed GAGE-C1). We synthesized 25-mer peptides corresponding to the C-termini of GAGE-2-8, PAGE-1 and PAGE-4 and examined their binding to the mAb. As shown in Figure 1D, USO9-13 mAb recognized only XAGE-1b57-81 peptide, indicating that it did not cross-react with C-terminal peptides from related GAGE/PAGE proteins.
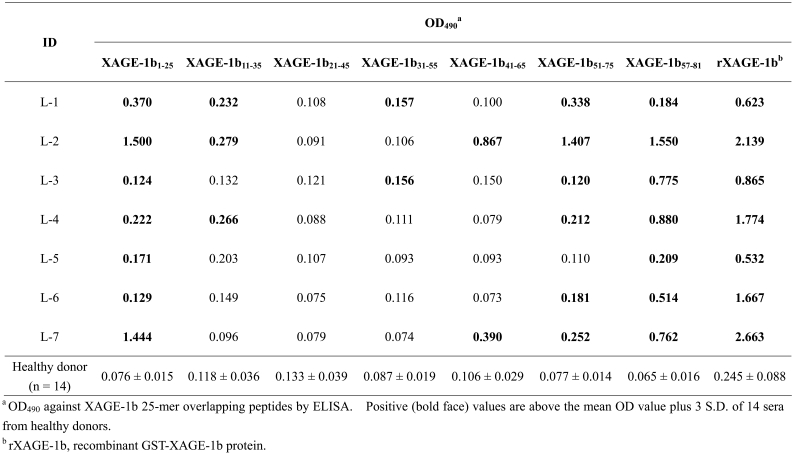
The XAGE-1b B cell epitopes recognized by sera from 7 seropositive lung adenocarcinoma patients (L-1 to L-7) were examined using overlapping peptides (Table 1). XAGE-1b57-81 peptide was recognized by all 7 sera. XAGE-1b1-25 and 51-75 peptides were recognized by 7 and 6 sera, respectively. XAGE-1b11-35, XAGE-1b31-55 and XAGE-1b41-65 peptides were recognized by 3, 2 and 2 sera, respectively.
Table 1.
Identification of B cell epitopes in XAGE-1b recognized by sera from 7 lung cancer patients.
Translation of XAGE-1b protein from XAGE-1a and b transcripts
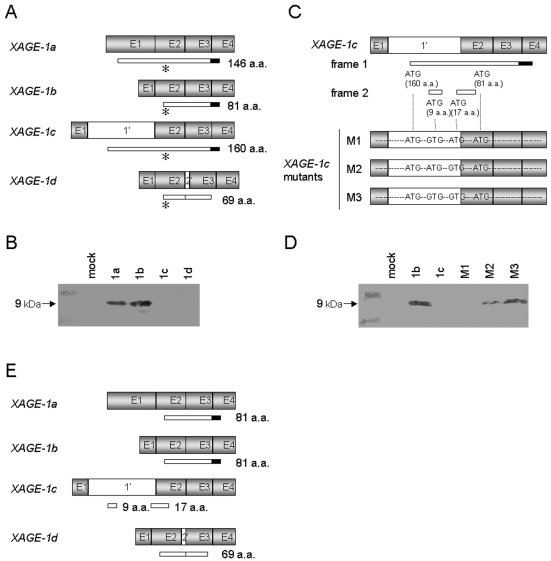
Figure 2A shows the alternative RNA splicing variants, XAGE-1a, b, c and d (11). We investigated the translation products of XAGE-1a, b, c and d transcripts by transfecting 293T cells with each XAGE-1 expression vector and analyzing the products by immunoblotting. As shown in Figure 2B, a 9-kDa molecule that was identical to XAGE-1b protein was detected from pcDNA3.1/XAGE-1a transfectants, indicating that translation of XAGE-1a protein starts at the third ATG, which is identical to the XAGE-1b protein initiation site. This was consistent with the previous finding using rabbit polyclonal antibody against XAGE-1 protein (12). On the other hand, no band was detected from pcDNA3.1/XAGE-1c transfectants by USO9-13 mAb despite the presence of the epitope at the C-terminus of the putative protein. There are 7 ATG codons in XAGE-1c mRNA, and translation initiation at the fourth ATG located in frame 1 results in the production of XAGE-1b protein (Figure 2, panels A and C). We hypothesized that the second and/or third ATG located in frame 2 was used for the translation from XAGE-1c mRNA. To address this possibility, XAGE-1c cDNA mutants that lacked the second and/or third ATG were prepared (Figure 2C). Transfection of pcDNA3.1/XAGE-1c M3, in which mutations were introduced into both of these ATGs, resulted in the production of a USO9-13 mAb-reactive 9-kDa molecule that was identical to XAGE-1b protein (Figure 2D). Transfection of pcDNA3.1/XAGE-1c M2, in which a mutation was introduced into the third ATG, yielded a small amount of the 9-kDa band. No specific band was detected upon transfection of pcDNA3.1/XAGE-1c M1, in which a mutation was introduced into the second ATG. These results suggested that XAGE-1c proteins would be polypeptides consisting of 9 and 17 amino acids translated from the second and the third ATG, respectively. No translation product of XAGE-1d transcript could be detected using USO9-13 mAb due to the lack of the C-terminal portion containing the epitope because of the insertion of exon 2' (Figure 2, panels A and B). We confirmed that XAGE-1d protein starts at the first ATG by immunoblotting of C-terminal Flag-tagged XAGE-1d using anti-Flag mAb (data not shown). Thus, XAGE-1b protein was translated from XAGE-1a and b transcripts (Figure 2E).
Figure 2.
Identification of the translation products of the XAGE-1 variants. (A) Schematic representation of the XAGE-1 variants. Exons (gray boxes), introns (open boxes) and putative open reading frames (narrow boxes) are shown. The solid box represents the epitope of USO9-13 mAb. The asterisk indicates the translation initiation site of XAGE-1b. (B) Immunoblotting of lysate of 293T cells transiently transfected with pcDNA3.1/XAGE-1a, b, c and d with USO9-13 mAb. (C) M1, M2 and M3 are pcDNA3.1/XAGE-1c mutants that lack the second and/or the third internal ATG codons. (D) Immunoblotting of lysate of 293T cells transiently transfected with pcDNA3.1/XAGE-1b and c, and M1, M2 and M3 using USO9-13 mAb. (E) Schematic representation of the translation products of the XAGE-1 variants. Exons (gray boxes), introns (open boxes) and defined open reading frames (narrow boxes) are shown. The solid box represents the epitope of USO9-13 mAb.
Nuclear localization of XAGE-1b protein in spermatogonia, spermatocytes and tumor cells
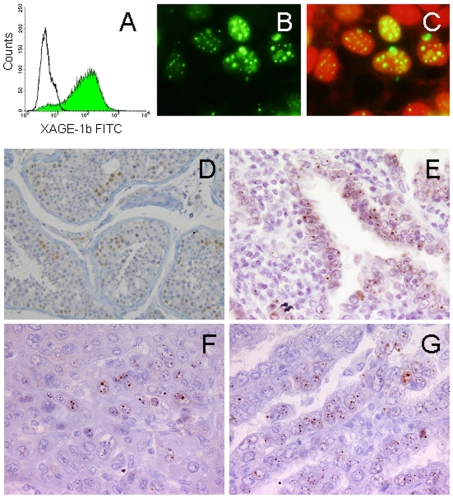
The cellular localization of XAGE-1b protein was analyzed in 293T cells stably transfected with the XAGE-1b expression vector by indirect immunofluorescence detected using a fluorescence microscope. As shown in Figure 3A, intracellular XAGE-1b protein expression was confirmed by flow cytometry using the USO9-13 mAb. The cellular localization of XAGE-1b protein was analyzed by doubly staining nuclei with propidium iodide and USO9-13 mAb. As shown in Figure 3, panels B and C, XAGE-1b protein was observed in the nuclei of the cells in a punctate pattern. No morphologic change was observed in the transfected 293T cells.
Figure 3.
Immunofluorescence (A-C) and IHC (D-G) analysis of XAGE-1b. (A) Expression of XAGE-1b in 293T cells transfected with pcDNA3.1/XAGE-1b (green) by flow cytometry. Solid line, empty vector. (B) Fluorescence microscopy analysis. (C) Nuclei are shown stained with PI (red). XAGE-1b derived fluorescence is merged with the corresponding PI recordings. (D), (E), (F) and (G) are IHC analysis of testis, lung cancer, hepatocellular carcinoma and gastric cancer, respectively. Magnification 400x.
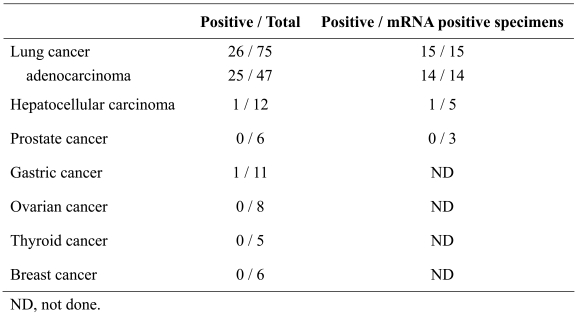
XAGE-1b protein expression in the normal and malignant cells was examined by IHC using USO9-13 mAb. No XAGE-1b protein expression was observed in the normal tissues except for testis, in which the nuclei of spermatogonia and spermatocytes were stained (Figure 3D). In tumors, similar nuclear staining was observed in 25/47 lung adenocarcinomas (Figure 3E), 1/12 hepatocellular carcinomas (Figure 3F) and 1/11 gastric cancers (Figure 3G). No XAGE-1b protein expression was observed in 6 prostate cancers, 8 ovarian cancers, 5 thyroid cancers or 6 breast cancers. In XAGE-1b mRNA-positive specimens, the positive expression of XAGE-1b protein was observed in 14 of 14 lung adenocarcinomas, 1 of 5 hepatocellular carcinomas and 0 of 3 prostate cancers (Table 2).
Table 2.
IHC analysis of XAGE-1b expression in various types of cancer.
Discussion
We showed that a single cycle of immunization of mice with DNA followed by the recombinant protein avoided the production of antibodies to bacterial products contaminating the recombinant protein and was enough to induce a sufficient titer for obtaining mAb against the protein. We have generated mAbs against XAGE-1b protein in this study, as well as against other tumor antigens, RFX-4 (21) and OY-TES-1, using this method. DNA as immunogen and unmethylated CpG motifs from the vector may contribute to the specific and vigorous antibody response (22).
In this study, we identified the translation products of 4 XAGE-1 transcripts by immunoblotting using a XAGE-1b mAb, USO9-13, and an anti-Flag mAb. Transfection of pcDNA3.1/XAGE-1a into 293T cells resulted in the production of XAGE-1b protein, as detected by immunoblotting using USO9-13 mAb. This finding indicated that the translation initiation from the third ATG in XAGE-1a mRNA is probably due to the lack of a Kozak consensus motif near the first ATG codon (23). This finding was consistent with the finding previously reported by others (12). On the other hand, no band was observed in pcDNA3.1/XAGE-1c transfectants upon immunoblotting using USO9-13 mAb despite the presence of the epitope in the putative XAGE-1c protein. The production of XAGE-1b protein as a result of the disruption of the second and the third ATG codons in an alternative open reading frame suggested that those sites were involved in translation, and 9- and 17-amino acid peptides were produced from those codons, respectively. The "leaky scanning" appeared to be due to deviation from the consensus sequence in both the second and the third ATG codons (23). For the final transcript, XAGE-1d, translation of a protein with the predicted size with a Flag sequence was detected by immunoblotting using anti-Flag antibody. Thus, XAGE-1a and XAGE-1b transcripts translated to XAGE-1b protein. On the other hand, it was suggested that XAGE-1c transcript translated to 9- and 17-aa polypeptides. The XAGE-1d transcript translated to a 69-aa protein.
We previously reported dominant expression of XAGE-1b protein in lung adenocarcinoma and the frequent production of antibody against XAGE-1b in patients (14, 15, 16). In this study, we determined the antibody epitopes of XAGE-1b protein recognized by antibodies from cancer patients. We showed that the C-terminal XAGE-1b57-81 peptide, which is the epitope of USO9-13 mAb and also the only epitope recognized by serum from immunized mice, was commonly recognized by all 7 lung adenocarcinoma patients examined. XAGE-1b1-25 and XAGE-1b51-75 peptides were also recognized by 7 and 6 sera, respectively, from the patients. Those three regions contributed to dominant epitopes, although additional epitopes were recognized by a fraction of sera from the patients. The recognition of additional epitopes may involve epitope spreading. B cell epitope spreading has been reported in autoimmune diseases, such as systemic lupus erythematosus, and viral diseases including infectious mononucleosis (24). T cell epitope spreading was also observed in tumor-associated antigens in patients who received peptide-based immunotherapy (25, 26).
IHC using USO9-13 mAb revealed the nuclear localization of XAGE-1b protein in pcDNA3.1/XAGE-1b transfected 293T cells. Nuclear localization of XAGE-1b protein was also observed in various types of cancer. When normal tissues were examined, positive staining was observed only in the nuclei of spermatogonia and spermatocytes in the testis. This finding is consistent with the presence of the bipartite nuclear localization signal in XAGE-1b protein (10). Although XAGE-1b staining revealed punctate nuclear patterns, further studies are necessary to elucidate the structural relationship. We previously reported that XAGE-1b mRNA is highly expressed in lung adenocarcinoma (45%) and most mRNA-positive specimens expressed XAGE-1b protein (15). We also observed XAGE-1b mRNA expression in hepatocellular carcinoma (13/39, 33%) and prostate cancer (14/54, 26%) (16). However, only 1 of 5 mRNA-positive hepatocellular carcinoma and none of the 3 mRNA-positive prostate cancer specimens showed XAGE-1b protein expression. The difference in the protein and mRNA expression in these different types of cancer could be due to differences in translation efficiency or protein accumulation of XAGE-1b protein in cells with different histological origin.
In conclusion, we investigated the translation products of 4 XAGE-1 transcripts, XAGE-1a, b, c and d, by immunoblotting using the XAGE-1b mAb, USO9-13, and an anti-Flag mAb, and showed that XAGE-1b protein was produced from XAGE-1a and b. We also identified the dominant epitope of XAGE-1b protein commonly recognized by USO9-13 mAb and cancer patients' sera. IHC analysis revealed the nuclear localization of XAGE-1b protein in the male germ cells and dominant expression in lung cancer. The CT antigen characteristics of XAGE-1b and its high immunogenicity provide a rationale for the use of XAGE-1b as a target for immunotherapy.
Abbreviations
- CT
cancer/testis
- GST
glutathione S-transferase
- IHC
immunohistochemistry
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a grant from the Cancer Research Institute, New York.
References
- 1.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. http://www.cancerimmunity.org/v4p1/031220.htm [PubMed] [Google Scholar]
- 2.Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayyoub M, Stevanovic S, Sahin U, Guillaume P, Servis C, Rimoldi D, Valmori D, Romero P, Cerottini JC, Rammensee HG, Pfreundschuh M, Speiser D, Levy F. Proteasome-assisted identification of a SSX-2-derived epitope recognized by tumor-reactive CTL infiltrating metastatic melanoma. J Immunol. 2002;168:1717–1722. doi: 10.4049/jimmunol.168.4.1717. [DOI] [PubMed] [Google Scholar]
- 4.Juretic A, Spagnoli GC, Schultz-Thater E, Sarcevic B. Cancer/testis tumour-associated antigens: immunohistochemical detection with monoclonal antibodies. Lancet Oncol. 2003;4:104–109. doi: 10.1016/s1470-2045(03)00982-3. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann U, Vasmatzis G, Lee B, Pastan I. Novel genes in the PAGE and GAGE family of tumor antigens found by homology walking in the dbEST database. Cancer Res. 1999;59:1445–1448. [PubMed] [Google Scholar]
- 6.Boon T, Old LJ. Tumor antigens. Curr Opin Immunol. 1997;9:681–683. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 7.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu XF, Helman LJ, Yeung C, Bera TK, Lee B, Pastan I. XAGE-1, a new gene that is frequently expressed in Ewing's sarcoma. Cancer Res. 2000;60:4752–4755. [PubMed] [Google Scholar]
- 9.Wang T, Fan L, Watanabe Y, McNeill P, Fanger GR, Persing DH, Reed SG. L552S, an alternatively spliced isoform of XAGE-1, is over-expressed in lung adenocarcinoma. Oncogene. 2001;20:7699–7709. doi: 10.1038/sj.onc.1204939. [DOI] [PubMed] [Google Scholar]
- 10.Zendman AJ, van Kraats AA, den Hollander AI, Weidle UH, Ruiter DJ, van Muijen GN. Characterization of XAGE-1b, a short major transcript of cancer/testis-associated gene XAGE-1, induced in melanoma metastasis. Int J Cancer. 2002;97:195–204. doi: 10.1002/ijc.1584. [DOI] [PubMed] [Google Scholar]
- 11.Zendman AJ, Van Kraats AA, Weidle UH, Ruiter DJ, Van Muijen GN. The XAGE family of cancer/testis-associated genes: alignment and expression profile in normal tissues, melanoma lesions and Ewing's sarcoma. Int J Cancer. 2002;99:361–369. doi: 10.1002/ijc.10371. [DOI] [PubMed] [Google Scholar]
- 12.Egland KA, Kumar V, Duray P, Pastan I. Characterization of overlapping XAGE-1 transcripts encoding a cancer testis antigen expressed in lung, breast, and other types of cancers. Mol Cancer Ther. 2002;1:441–450. [PubMed] [Google Scholar]
- 13.Lim JH, Kim SP, Gabrielson E, Park YB, Park JW, Kwon TK. Activation of human cancer/testis antigen gene, XAGE-1, in tumor cells is correlated with CpG island hypomethylation. Int J Cancer. 2005;116:200–206. doi: 10.1002/ijc.21007. [DOI] [PubMed] [Google Scholar]
- 14.Ali Eldib AM, Ono T, Shimono M, Kaneko M, Nakagawa K, Tanaka R, Noguchi Y, Nakayama E. Immunoscreening of a cDNA library from a lung cancer cell line using autologous patient serum: Identification of XAGE-1b as a dominant antigen and its immunogenicity in lung adenocarcinoma. Int J Cancer. 2004;108:558–563. doi: 10.1002/ijc.11587. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Noguchi Y, Uenaka A, Sato S, Okumura H, Tanaka M, Shimono M, Ali Eldib AM, Ono T, Ohara N, Yoshino T, Yamashita K, Tsunoda T, Aoe M, Shimizu N, Nakayama E. XAGE-1 expression in non-small cell lung cancer and antibody response in patients. Clin Cancer Res. 2005;11:5496–5503. doi: 10.1158/1078-0432.CCR-05-0216. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi F, Noguchi Y, Saika T, Nakagawa K, Sato S, Eldib AM, Nasu Y, Kumon H, Nakayama E. XAGE-1 mRNA expression in prostate cancer and antibody response in patients. Microbiol Immunol. 2005;49:471–476. doi: 10.1111/j.1348-0421.2005.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 17.Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Backer O, Arden KC, Boretti M, Vantomme V, De Smet C, Czekay S, Viars CS, De Plaen E, Brasseur F, Chomez P, Van den Eynde B, Boon T, van der Bruggen P. Characterization of the GAGE genes that are expressed in various human cancers and in normal testis. Cancer Res. 1999;59:3157–3165. [PubMed] [Google Scholar]
- 19.Chen ME, Lin SH, Chung LW, Sikes RA. Isolation and characterization of PAGE-1 and GAGE-7. New genes expressed in the LNCaP prostate cancer progression model that share homology with melanoma-associated antigens. J Biol Chem. 1998;273:17618–17625. doi: 10.1074/jbc.273.28.17618. [DOI] [PubMed] [Google Scholar]
- 20.Brinkmann U, Vasmatzis G, Lee B, Yerushalmi N, Essand M, Pastan I. PAGE-1, an X chromosome-linked GAGE-like gene that is expressed in normal and neoplastic prostate, testis, and uterus. Proc Natl Acad Sci U S A. 1998;95:10757–10762. doi: 10.1073/pnas.95.18.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita H, Uenaka A, Ono T, Hasegawa K, Sato S, Koizumi F, Nakagawa K, Toda M, Shingo T, Ichikawa T, Noguchi Y, Tamiya T, Furuta T, Kawase T, Date I, Nakayama E. Identification of glioma-specific RFX4-E and -F isoforms and humoral immune response in patients. Cancer Sci. 2005;96:801–809. doi: 10.1111/j.1349-7006.2005.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 23.Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderlugt CJ, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8:831–836. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribas A, Timmerman JM, Butterfield LH, Economou JS. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 2003;24:58–61. doi: 10.1016/s1471-4906(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 26.Germeau C, Ma W, Schiavetti F, Lurquin C, Henry E, Vigneron N, Brasseur F, Lethe B, De Plaen E, Velu T, Boon T, Coulie PG. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J Exp Med. 2005;201:241–248. doi: 10.1084/jem.20041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler G, Howe SC, Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976;6:292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- 28.DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurashige T, Noguchi Y, Saika T, Ono T, Nagata Y, Jungbluth A, Ritter G, Chen YT, Stockert E, Tsushima T, Kumon H, Old LJ, Nakayama E. NY-ESO-1 expression and immunogenicity associated with transitional cell carcinoma: correlation with tumor grade. Cancer Res. 2001;61:4671–4674. [PubMed] [Google Scholar]
Materials and methods
Mice
Female BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). The mice were bred at the Laboratory Animal Center in Okayama University. All experiments were carried out in accordance with the Guidelines for Animal Experiments at Okayama University, the Japanese Government Animal Protection and Management Law (No. 105) and the Japanese Government Notification on Feeding and Safekeeping of Animals (No. 6).
Cell lines
NS-1 is a non-secreting clone derived from BALB/c MOPC-21 cells, which were derived from a mineral oil induced plasmacytoma (27). 293T is a derivative of 293 human embryonic kidney cells into which the gene for the SV40 T antigen had been inserted (28). These cell lines were maintained in RPMI 1640 medium supplemented with 10% FCS. A 293T-derived cell line overexpressing XAGE-1b was generated by stable transfection with pcDNA3.1/Zeo(+) containing XAGE-1b cDNA (Invitrogen, Carlsbad, CA).
Tumor specimens
Tumor specimens were obtained from patients who underwent surgery at Okayama University Hospital and were fixed with buffered formalin and embedded in paraffin. All tumor specimens were histologically diagnosed as cancer. Informed consent for the use of specimens was obtained from all of the patients.
Plasmid construction
XAGE-1a (nt 23-551, RefSeq accession no. NM_020411), XAGE-1b (nt 31-395, GenBank accession no. AJ290447) and XAGE-1c (nt 101-715, NM_133431) cDNAs were amplified by PCR and cloned into the pcDNA3.1(+) expression plasmid vector (Invitrogen). The constructs for the XAGE-1c mutants and XAGE-1d were generated by site-directed mutagenesis. XAGE-1c mutants (pcDNA3.1/XAGE-1c M1, M2 and M3) were generated by mutating the internal ATG sequence to GTG. pcDNA3.1/XAGE-1d was generated in the pcDNA3.1/XAGE-1b template by inserting a XAGE-1d-unique sequence (GTGCTGGGAAGGGAAA; nt 221-236, NM_133430). XAGE-1d-Flag was constructed in pFLAG-CMV-14 (Sigma-Aldrich, St. Louis, MO). The nucleotide sequence of the cloned cDNA was confirmed by DNA sequencing.
Preparation of the recombinant protein
XAGE-1b cDNA was amplified by PCR and cloned into pGEX-6P-1 vector (Amersham Biosciences, Piscataway, NJ). XAGE-1b protein was expressed as a GST fusion protein in BL21 E. coli cells and purified by affinity chromatography under native conditions.
Peptide synthesis
Peptides were synthesized by standard solid phase methods using Fmoc chemistry and a peptide synthesizer (model 430A; Applied Biosystems, Foster City, CA). Cleavage of the peptide from the resin and removal of the side chain-protecting groups were carried out using 95% trifluoroacetic acid.
Immunization of mice and generation of USO9-13 mAb
The plasmid for in vivo electroporation was prepared using an Endfree Plasmid Mega Kit (QIAGEN, Hilden, Germany). Five BALB/c mice were injected with 100 µg of pcDNA3.1/XAGE-1b into the anterior tibial muscle and pulsed by an electric pulse generator (CUY-21; BEX, Tokyo, Japan) using a 1.0-cm diameter round plate electrode twice at a 2-week interval. The mouse with the highest antibody titer was boosted intraperitoneally with 100 µg of GST-XAGE-1b protein 3 days prior to fusion. Spleen cells fused with NS-1 cells were suspended in ClonaCell-HY soft agar (StemCell Technologies Inc, Vancouver, BC, Canada). Hybridomas were tested for the production of antibody against XAGE-1b by ELISA assay. A hybridoma clone designated USO9-13 was obtained by limiting dilution. The USO9-13 mAb was purified from ascites via Protein G affinity chromatography (Amersham Biosciences).
ELISA
Ninety-six-well Immuno-Plates (Nalge Nunc International, Rochester, NY) were coated with 100 ng/well of proteins or 200 ng/well of peptides. ELISA was performed as described previously (29). Optical density at 490 nm was determined using a microplate reader benchmark (Bio-Rad Laboratories, Hercules, CA).
Immunoblot analysis
293T cells were transiently transfected with plasmids using Lipofectamine 2000 reagent (Invitrogen). Total cell extracts were separated by electrophoresis under reducing conditions and transferred to a PVDF membrane (Hybond-P; Amersham Biosciences). The membrane was probed with USO9-13 or anti-Flag M2 (Sigma-Aldrich) mAb, followed by alkaline phosphatase-conjugated goat anti-mouse IgG antibody. Reactive protein bands were visualized using an AP Conjugate Substrate Kit (Bio-Rad Laboratories).
Immunofluorescence staining
Intracellular staining of XAGE-1b was done using a BD Cytofix/Cytoperm Kit (BD Biosciences Pharmingen, San Diego, CA) for flow cytometry analysis according to the manufacturer's instructions. USO9-13 mAb and FITC-conjugated goat anti-mouse IgG antibody were used for detection. Cells were analyzed on a FACScan using CellQuest software (BD Biosciences Immunocytometry Systems, San Jose, CA). For immunofluorescence microscopy, cells growing on glass slides were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 buffer. USO9-13 mAb and FITC-conjugated goat anti-mouse IgG antibody were used for detection. Nuclei were stained with propidium iodide. Slides were visualized under a fluorescence microscope.
IHC staining
Tissue specimens were deparaffinized and microwave-heated in an antigen retrieval buffer (10 mM citrate buffer, pH 6.0) with a pressure cooker for 10 minutes. Staining was performed as described elsewhere (15).