Abstract
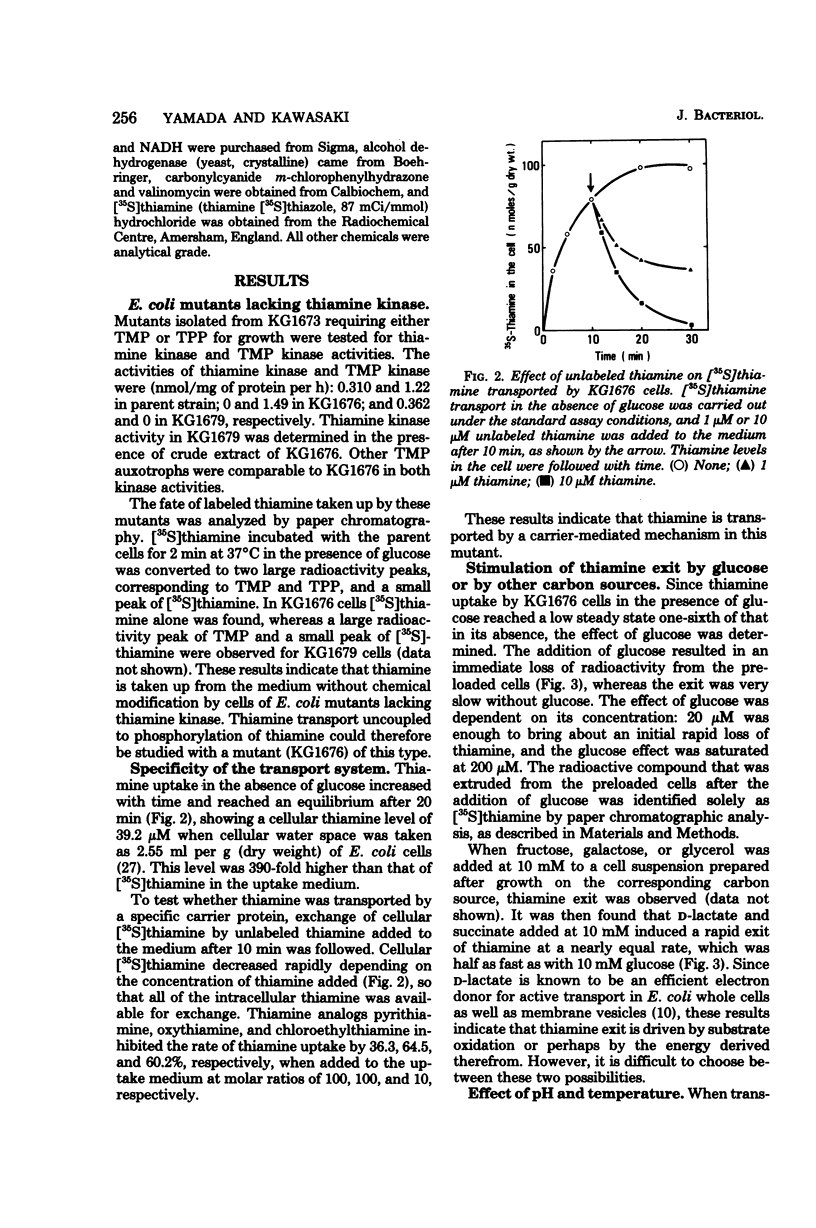
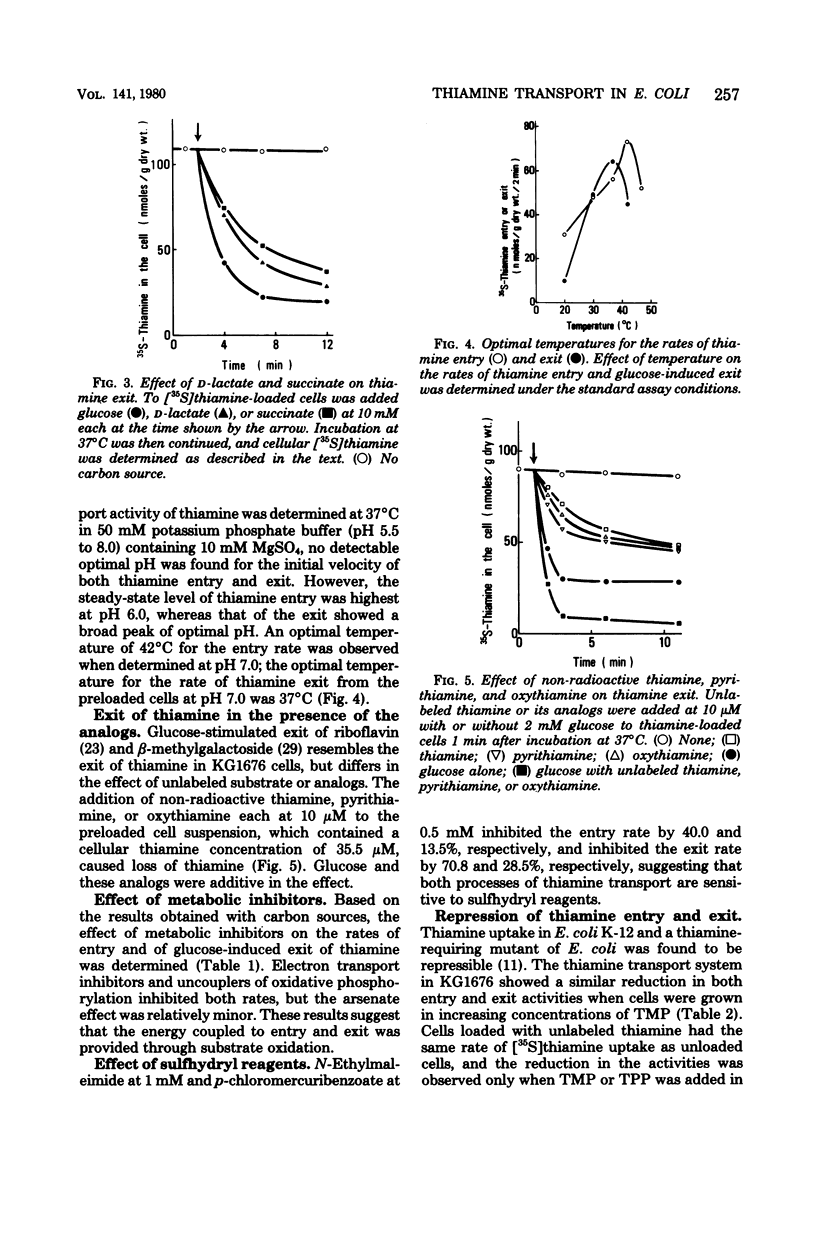
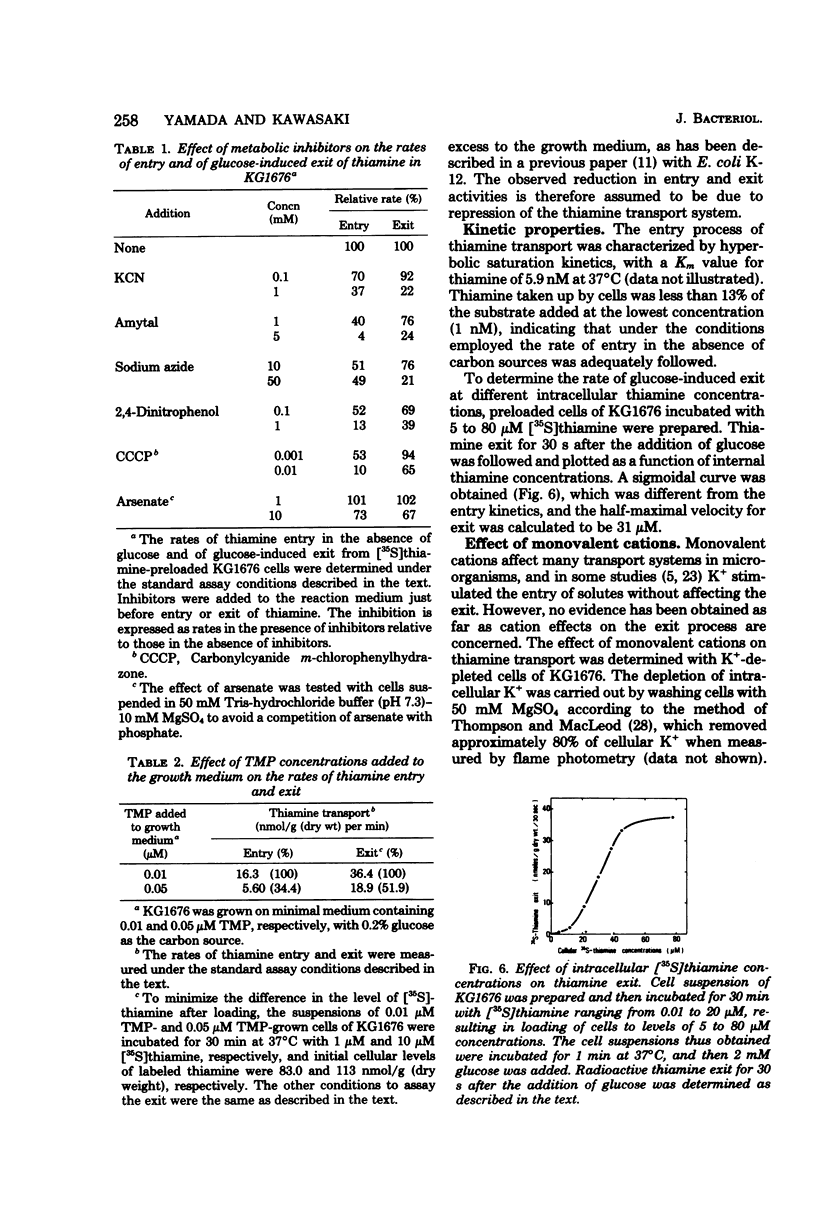
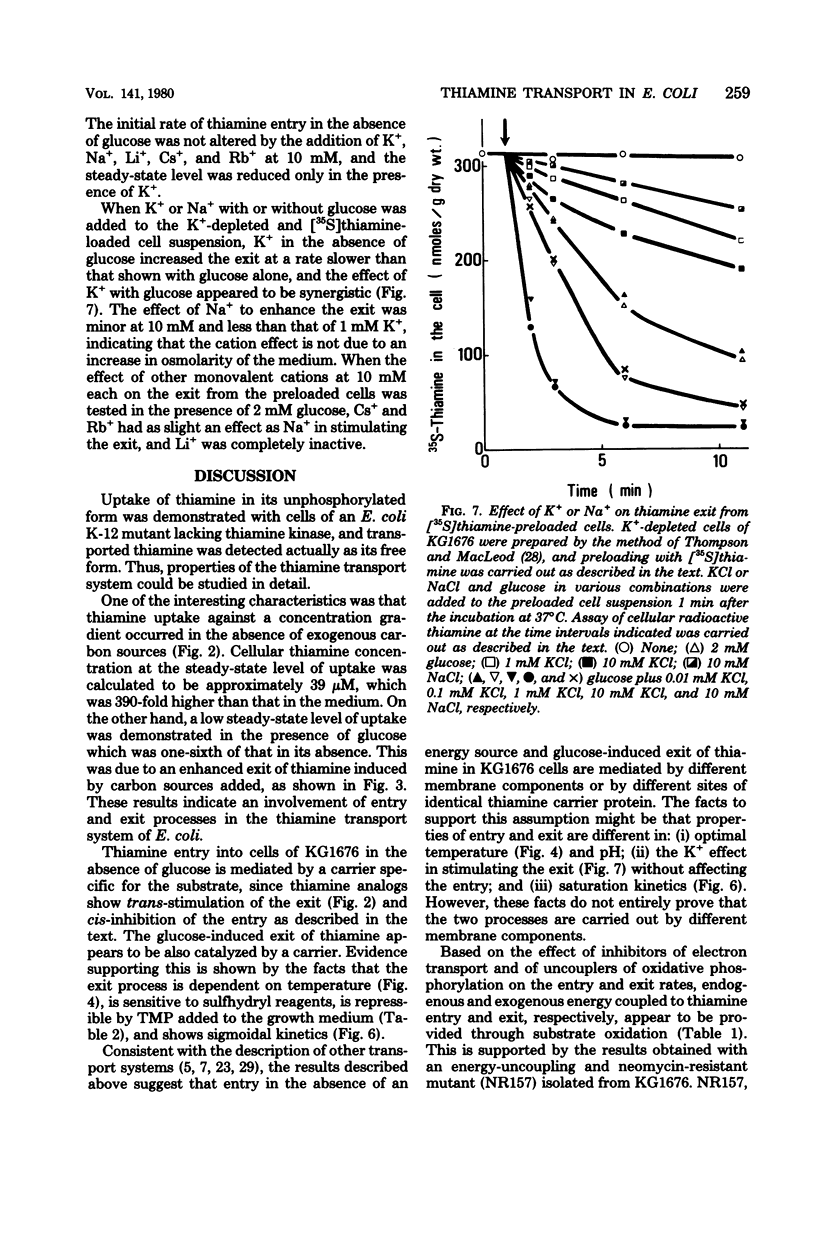
Thiamine transport was studied with a mutant (KG1976) of Escherichia coli K-12 deficient in thiamine kinase (EC 2.7.1.89), which catalyzes the formation of thiamine monophosphate from thiamine. Mutant cells accumulated thiamine 390-fold as the free form against a concentration gradient in the absence of added carbon sources at the steady state. Thiamine taken up from the medium, or thiamine preloaded in the absence of glucose, was expelled into the medium when glucose, d-lactate, or succinate was added, whereas exit in the absence of glucose was very slow. The rate of thiamine entry was therefore determined in the absence of glucose, and that of thiamine exit was followed by the addition of glucose to thiamine-preloaded cells. The activities of thiamine entry and exit were optimal at 42 and 37°C, respectively. Hyperbolic saturation kinetics were obtained for the entry rate with a Km value of 5.9 nM. The exit rate showed a sigmoidal dependence on cellular thiamine concentrations, and a half-maximal velocity was observed at 31 μM. The rates of both entry and exit were lowered by electron transport inhibitors and uncouplers, suggesting that the energy coupled to both processes was provided through substrate oxidation. Thiamine exit from K+-depleted cells was enhanced by K+ alone and by Na+ to a much lesser extent, and K+ and glucose were found to be synergistic for thiamine exit. These cations had no effect on the entry of thiamine into KG1676 cells in the absence of glucose. These properties of the entry and exit of thiamine in KG1676 are discussed from the standpoint of the possible involvement of different membrane components or different sites of identical thiamine carrier protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. II. Characteristics of the exit process. J Biol Chem. 1960 Jun;235:1586–1590. [PubMed] [Google Scholar]
- Halpern Y. S., Barash H., Druck K. Glutamate transport in Escherichia coli K-12: nonidentity of carriers mediating entry and exit. J Bacteriol. 1973 Jan;113(1):51–57. doi: 10.1128/jb.113.1.51-57.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israeli E., Kalra V. K., Brodie A. F. Different binding sites for entry and exit of amino acids in whole cells of Mycobacterium phlei. J Bacteriol. 1977 May;130(2):729–735. doi: 10.1128/jb.130.2.729-735.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashima A., Nishino H., Nose Y. Conversion of thiamine to thiamine monophosphate by cell-free extracts of Escherichia coli. Biochim Biophys Acta. 1972 Jan 20;258(1):333–336. doi: 10.1016/0005-2744(72)90991-6. [DOI] [PubMed] [Google Scholar]
- Iwashima A., Nose Y. Inhibition of thiamine transport by chloroethylthiamine in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1438–1440. doi: 10.1128/jb.112.3.1438-1440.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Esaki K. Thiamine uptake in Escherichia coli. 3. Regulation of thiamine uptake in Escherichia coli. Arch Biochem Biophys. 1971 Jan;142(1):163–169. doi: 10.1016/0003-9861(71)90271-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Miyata I., Esaki K., Nose Y. Thiamine uptake in Escherichia coli. I. General properties of thiamine uptake system in Escherichia coli. Arch Biochem Biophys. 1969 Apr;131(1):223–230. doi: 10.1016/0003-9861(69)90125-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Nakata T., Nose Y. Genetic mapping with a thiamine-requiring auxotroph of Escherichia coli K-12 defective in thiamine phosphate pyrophosphorylase. J Bacteriol. 1968 Apr;95(4):1483–1485. doi: 10.1128/jb.95.4.1483-1485.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Sanemori H., Egi Y., Yoshida S., Yamada K. Biochemical studies of pyrithiamine-resistant mutants of Escherichia coli K12. J Biochem. 1976 May;79(5):1035–1042. doi: 10.1093/oxfordjournals.jbchem.a131144. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Yamada K. The uptake system of free thiamine in mutants of Escherichia coli. Biochem Biophys Res Commun. 1972 Apr 28;47(2):465–471. doi: 10.1016/0006-291x(72)90737-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakayama H., Hayashi R. Biosynthesis of thiamine pyrophosphate in Escherichia coli. J Bacteriol. 1972 Feb;109(2):936–938. doi: 10.1128/jb.109.2.936-938.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Hayashi R. Biosynthetic pathway of thiamine pyrophosphate: a special reference to the thiamine monophosphate-requiring mutant and the thiamine pyrophosphate-requiring mutant of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1118–1126. doi: 10.1128/jb.112.3.1118-1126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino H. Biogenesis of cocarboxylase in Escherichia coli. Partial purification and some properties of thiamine monophosphate kinase. J Biochem. 1972 Nov;72(5):1093–1100. doi: 10.1093/oxfordjournals.jbchem.a129996. [DOI] [PubMed] [Google Scholar]
- Nishino H., Iwashima A., Nose Y. Biogenesis of cocarboxylase in Escherichia coli: a novel enzyme catalyzing the formation of thiamine pyrophosphate from thiamine monophosphate. Biochem Biophys Res Commun. 1971 Oct 15;45(2):363–368. doi: 10.1016/0006-291x(71)90827-8. [DOI] [PubMed] [Google Scholar]
- Perl M., Kearney E. B., Singer T. P. Transport of riboflavin into yeast cells. J Biol Chem. 1976 Jun 10;251(11):3221–3228. [PubMed] [Google Scholar]
- Rosen B. P., Adler L. W. The maintenance of the energized membrane state and its relation to active transport in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):23–36. doi: 10.1016/0005-2728(75)90049-3. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Cation transport in Escherichia coli. I. Intracellular Na and K concentrations and net cation movement. J Gen Physiol. 1961 Nov;45:355–369. doi: 10.1085/jgp.45.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanemori H., Egi Y., Kawasaki T. Pathway of thiamine pyrophosphate synthesis in Micrococcus denitrificans. J Bacteriol. 1976 Jun;126(3):1030–1036. doi: 10.1128/jb.126.3.1030-1036.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]
- Wilson D. B. Properties of the entry and exit reactions of the beta-methyl galactoside transport system in Escherichia coli. J Bacteriol. 1976 Jun;126(3):1156–1165. doi: 10.1128/jb.126.3.1156-1165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Sanemori H., Yamada K., Kawasaki T. Hydroxyethylthiazole uptake in Escherichia coli: general properties and relationship between uptake and thiamine biosynthesis. J Bacteriol. 1973 Dec;116(3):1280–1286. doi: 10.1128/jb.116.3.1280-1286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



