Abstract
Novel therapeutic agents that are safe and effective are needed for the treatment of pancreatic, ovarian, lung adenocarcinomas and mesotheliomas. Mesothelin is a glycosyl-phosphatidyl inositol (GPI)-linked membrane protein of 40 kDa over-expressed in all pancreatic adenocarcinoma and mesothelioma, in >70% of ovarian adenocarcinoma, and in non-small cell lung and colorectal cancers. The biological functions of mesothelin are not known, although it appears to be involved in cell adhesion via its interaction with MUC16. We have recently developed MORAb-009, a mouse-human chimeric IgG1κ monoclonal antibody with an affinity of 1.5 nM for human mesothelin. Here we provide evidence that MORAb-009 prevents adhesion of mesothelin-bearing tumor cells to MUC16 positive cells and can elicit cell-mediated cytotoxicity on mesothelin-bearing tumor cells. Treatment that included MORAb-009 in combination with chemotherapy led to a marked reduction in tumor growth of mesothelin-expressing tumors in nude mice compared to chemotherapy or MORAb-009 treatment alone. No adverse effects of MORAb-009 were noted during toxicology studies conducted in non-human primates. The preclinical data obtained from our studies warrants pursuing clinical testing of MORAb-009. We have in fact initiated a Phase I clinical study enrolling patients with mesothelin-positive pancreatic, mesothelioma, non-small cell lung and ovarian cancers.
Keywords: preclinical drug evaluation, monoclonal antibody, mesothelin, antibody-dependent cellular cytotoxicity
Introduction
Mesothelin is a glycosyl-phosphatidyl inositol-linked membrane glycoprotein expressed in a variety of cancers (1, 2). Immunohistochemistry studies have shown that mesothelin is over-expressed in virtually all pancreatic ductal adenocarcinomas and mesotheliomas (3-5), a high percentage of epithelial ovarian cancers (including fallopian tube and primary peritoneal malignancies), as well as non-small cell carcinomas of the lung (6-10). Expression in normal tissues is limited to mesothelial cells lining the pleura, pericardium and peritoneum (6).
Since mesothelin has recently been reported to bind to CA125/MUC16, one possible biological role of this membrane protein may be linked to heterotypic cell adhesion, which may facilitate the metastatic spread of mesothelin-bearing cancer cells (11). Notwithstanding, the biologic functions of mesothelin remain to be fully defined. Analysis of the mesothelin amino acid sequence yielded no strong homologies to known protein functional domains, other than a C-terminal GPI-anchor motif (2). Moreover, mesothelin does not appear to be required for either normal development or reproduction since there were no apparent abnormalities in mesothelin knockout mice (12).
Two lines of clinical evidence suggest that mesothelin may be an important target for the treatment of mesothelin-bearing tumors. First, tumor responses were observed in a Phase I trial of the anti-mesothelin immunotoxin, SS1P, which included patients with mesotheliomas, ovarian and pancreatic cancer (13). In a different clinical trial, induction of cytotoxic T cell responses to mesothelin epitopes were exclusively obtained in three patients vaccinated with autologous mesothelin-positive tumor cells who had shown delayed-type hypersensitivity after vaccination (14).
Novel therapeutic agents that are safe and effective are needed for the treatment of pancreatic, ovarian, mesothelioma, and lung cancers. Over the years, monoclonal antibodies have proven to be well tolerated, disease antigen-specific, and able to deliver clinical benefits to patients with conditions that include chronic inflammation, lymphomas, and metastatic solid tumors (15). Here we report the preclinical evaluation of MORAb-009, a chimeric monoclonal antibody antagonizing human mesothelin to determine its suitability as an anti-tumor agent. MORAb-009 was able to mediate biological responses in vitro, including inhibition of mesothelin-dependent cell adhesion, antibody-dependent cellular cytotoxicity (ADCC), as well as the reduction of tumor growth in mice implanted with human cancer cells, while exhibiting no adverse effects in non-human primates.
Results
Generation of MORAb-009
The mouse antibody precursor of MORAb-009 was first isolated by panning a phage display library made from splenic mRNA of a mouse immunized with mesothelin cDNA on mesothelin-positive cells (16). The affinity of this mouse single-chain anti-mesothelin antibody was further optimized by re-engineering its variable regions to derive SS1(scFv) (17). Subsequently, we have cloned the heavy and light chain variable regions of SS1(scFv) and grafted them in frame with human IgG1 and kappa constant regions, respectively, yielding the genes coding for a chimeric (mouse/human) antibody designated MORAb-009 showing an affinity (KD) of 1.5 nM. When aligned to its closest human germline immunoglobulin match, MORAb-009 exhibits a high amino acid sequence identity of 82.6% (excluding the complementarity determining regions), compared to a typical 90% identity of a humanized antibody.
MORAb-009 binds to cell surface mesothelin expressed by tumor cell lines
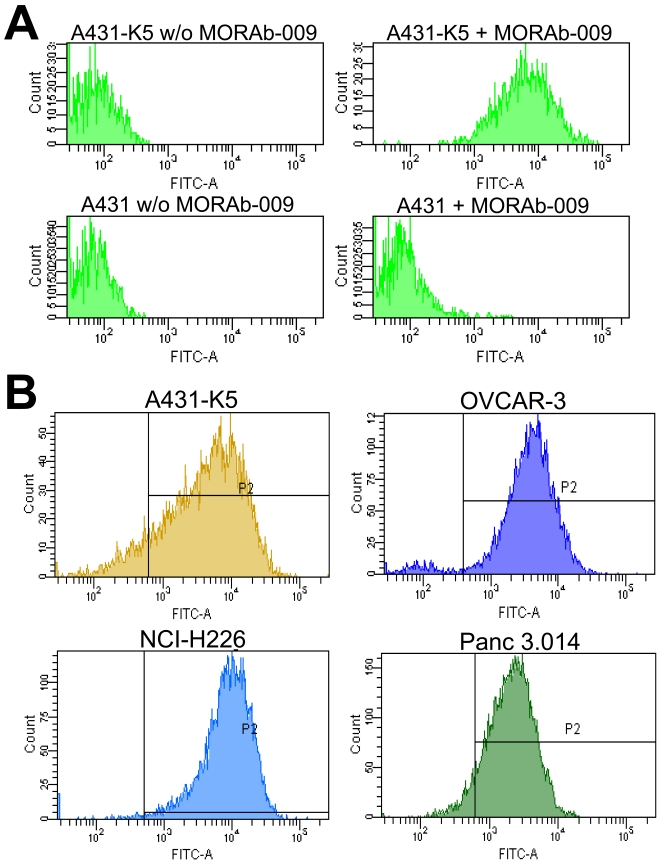
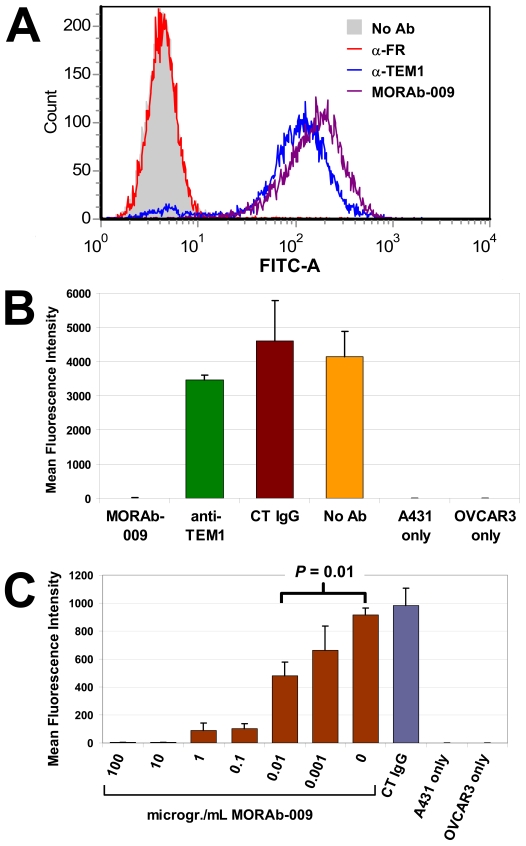
To test the binding specificity of MORAb-009, we conducted FACS analyses using the mesothelin negative and positive cells, A431 and A431-K5, respectively. A431-K5 cells were derived from human epidermoid carcinoma cells (A431) ectopically expressing membrane-bound human mesothelin (6). Surface binding by MORAb-009 was only observed on A431-K5 cells (Figure 1A, top right panel), indicating that MORAb-009 specifically bound mesothelin but not other membrane proteins expressed by these cancer cells. Mesothelin is frequently expressed in pancreatic, mesothelioma and ovarian cancers. We therefore tested whether MORAb-009 could recognize mesothelin on the cell surface of ovarian (OVCAR-3), mesothelioma (NCI-H226), and pancreatic (Panc 3.014) cancer cell lines known to express mesothelin. Significant MORAb-009 binding could be detected in all cell lines tested (Figure 1B) suggesting that this antibody is suitable for targeting tumor-associated mesothelin.
Figure 1.
FACS analyses of tumor cells stained with MORAb-009. (A) Parental A431 cells, as well as cells expressing mesothelin (A431-K5), were reacted with FITC-labeled anti-human IgG antibody, with or without MORAb-009. Positive staining was observed only in the presence of MORAb-009 in A431-K5 cells, demonstrating specificity of the immune staining and lack of reactivity of MORAb-009 with other unrelated membrane proteins. (B) Tumor cells were reacted with FITC-labeled anti-human IgG antibody alone to determine background staining (not shown) and to create gate P2 to capture fluorescent signal above background. MORAb-009 was able to bind mesothelin expressed by ovarian (OVCAR-3), mesothelioma (NCI-H226), and pancreatic (Panc 3.014) cancer cells.
MORAb-009 targets tumor-associated mesothelin
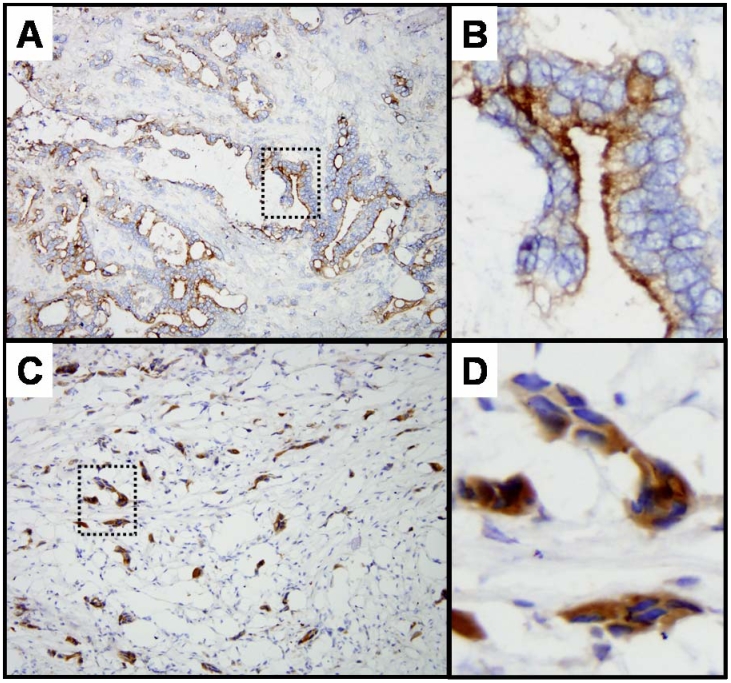
Immunohistochemistry studies supported the ability of MORAb-009 to target mesothelin protein expressed in human pancreatic tumors. We detected membranous staining at the luminal and apical surface of neoplastic cells, although some cytoplasmic staining was also observed (Figure 2). Of the ten frozen pancreatic ductal adenocarcinomas (primary and metastatic disease) studied, all were found to be positive for MORAb-009 staining, with 50% of pancreatic cancer specimens showing strong positive labeling (3+) of at least 75% of the neoplastic cells and the remaining specimens exhibiting a less intensive staining (data not shown). This frequency of mesothelin staining seen with MORAb-009 in pancreatic cancer specimens matched that we have observed using the murine 5B2 antibody (data not shown) and is in line with previous immunohistochemistry studies using the same murine antibody (3). No relationship between tumor stage (primary versus metastasis) and staining intensity was found, although we observed that well differentiated carcinomas tended to stain more robustly than poorly differentiated carcinomas.
Figure 2.
Immunohistochemical staining of human pancreatic tumor tissues. Frozen sections of ductal pancreatic adenocarcinomas (A and C) were stained with MORAb-009. Membranous staining at the luminal and apical surface of neoplastic cells was detected. (B and D) Five-fold enlargement of dotted areas in A and C. Shown are clusters of neoplastic cells where approximately 75% of the cells stained positive with MORAb-009.
Internalization of MORAb-009 upon binding to cell surface mesothelin
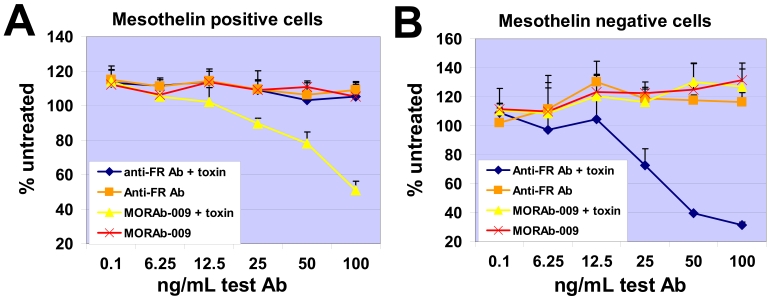
Saporin is a toxin that inhibits protein translation causing cell death only if it is internalized by cells and can be used to determine receptor internalization (18). To address whether MORAb-009 internalizes after binding cell surface mesothelin, we reacted MORAb-009 with an anti-human IgG conjugated with saporin, treated NCI-H226 cells (mesothelin positive) or IGROV-1 cells (mesothelin negative) with this complex, and measured cell death. The MORAb-009-saporin complex, but not MORAb-009 alone, caused significant death of NCI-H226 cells (Figure 3A). A control antibody specific to folate receptor α (FRα), which is not expressed in NCI-H226 cells, expectedly had no effect on cell viability whether alone or complexed with saporin-conjugated anti-human IgG. Neither MORAb-009-saporin complex nor MORAb-009 alone caused significant death of mesothelin negative, FRα positive IGROV-1 cells (Figure 3B). These results suggest that MORAb-009 can internalize after binding mesothelin on the cell surface.
Figure 3.
Internalization of MORAb-009-mesothelin complex. (A) Mesothelin positive, FRα-negative cells were reacted with MORAb-009 alone, MORAb-009 complexed with saporin-conjugated anti-human mAb (toxin), anti-FRα mAb alone, or anti-FRα mAb complexed with toxin. Cell killing, and thus internalization, of MORAb-009-mesothelin complex was only seen with MORAb-009 complexed with toxin. (B) Same as in A using mesothelin-negative, FRα-positive cells. Internalization was only seen with anti-FRα mAb complexed with toxin.
MORAb-009 can exert antibody-dependent cellular cytotoxicity
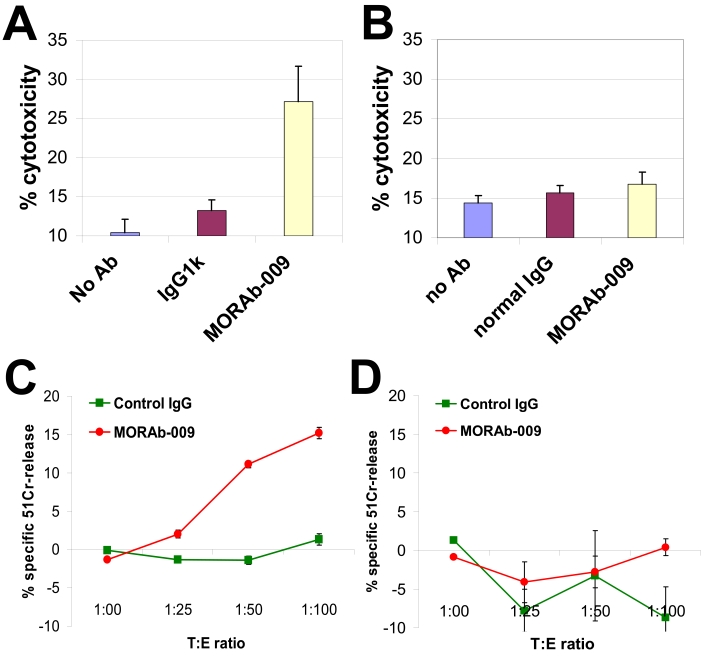
Antibody opsonization of tumor cells followed by the recruitment of immune killer cells eliciting cell-mediated cytotoxicity (ADCC) is thought to be an important mechanism of tumor cell cytotoxicity in vivo. To assess the ability of MORAb-009 to elicit this effector function, we used human cancer cell lines as targets for ADCC. In one strategy, measurement of lactate dehydrogenase (LDH) released by lysed cells was used to determine the extent of tumor cell killing using a constant target to effector ratio of 1:25. MORAb-009 exerted significant ADCC activity against OVCAR-3 cells (Figure 4A) but not on mesothelin negative pancreatic cancer cells, Panc 3.27 (Figure 4B). MORAb-009 also elicited significant ADCC activity on pancreatic cancer cells, Panc 3.014 (data not shown). We also used 51Cr release to measure cell cytotoxicity and tested various target to effector ratios. MORAb-009 again mediated ADCC activity on mesothelin-expressing mesothelioma cells NCI-H226 (Figure 4C) but had no effect on human pancreatic cancer cells, Panc 10.05 (Figure 4D), which express low or undetectable mesothelin levels (data not shown). Maximum effect was seen at a target to effector ratio of 1:100. We did not measure significant complement-mediated cytotoxicity (CDC) by MORAb-009 (data not shown). Since our preliminary mapping identified an epitope for MORAb-009 at the amino terminus of mesothelin, one untested hypothesis is that mesothelin-bound MORAb-009 may be too far from the cell surface for the complement membrane attack complex to be effective.
Figure 4.
MORAb-009 induces ADCC. (A) OVCAR-3 cells were used as target cells and reacted with 10 µg/mL of MORAb-009 or control IgG in the presence of human PBMCs at a ratio of 1:25. ADCC activity was measured by LDH release and only noted with MORAb-009 treatment. (B) Same as in A, whereby target cells (Panc 3.27) express low or no mesothelin. (C) NCI-H226 cells were used as target cells and reacted with 10 µg/mL of MORAb-009 or control IgG in the presence of human PBMCs at different ratios. ADCC activity was measured by 51Cr release and only noted with MORAb-009 treatment. (D) Same as in C, whereby target cells (Panc 10.05) express no or low levels of mesothelin.
CA125/MUC16 and mesothelin-dependent cell adhesion is blocked by MORAb-009
CA125/MUC16 has been identified as a ligand for mesothelin (11). CA125/MUC16 and mesothelin are both expressed in the peritoneal mesothelium as well as in some ovarian cancer cells and are believed to facilitate metastasis of cancer cells to the peritoneum by promoting cell attachment (19). In our preliminary studies, A431-K5 cells, but not parental A431 cells, could bind to CA125/MUC16 positive cells, demonstrating that this heterotypic cell interaction depends on mesothelin expression (data not shown). To explore the possibility that MORAb-009 interferes with mesothelin-CA125/MUC16 interaction, we established A431 cells (A431-K5-TEM) that expressed mesothelin as well as another surface receptor, endosialin/TEM1, but are negative for FRα. Figure 5A shows FACS analysis of A431-K5-TEM cells that exhibited positive staining after being reacted with either MORAb-009 or an anti-endosialin/TEM1 antibody. Expectedly, A431-K5-TEM cells did not stain with an anti-FRα antibody, demonstrating specificity of binding. We then measured adhesion of fluorescently labeled A431-K5-TEM cells onto CA125/MUC16 positive OVCAR-3 cells. Figure 5B outlines the results of this study. In the absence of OVCAR-3 cells, no adhesion of A431-K5-TEM cells to the uncoated surface of the microplate could be observed. Significant heterotypic cell binding was seen on a monolayer of OVCAR-3 cells in the absence of antibody treatment or with control IgG. In contrast, MORAb-009 completely abolished A431-K5-TEM cell adhesion onto OVCAR-3 cells. Although anti-endosialin/TEM1 antibody could bind A431-K5-TEM cells as strongly as MORAb-009 (Figure 5A), it did not inhibit cell-cell interaction, suggesting that MORAb-009-mediated inhibition of cell adhesion was due to specific disruption of mesothelin-CA125/MUC16 interaction rather than steric hindrance at the cell-cell interface. The ED90 of this activity was estimated around 100 ng/mL of MORAb-009 and a statistically significant inhibition was detected with concentrations as low as 10 ng/mL (Figure 5C).
Figure 5.
MORAb-009 blocks CA125/MUC16-mesothelin interaction. (A) FACS analysis of A431-K5-TEM cells reacted with no mAb, anti-FRα mAb, anti-TEM1 mAb, or MORAb-009. Equivalent positive staining was associated with the expression of cognate proteins (TEM-1 and mesothelin) but not with FRα, which is not expressed in these cells. (B) Fluorescently-labeled A431-K5-TEM cells were reacted with no mAb, anti-FRα mAb (CT IgG), anti-TEM1 mAb, or MORAb-009, and seeded on a monolayer of CA125/MUC16 positive OVCAR-3 cells (first four bars). Cell adhesion was completely blocked by MORAb-009 compared to the other treatments. (C) Same as in B, whereby MORAb-009 concentration ranged from 1 ng/mL to 100 µg/mL. Blockade of CA125/MUC16-mesothelin interaction was seen with concentrations as low as 10 ng/mL.
MORAb-009 enhances the anti-tumor effects of chemotherapy in vivo
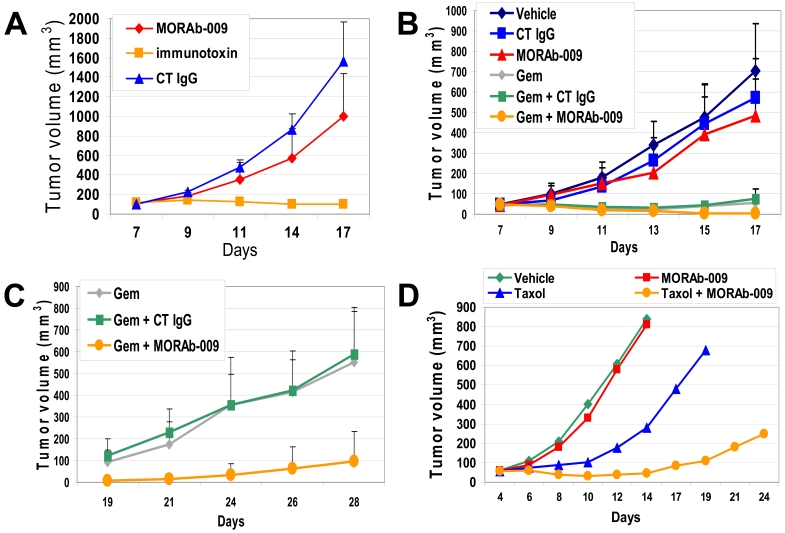
The in vivo anti-tumor effect of MORAb-009 in combination with chemotherapy was evaluated in immunodeficient mice bearing A431-K5 tumor xenografts. The number of receptor sites in these cells is comparable to that of other tumor cells endogenously expressing mesothelin (Figure 1B) and their implantation in mice consistently results in aggressive tumor growth when compared to other mesothelin-positive cells. Preliminary studies using the A431-K5 tumor xenograft model showed moderate but statistically significant (P = 0.01) anti-tumor activity of MORAb-009 alone compared to the isotype control Rituximab, an IgG1 monoclonal antibody that targets the CD20 antigen not expressed on A431-K5 cells (Figure 6A). In this model, the mesothelin-specific immunotoxin SS1(scFv) could completely inhibit tumor growth. In subsequent studies, athymic nude mice bearing A431-K5 tumors were treated with MORAb-009 alone, gemcitabine alone (at a dose that can delay tumor growth without causing regression) or with the combination of the two agents. Seventeen days after inoculation of tumor cells, the average tumor size in mice treated with MORAb-009 alone was reduced compared to vehicle control and Rituximab alone treated mice, albeit this response was moderate and did not reach statistical significance (P = 0.071, Figure 6B). We observed significant tumor growth inhibition in mice treated with gemcitabine alone or in combination with MORAb-009 (P <0.001), compared to control IgG (Rituximab) or MORAb-009 alone groups. Due to their tumor burden, animals in the vehicle control, Rituximab, and MORAb-009 single agent groups were sacrificed around day 17-18. The last dose of MORAb-009 or control IgG was administered on day 17, while we continued monitoring tumor volumes in the remaining groups for an additional 11 days (Figure 6C). Whereas tumors resumed vigorous growth in mice treated with gemcitabine alone, reaching an average volume of 600 mm3 by day 28, the average tumor volume in mice that also received MORAb-009 remained significantly smaller than 100 mm3 (P = 0.001, Figure 6C). Importantly, transient tumor remissions (tumor volumes 0-8 mm3) were only noted in the gemcitabine/MORAb-009 treatment group (6 of the 10 mice) compared to none in the other groups, with two mice remaining tumor-free for the entire course of the study (35 days). Expectedly, the control IgG (Rituximab) had no effect on tumor growth whether administered alone or in combination with gemcitabine (P = 0.548). Since Taxol® is frequently used in the clinical setting as the first line therapy of mesothelin-expressing ovarian and lung adenocarcinomas, we also evaluated possible synergistic anti-tumor activity of MORAb-009 in combination with Taxol® using the above A431-K5 tumor xenograft model. As shown in Figure 6D, while treatment with MORAb-009 alone showed little tumor volume reduction and treatment with Taxol® alone only delayed tumor growth, we observed a more robust anti-tumor effect when Taxol® and MORAb-009 were used in combination. Importantly, four of the seven mice in the Taxol®/MORAb-009 combination treatment group exhibited complete tumor regression compared to none in the other groups.
Figure 6.
Effect of MORAb-009 on tumor growth. (A) A431-K5 cells were inoculated in the flank of nude mice to establish tumors of approximately 50 mm3 in size. On day 7, mice were treated with the control IgG1 Rituximab (CT IgG, 50 mg/kg), MORAb-009 (50 mg/kg), or mesothelin-specific immunotoxin SS1(scFv) (immunotoxin, 0.2 mg/kg). Average tumor size for each treatment group was calculated on day 7-17. (B and C) A431-K5 cells were inoculated as described in A. On day 7, mice were treated with vehicle, control IgG (CT IgG, 50 mg/kg), MORAb-009 (50 mg/kg), gemcitabine (Gem, 80 mg/kg), or combinations of these drugs (see Material and methods for regimens). Average tumor size for each treatment group was calculated on day 7-17 (panel B) and day 19-28 (panel C). Best anti-tumor responses were observed with gemcitabine plus MORAb-009. (D) Same model as in panels A-C, whereby mice were treated with vehicle, MORAb-009 (50 mg/kg), Taxol® (50 mg/kg), or combinations of these drugs. MORAb-009 enhanced the anti-tumor effect of Taxol®.
MORAb-009 safety profile
Western blot analysis utilizing mesothelin-expressing tissues from rat, mouse and cynomolgus monkeys indicated lack of cross-reactivity of MORAb-009 to rodent species but significant binding to monkey tissues (data not shown). Immunohistochemistry (IHC) analysis confirmed similar staining pattern in normal tissues from human and cynomolgus macaque, with staining observed only in mesothelia. Therefore, a 23-day toxicology study with MORAb-009 was conducted in cynomolgus monkeys. This study employed relatively high doses of MORAb-009 administered over a short period to establish an initial safety profile of MORAb-009. There were no abnormal clinical observations, macroscopic or microscopic findings related to the administration of MORAb-009 and the data indicated that the no observed adverse effect level (NOAEL) of MORAb-009 was greater than the seven doses of 15 mg/kg administered over 23 days.
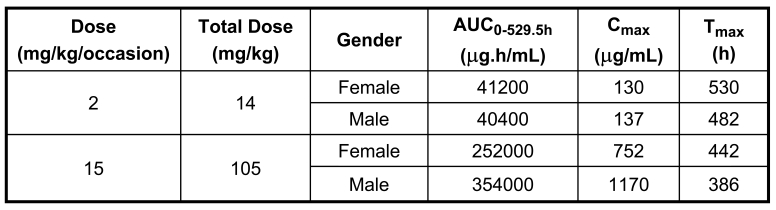
Toxicokinetic analyses were performed and the results are summarized in Table 1. Systemic exposure of female and male monkeys to MORAb-009 increased proportionately with increasing dose. There were no gender differences at 2 mg/kg/h/dose (total dose = 14 mg/kg); however, exposures in males were greater than females at 15 mg/kg/h/dose (total dose = 105 mg/kg). Over a 7.5-fold dose range, AUC0-529.5h values for females and males increased 6.12 and 8.76-fold, respectively, and Cmax values for females and males increased 5.78 and 8.54-fold, respectively. The serum concentration levels of MORAb-009 at 30 minutes prior to and 30 minutes after the last infusion were greater than those after the second infusion, indicating a possible accumulation after repeated intravenous infusions. MORAb-009 was measurable in serum up to 1224 hours after starting the first infusion in the designated monkeys for the recovery period. MORAb-009 half-life was relatively long in designated female and male monkeys for the recovery period (341 and 285 hours, respectively). Overall, the pharmacokinetics of MORAb-009 followed the typical pattern of large biomolecules administered intravenously. The Tmax occurred at the first time point measured after the intravenous infusion was complete (30 minutes post-infusion in this study). There was drug accumulation in the plasma following multiple infusions. The volume of distribution was initially equal to the plasma volume.
Table 1.
Mean serum pharmacokinetic parameters of MORAb-009 in male and female monkeys following intravenous infusion.
Discussion
To advance new therapeutic candidates for solid tumors expressing mesothelin, we conducted the preclinical evaluation of MORAb-009, a high affinity chimeric anti-mesothelin monoclonal antibody, and determined its suitability as an anti-tumor agent. Our results show that MORAb-009 is able to mediate biological responses in vitro, including inhibition of mesothelin-dependent cell adhesion and ADCC activity. In addition, MORAb-009 reduced tumor growth and enhanced the effect of chemotherapy in mice implanted with human tumor cells while exhibiting no adverse effects in non-human primates.
In order to detect possible cross-reactivity, MORAb-009 was applied to a human tissue panel that included all tissues recommended by the US Food and Drug Administration. The binding profile was essentially similar to that reported for other mesothelin-specific monoclonal antibodies, whereby reactivity to normal tissues was limited to mesothelium (data not shown). As part of a safety study, we attempted to determine the dose level at which potential toxicity may occur due to binding of MORAb-009. Since MORAb-009 does not bind to rodent tissues and its staining pattern in human and cynomolgus monkey tissues was similar, only non-human primates were used to determine potential toxicity. The results of these studies indicated that the estimated no observed adverse effect level for MORAb-009 in cynomolgus monkeys was greater than the seven doses of 15 mg/kg administered over 23 days. The lack of toxicity observed could be explained by the limited mesothelin expression in normal tissues as evidenced by the IHC studies. Furthermore, although MORAb-009 was derived from a mouse antibody via chimerization with a human IgG1 backbone, it shares 82.6% amino acid sequence identity to a human IgG1κ. This may explain the lack of monkey anti-chimeric antibody responses observed.
Although MORAb-009 as a single agent had moderate anti-tumor activity against mesothelin-expressing tumor xenografts in vivo, this effect was markedly increased in combination with either gemcitabine or Taxol®. Presently, the mechanism(s) by which MORAb-009 enhances the anti-tumor effects of chemotherapeutic agents such as gemcitabine and Taxol® is unknown. One hypothesis is that binding of MORAb-009 leads to opsonization of tumor cells and their subsequent killing by cytotoxic immune cells, whereby this effect is additive or synergistic to that of chemotherapy. Mesothelin has been suggested to play a role in cell adhesion and we have shown that MORAb-009 can block mesothelin binding to CA125/MUC16 (Figure 5). Although we have no evidence that in our animal models tumor growth depends on cell adhesion, specifically on CA125/MUC16-mesothelin interaction, it is still possible that binding of MORAb-009 to mesothelin interferes with other tumor-host interactions, creating a condition whereby tumor cells are more susceptible to chemotherapy. Alternatively, gemcitabine and Taxol® can enhance MORAb-009 activity by improving tumor blood flow (20), facilitating the penetration and diffusion of MORAb-009 into the tumor. Two studies, one in mice and the other in humans, showed that Taxol® increased tumor uptake of radiolabeled antibody-interleukin 2 immunocytokine (21, 22). Regardless of the exact mechanism of the synergy between MORAb-009 and chemotherapy, this interaction could be useful in the clinic for the treatment of mesothelin-expressing cancers with MORAb-009 in combination with gemcitabine or Taxol®, two commonly used drugs for the treatment of pancreatic, ovarian, lung adenocarcinomas, and mesotheliomas.
In view of the encouraging preclinical data obtained from our studies, and to evaluate the safety profile of MORAb-009 in humans, we have initiated a Phase I clinical study enrolling patients with mesothelin-positive pancreatic, mesothelioma, non-small cell lung and ovarian cancers. Results obtained from this study will help guide the development of MORAb-009 alone or in combination with chemotherapy for the treatment of mesothelin-expressing cancers.
Abbreviations
- FRα
folate receptor α
References
- 1.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 3.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 4.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 5.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 6.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 7.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 9.Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, Pastan I. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Sarlomo-Rikala M. Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am J Surg Pathol. 2003;27:150–158. doi: 10.1097/00000478-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 12.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham M, Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin for targeted therapy of mesothelin expressing mesotheliomas, ovarian and pancreatic cancer [meeting abstract].; J Clin Oncol; 2007. p. 3553. [DOI] [PubMed] [Google Scholar]
- 14.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolaides NC, Sass PM, Grasso L. Monoclonal antibodies: a morphing landscape for therapeutics. Drug Dev Res. 2006;67:781–789. [Google Scholar]
- 16.Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci U S A. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury PS, Pastan I. Analysis of cloned Fvs from a phage display library indicates that DNA immunization can mimic antibody response generated by cell immunizations. J Immunol Methods. 1999;231:83–91. doi: 10.1016/s0022-1759(99)00142-8. [DOI] [PubMed] [Google Scholar]
- 18.Fransson J, Borrebaeck CA. The nuclear DNA repair protein Ku70/80 is a tumor-associated antigen displaying rapid receptor mediated endocytosis. Int J Cancer. 2006;119:2492–2496. doi: 10.1002/ijc.22212. [DOI] [PubMed] [Google Scholar]
- 19.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, Pastan I, Patankar MS. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 21.Holden SA, Lan Y, Pardo AM, Wesolowski JS, Gillies SD. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin Cancer Res. 2001;7:2862–2869. [PubMed] [Google Scholar]
- 22.Miers L, Lamborn K, Yuan A, Richman C, Natarajan A, DeNardo S, DeNardo G. Does paclitaxel (Taxol) given after (111)In-labeled monoclonal antibodies increase tumor-cumulated activity in epithelial cancers? Clin Cancer Res. 2005;11:7158s–7163s. doi: 10.1158/1078-0432.CCR-1004-0012. [DOI] [PubMed] [Google Scholar]
- 23.Jaffee EM, Schutte M, Gossett J, Morsberger LA, Adler AJ, Thomas M, Greten TF, Hruban RH, Yeo CJ, Griffin CA. Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am. 1998;4:194–203. [PubMed] [Google Scholar]
- 24.Embuscado EE, Laheru D, Ricci F, Yun KJ, de Boom Witzel S, Seigel A, Flickinger K, Hidalgo M, Bova GS, Iacobuzio-Donahue CA. Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther. 2005;4:548–554. doi: 10.4161/cbt.4.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Materials and methods
Cells
NCI-H226 and OVCAR-3 cells were purchased from the American Type Culture Collection (ATCC). IGROV-1 cells were obtained from the NCI cell collection. Panc 10.05 cells were previously described (23). Panc 3.27 and Panc 3.014 cells were generated in Dr. Elizabeth Jaffee's laboratory. A431-K5 cells were derived from A431, an epidermoid carcinoma cell line that has been stably transfected with a plasmid encoding mesothelin (6). A431-K5-TEM cells were derived in Dr. Grasso’s laboratory from A431-K5 cells by transfection with an expression vector containing the full-length endosialin/TEM1 cDNA.
FACS analyses
Cells were harvested in cell dissociation solution (Invitrogen), washed, and resuspended in ice-cold PBS containing 1% FBS. Primary antibodies were added at 10 µg/mL and incubated for 1 hour on ice. Next, the cells were washed with PBS, incubated with FITC-conjugated goat-anti-human secondary antibody (Southern Biotech, Birmingham, AL) diluted to 1 µg/mL, washed as above and analyzed on an EasyCyte Flow Cytometer (Guava Technologies, Hayward, CA).
Immunohistochemistry
Samples of snap frozen pancreatic cancer were obtained from pancreaticoduodenectomy specimens or from tissues harvested in accordance with the Gastrointestinal Cancer Rapid Medical Donation Program at The Johns Hopkins Hospital (24). Five micron sections were cut from each tissue onto sterile glass slides and fixed in 100% ethanol for 1 minute, followed by rinsing in 1x TBS with Tween (TBST) for 5 minutes. Next, endogenous peroxidases were blocked by incubation with 3% hydrogen peroxide for 10 minutes followed by a second rinse in 1x TBST. Next, slides were incubated with a 0.5 µg/mL concentration of MORAb-009 antibody (Morphotek Inc., Exton, PA) diluted in 0.2 M Tris-HCL pH 7.5 for 1 hour at room temperature and again rinsed in 1x TBST for 5 minutes. Immunolabeling was detected using the LSAB+ Kit (DAKO) according to the manufacturer's instructions. Slides were counterstained in hematoxylin for 30 seconds and coverslipped. Staining patterns were evaluated by two of the authors (S.M. and C.I.D.) as negative (0), weakly positive (1+), positive (2+) and strongly positive (3+), and the percent labeling was evaluated on a 10-tiered scale ranging from 10% to 100%.
Internalization of MORAb-009
The human cell lines NCI-H226 and IGROV-1 were cultured in complete RPMI 1640 medium (1 mM sodium pyruvate, 5 U/mL penicillin, 5 µg/mL streptomycin, 2 mM L-glutamine) containing 10% FBS (Invitrogen) at 37˚C, in a humidified atmosphere containing 5% CO2. One day prior to addition of antibodies, cells were collected by trypsinization and plated at a density of 500 cells/well in a 96-well tissue culture plate (Nalgene Nunc) in complete RPMI 1640 medium. MORAb-009, polyclonal, saporin-conjugated goat anti-human antibodies (Hum-ZAP, Advanced Targeting Systems) or control antibodies were mixed in various ratios and incubated for 30 minutes at room temperature, prior to addition to cultured cells. Cells were incubated at 37˚C, in a humidified atmosphere containing 5% CO2, for 5 days prior to harvesting. Relative changes in cell numbers were quantified by the sulforhodamine B method. Briefly, cells were killed and fixed for one hour on ice by addition of ice-cold 100% trichloroacetic acid (TCA) directly into the growth medium, to a final concentration of 10%. TCA was removed by washing fixed cells 5 times using distilled, deionized water, and allowed to dry in open atmosphere. 100 µL of a solution of 0.4% sulforhodamine B in distilled, deionized water were added to each well, and incubated for 10 minutes. The wells were washed 4 times using 1% acetic acid and allowed to dry in open atmosphere. The resulting precipitate was dissolved in 100 µL of 10 mM Tris-Cl pH 8.0, and the absorbance at 540 nm was measured with a microplate reader. Relative cell numbers were determined by interpolation from a standard curve constructed by measuring absorbance of known cell numbers from each cell line, deposited in the bottom of a 96-well tissue culture plate. Results were expressed as % growth, by comparison to untreated cells.
ADCC assay
Donor peripheral blood mononuclear cells (PBMCs) used as effector cells were thawed and kept overnight in Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 10% fetal calf serum. The cells were resuspended in medium at a concentration of 107 cells/mL. The tumor cells used as target cells were detached from the culture flask and 106 cells in 100 µL fetal calf serum were labeled with 100 µCi (3.7 MBq) 51Cr for 2 hours at 37˚C. Alternatively, lysing of cells was monitored by a standard lactate dehydrogenase (LDH) release assay following the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN). Cells were washed three times with 5 mL of medium and resuspended in medium at a concentration of 105 cells/mL. Fifty microliters of the tumor cells were seeded in V-bottom 96-well culture plates. Cells were then incubated with 50 µL medium containing MORAb-009 or control antibody at concentrations indicated in the text. After 30 minutes of incubation at 37˚C, 50 µL of the PBMCs were seeded in V-bottom 96-well culture plates at various target-effector cell ratios (1:0, 1:25, 1:50 and 1:100) and the plates were further incubated for 18 hours at 37˚C. The release of 51Cr in the supernatant was determined in a gamma counter. Each measurement was carried out in triplicate.
CA125/MUC16-mesothelin interaction assay
OVCAR-3 cells (1 x 105) were seeded in triplicate in microplates, incubated overnight at 37˚C/5% CO2 and allowed to form a monolayer. The following day, A431-K5-TEM cells (mesothelin+/TEM1+) were harvested and loaded with Calcein AM cell dye (Invitrogen Corp., Carlsbad, CA) as per the instruction manual. Labeled cells (2 x 105) were pre-incubated for 1 hour at 4˚C with MORAb-009 or control antibodies at a final concentration indicated in the figure legends. Both normal human polyclonal IgG (Jackson Immunoresearch, WestGrove, PA) and anti-TEM1 antibody (Morphotek Inc., Exton, PA) were included to control for the specificity of the MORAb-009 inhibition of the mesothelin-MUC16 interaction. The OVCAR-3 monolayers were washed once with 200 µL 10% complete RPMI (cRPMI) and the cells/antibody mixtures added to triplicate wells for 45 minutes at 4˚C. Wells were gently washed five times with 200 µL PBS by inverting the plate on paper towels. Cell adherence and its inhibition by MORAb-009 were quantitated using the MetaMorph Fluorescence Imaging System (ver.6, Molecular Dynamics, Downingtown, PA).
In vivo efficacy studies
Gemcitabine and Taxol® were provided by the Division of Veterinary Resources (NIH). A431-K5 cells were grown in DMEM with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 units/mL penicillin G, 100 µg/mL streptomycin and 750 µg/mL G418. Tumor experiments evaluating MORAb-009 in combination with gemcitabine were conducted using A431-K5 xenografts in nude mice. Four to six week old female athymic nude mice (National Cancer Institute-Frederick Animal Production Area, Frederick, MD) were housed in microisolator cages for the course of the experiment. The research protocol was approved and the mice were maintained as per institutional guidelines of the NIH. Two million A431-K5 cells were inoculated subcutaneously into the right flank of the mice. Tumor dimensions were determined using calipers and the tumor volume (mm3) was calculated by the formula: length x (width)2 x 0.4. Treatment was initiated when tumors reached approximately 50 mm3 in size. The different treatment regimens included: gemcitabine (80 mg/kg) alone via i.p. injection on days 7, 9 and 11 after tumor inoculation; MORAb-009 or Rituximab (50 mg/kg) alone given i.v. on days 7, 9 and 11 after tumor inoculation; gemcitabine (80 mg/kg) i.p. on days 7, 9 and 11 in combination with either MORAb-009 or Rituximab (50 mg/kg) i.v. on days 7, 9, 11, 13, 15 and 17 after tumor inoculation. Mice were sacrificed when the tumors reached approximately 500 mm3. To test the in vivo activity of MORAb-009 in combination with Taxol®, the same A431-K5 tumor xenograft model was used. On day 4 after tumor cell inoculation when the tumor size was approximately 60 mm3, mice (n = 7 per treatment group) were treated with either Taxol® 50 mg/kg i.p. on day 4, MORAb-009 50 mg/kg i.v. on days 4, 6 and 8 alone or the combination of Taxol® 50 mg/kg on day 4 and MORAb-009 50 mg/kg i.v. on days 4, 6, 8, 10, 12 and 14. Mice were sacrificed when the tumors reached approximately 500 mm3.
Non-human primate toxicity study
After preliminary dose-ranging studies, a 23-day intravenous infusion toxicity study in cynomolgus monkeys with a 28-day recovery period was conducted under Good Laboratory Practice (GLP). At the saline control and 15 mg/kg dose levels, three monkeys per sex were dosed with MORAb-009 twice per week over 23 days (seven total doses). At the 2 mg/kg dose level, two monkeys per sex were dosed at the same frequency. Two days after the last dose, two monkeys per sex were euthanized and their necropsy carried out. The remaining control and high dose monkeys in the respective recovery groups were observed for 28 additional days before euthanasia and necropsy. Clinical evaluations of the monkeys included the following: physical examinations pre- and post-dosing; daily clinical observations, including mortality checks; body weight measurements prior to study start, and twice weekly throughout the study; daily food consumption estimates beginning one week prior to treatment throughout the study; and hematology, coagulation, clinical chemistry and urinalysis measurements prior to study start and at study termination. Postmortem evaluations included the following: complete macroscopic examinations on all monkeys; selected organ weights; and complete histopathology examination. Blood was drawn before and after each dose, as well as during the recovery period for the determination of the toxicokinetics of MORAb-009 and for the detection of the possible generation of monkey anti-chimeric antibodies, which were determined by using ELISA.