Abstract
Human colorectal cancer tissues are infiltrated by various immune/inflammatory cells, usually along the invasive margin. These responses tend be regarded as “non-specific”. However, it is now clear that these cellular responses, particularly lymphocytic reactions, are independent prognostic factors for a better survival. Immunohistochemical subset analyses have generally disclosed that the number of T-lymphocytes is important. The effects of these T cells tend to be more manifest when the observation periods are longer. These data suggest that some degrees of anti-tumor immunity exist in human colorectal carcinomas. However, human tumors are generally composed of various histologic subtypes, which sometimes complicates these analyses and simple explanations may be misleading. Considered with precaution, these morphologic analyses on immune cell subtypes are important tools to understand the immune responses in human cancers.
Keywords: human, colorectal cancer, tumor infiltrating lymphocytes, prognosis
General aspects of colorectal cancer
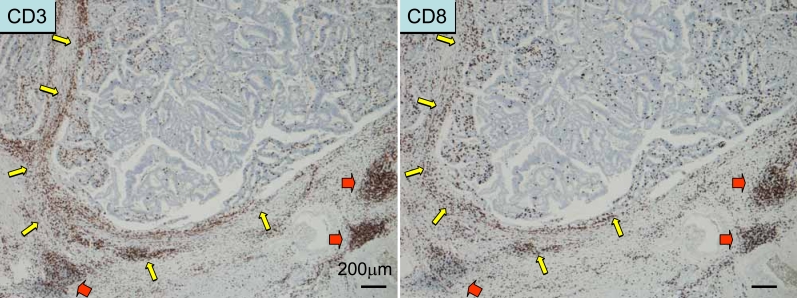
Most cases of colorectal cancer are adenocarcinomas, of which the majority of cases are of the differentiated type (well differentiated or moderately differentiated adenocarcinoma) (1). With this preponderance of the differentiated type, colorectal cancer is composed of rather homogenous histologic types. This is one of the significant features that should be borne in mind in dealing with the current topic. Colorectal cancers become clinically malignant after cancer cells invade through the muscularis mucosa into the submucosa (1, 2). With this invasion, various host reactions take place, which include desmoplasia of the stroma (fibrogenic reaction) and various immune/inflammatory responses that are more concentrated along the invasive margin (tumor-host interface) (3). Immune/inflammatory infiltrates include lymphocytes, neutrophils, and macrophages (Figure 1, Figure 2, Figure 3, and Figure 4). With this feature, these infiltrates tend to be regarded as non-specific, leading to the assumption that they may not be relevant to the patients' prognosis. The present review discusses the significance of immune/inflammatory infiltrates in colorectal cancer.
Figure 1.
Immune cell responses in colorectal cancer tissue. The tumor can be regarded as “peripheral inflamed tissue”, whereas the lymphoid infiltrate is similar to, but different from, “secondary lymphoid tissue”. Lymphocytic infiltration along the invasive margin (yellow arrows) and “Crohn's-like lymphoid aggregates” (red arrows) are seen. Areas within the cancer are usually sparse in lymphocytic responses; this can be the effect of immune evasion mechanisms.
Figure 2.
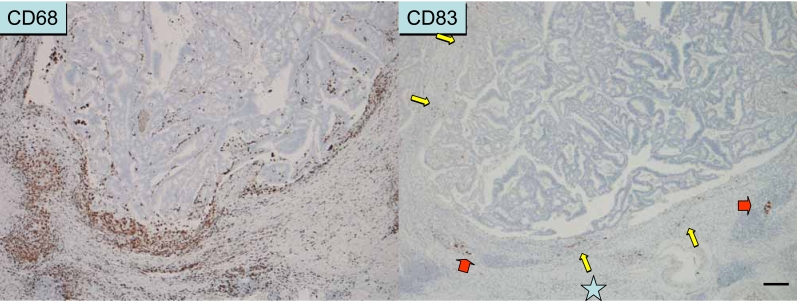
Immune response observed in areas along the invasive margin. These areas are rich in inflammatory cells, including macrophages (as shown by CD68 immunostaining) and neutrophils (not shown). Mature dendritic cells (shown by CD83 immunostaining) are usually distributed along the invasive margin with T cells, but not in the cancer tissue.
Figure 3.
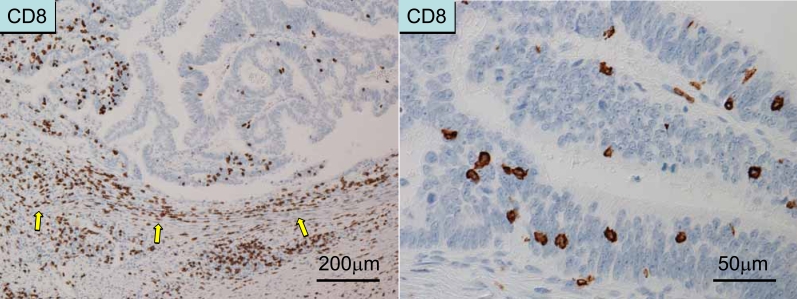
Localization of T cells. Left panel: T cells along the invasive margin (peritumoral T cells) as detected by CD8 immunostaining. Right panel: Intraepithelial T cells (as detected by CD8 immunostaining).
Figure 4.
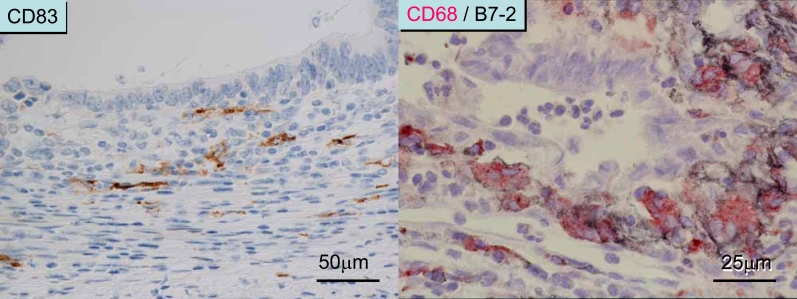
Immune/inflammatory infiltrates in colorectal cancer tissue. Left panel: The T cell infiltrate also co-localizes with mature DCs (as shown by CD83 immunostaining). The number of DCs is not so abundant compared with that in secondary lymphoid tissue. Right panel: CD68+ macrophages abundantly distributed along the invasive margin also express B7-2 (CD86) and HLA-DR (not shown), a phenotype similar to that of mature DCs.
Prognostic impact of tumor-infiltrating lymphocytes (TILs) observed in colorectal cancer by conventional histopathological methods (Boxes 1 and 2)
Lymphocytes are recognized as small round cells by HE-stained sections. However, for well-trained pathologists, these cells are usually differentiated from plasma cells, neutrophils, eosinophils, macrophages, or mast cells by their histologic features. Jass et al. (4) pointed out that conspicuous lymphocytic infiltration along the invasive margin of rectal cancer is an independent prognostic factor for improved survival using multivariate analysis. The other significant factors included the number of lymph node metastases, the pattern of invasive margin (expanding or infiltrating) and limitation of the tumor growth to the bowel wall. Ropponen et al. (5) confirmed the concept of a prognostic impact of TILs in colorectal cancer. They semi-quantified the degree of TILs in the stroma and those around the tumor (along the invasive margin) into four groups, and showed that TILs are a significant predictor of improved overall- and recurrence-free survival by both univariate- and multivariate analysis (Cox proportional hazards model). They also showed an inverse correlation between the presence of TILs and tumor stage; i.e. TILs are more prominent in the early stages (Dukes A and B) and decrease in the advanced stages (Dukes C and D). The concept of TILs was then expanded to include lymphoid aggregates that are formed apposing the invasive margin (termed “Crohn's-like lymphoid reaction”) (6). In their report, lymphoid aggregates were found to be independently prognostic for improved survival in right-sided colon cancer in multivariate analysis.
Box 1. Prognostic factors.
-
The most important one is the stage by definition.
Stage grouping of colorectal carcinoma
I (Dukes A): no penetration of muscle layer
II (Dukes B): penetration of muscle layer
III (Dukes C): lymph node metastasis
IV (Dukes D): distant metastasis
For more details, see the TNM classification.
Other factors (including TILs) are generally regarded as “modulatory factors”.
Box 2. How to understand the results of multivariate analysis.
Multiple co-variates are analyzed at the same time
For each co-variate, patients are grouped, for example into two, without any intention of the researchers (if the co-variate is consecutive, the patients are usually divided at the median value).
The results are usually expressed in terms of the following:
hazard ratio (indicating the power of co-variate to affect the prognosis)
95% confidence interval of hazard ratio
p-value
In addition to TILs, follicular and paracortical hyperplasia in regional lymph nodes are important for prognosis of colorectal cancer (7) (here “paracortex” means T cell zone). In the analyses by Pihl et al. (7), the most significant result was noted in the paracortical hyperplasia in tumor-involved lymph nodes in stage III patients (i.e., patients with lymph node metastasis). The study clearly demonstrated that immune responses taking place in regional lymph nodes are also important for the patients' survival. The study by Pihl et al. (7) is of particular importance because of the interplay between immune reactivity in the primary tumor and that in the regional lymph nodes. Both responses are closely linked via, for example, the migration of dendritic cells (DCs) from the primary sites to the regional lymph nodes (8). Immature DCs can uptake antigens in the primary sites (that are regarded as “inflammatory sites”) and then migrate to the regional lymph nodes to become mature DCs and prime antigen-specific T cells. In this regard, a recent review stressed the importance of immunosuppressive effects by cancer cells in sentinel lymph nodes, which make the microenvironment more suitable for the establishment of metastasis (9). These reports, based on conventional histopathological methods, suggest that immune responses taking place at the primary tumor sites or those in the regional lymph nodes may reflect anti-tumor activity, although it could be argued that most immune reactivity would be non-specific and probably related to the intestinal bacterial flora in colorectal carcinoma.
Epstein-Barr virus-associated gastric cancer
Before discussing colorectal cancer, it is relevant to mention features of Epstein-Barr virus (EBV)-associated gastric cancers, as these could be considered representative of lymphocyte-rich cancers. These cancers are composed of the spectrum ranging from the lymphocyte-rich “medullary carcinoma with lymphoid stroma” (“lymphoepithelioma-like carcinoma”) to the usual adenocarcinoma that is not associated with lymphoid stroma (10). However, as Saiki et al. (11) pointed out, these EBV-associated gastric cancers feature prominent CD8+ T cell infiltration of the cancer tissues (particularly into the cancer cell nests), sharply contrasting with the EBV-negative adenocarcinoma which may be associated with a T cell response only along the invasive margin. Unexpectedly these CD8+ T cells in cancer tissues bore significant degrees of labeling for Ki-67 (13% on average), suggesting that these T cells are actively proliferating. This suggests that attention should be paid to these CD8+ T cells that have infiltrated the cancer cell nests (i.e., intraepithelial CD8+ T cells) in the analyses of host immune responses in cancer tissues.
Subset analysis by immunohistochemistry: The presence of T cell infiltrate is an indicator for a better prognosis in colorectal carcinoma (Figure 1)
In most colorectal cancers, cancer tissues are infiltrated by few lymphocytes and only the area along the invasive margin is infiltrated by lymphocytes and other inflammatory cells, ranging from a thick continuous pattern to nearly none. It is also worthy to note that lymphocytic infiltrate is nearly always accompanied by neutrophil and macrophage infiltration in colorectal cancer. In most cases these lymphocytes are either CD4+ or CD8+ T cells (12, 13). B cells are usually observed in lymphoid follicles, which are occasionally observed. Intraepithelial lymphocytes (i.e., lymphocytes within cancer cell nests or lymphocytic infiltrate in neoplastic epithelium) are usually CD8+ T cells. Although small in number, the number of these intraepithelial CD8+ T cells, as quantified in the most abundant areas, significantly correlated with patients' longer disease-specific survival in an analysis of 138 patients (14) and another of 385 patients (15) by both uni- and multivariate analysis, including N (lymph node metastasis)- and M (distant metastasis)-categories as co-variates. Similar results were obtained when patients were categorized as those who had undergone curative surgery (i.e., patients who were judged to be cancer-free at the completion of surgery) (14). In contrast, the degree of CD8+ T cells in cancer stroma and the degree of CD8+ T cells along the invasive margin are not a significant prognostic factor for patients' survival (14). These results suggest that tumor-infiltrating lymphocytes, particularly intraepithelial CD8+ T cells, have anti-tumor activity as judged by their favorable effect on patients' survival.
In these analyses, patients were divided into two groups at the median value of the number of intraepithelial CD8+ T cells. In addition, the significance at other dichotomy points was analyzed. For example, patients were divided into two groups at the 75 percentile or 80 percentile values. These analyses were performed to examine the significance of cases with a higher number of intraepithelial CD8+ T cells. However, no significant results were obtained at either the 75 or 80 percentile level. Additional factors, such as the location of the primary tumor (right-sided colon, left-sided colon, or rectum) did not affect survival significantly.
Further analysis of TILs in colorectal carcinoma
Diederichsen et al. (16) showed that a low CD4/CD8 ratio by flow cytometric analysis is an independent prognostic factor for a longer survival by multivariate analysis. Although the study did not discriminate the precise in situ location of TILs, it clearly showed that the CD4/CD8 ratio is important and that this may be related to the current concept of the role of CD4+ CD25+ FOXP3+ regulatory T cells in tumor tissue (17).
To further analyze immune responses in colorectal cancer, Pages et al. (18) performed a comprehensive analysis of TILs. They focused on “early metastatic invasion”, which included the following histopathological findings: vascular emboli (by tumor cells), lymphatic invasion, and perineural invasion (collectively referred to VELIPI). They first showed that VELIPI is an independent prognostic factor in an analysis of 959 patients. Real-time PCR analysis of 75 patients showed that the levels of mRNAs for CD8, granzyme B, and granulysin are higher in VELIPI-negative tumors than in VELIPI-positive tumors. They also performed a large-scale flow cytometric analysis in 39 patients to differentiate between naive (CD3+ CCR7+), early memory (CD45RO+ CCR7- CD28+ CD27+) and effector memory (CD45RO+ CCR7- CD28- CD27-) T cells in tumor tissue. VELIPI-negative tumors contained a high proportion of mature CD8+ T cells. Furthermore, they showed that VELIPI-negative tumors from 377 patients contained a high number of such CD45RO+ cells by immunohistochemistry. VELIPI-negative tumors from patients without relapse had a significant increase in the Th1 mediator T-BET, interferon regulatory factor 1, and interferon-γ compared to VELIPI-positive tumors from patients which had experienced a relapse. The number of CD45RO+ cells was found to be an independent prognostic factor, together with the TNM category, by Cox proportional hazards regression.
There are two main reservations about this study. First, there was no clear rationale for subdividing patients at a count of 250 cells per square millimeter. Second, no distinction was made regarding the intraepithelial or stromal location of CD45RO+ cells. Despite these limitations, this study strongly supports the notion that immune surveillance has a significant prognostic impact on colorectal cancer.
Recently, the same group extended their study of TILs in colorectal cancer further (19). In this paper, they first analyzed gene expression levels by real-time PCR in 75 patients with colorectal cancer and showed that the expression of Th1 adaptive immunity is inversely correlated with tumor recurrence. Particularly, patients with a homogeneously increased expression of genes for Th1 adaptive immunity had the best prognosis. Genes for Th1 adaptive immunity included interferon regulatory factor 1, CD3, CD8, granzyme B, granulysin, interferon-γ, and T-box 21 (TBET). They next performed immunohistochemistry for CD3, CD8, granzyme B and CD45RO in 415 patients with colorectal cancer using tissue microarray. In the study, they analyzed both the tumor center and invasive margin. They showed that patients without recurrence had a higher immune cell density in both the tumor center and invasive margin. They confirmed these results in patients from three institutions. They finally revealed that patients with CD3CThi, CD3IMhi plus CD45ROCThi CD45IMhi showed longer recurrence-free survival, while patients with CD3CTlo, CD3IMlo plus CD45ROCTlo CD45IMlo showed shorter survival, suggesting that immune cell density is a stronger prognostic factor than TNM staging.
The methods used in the study are innovative and the results provocative. However, a closer look at Figure 3A in the paper shows that the disease-free survival curve of stage III patients (with lymph node metastasis) suddenly plateaus at 37-38 months after surgery, which makes the survival rate of the stage III patients not so different from that of stage I and II patients. Therefore, there is a need for more analyses to determine whether immune cell factors are better predictors of survival than TNM staging.
NKT cells in colorectal cancer
By adopting a highly sensitive method, Tachibana et al. (20) succeeded in the immunoperoxidase- and immunofluorescent staining of T cell receptor repertoires Vα24 and Vβ11 in formalin-fixed, paraffin-embedded sections to identify NKT cells. By double staining they showed that most NKT cells in colorectal cancer express the early activation marker CD69, contrasting with the lower number of NKT cells with a lower rate of CD69 expression in the normal colon mucosa. NKT cell infiltration positively correlated with fewer lymph node metastases, and the number of NKT cells was an independent prognostic factor for longer overall- and disease-free survival. Although their paper did not specify the exact location of NKT cell infiltration in colon cancer tissues, a subsequent report indicated that the cells were located along the invasive margin (21). These findings suggest that NKT cells co-localize with other immune or inflammatory cells. Their method is innovative and could significantly contribute to the immunological analysis of human tissue.
Microsatellite instability and lymphocytic infiltration in cancer tissues
A high level of DNA microsatellite instability (MSI) is a feature of hereditary nonpolyposis colorectal cancer (HNPCC) and the same change is observed in sporadic colorectal cancers (1). These cases are characterized by abundant CD3+ CD8+ lymphocytic infiltrate in neoplastic epithelium that could result from the immunologic recognition of frameshift-mutated peptides (22, 23). However, in the analysis by Chiba et al. (15), there was no significant increase in the number of intraepithelial CD8+ T cells in cases with loss of mismatch repair enzymes. Therefore, it appears that although MSI could be associated with high infiltration of T cells into tumor tissues, additional mechanisms may exist to attract CD8+ T cells into colonic neoplastic epithelium.
T cell and NK cell infiltration into cancer cell nests, as well as downregulation of MHC class I molecule, correlate with improved survival
The expression of MHC class I molecule on the cell surface is a prerequisite for the T cell recognition of target cells. Menon et al. (24) reported that decreased expression of MHC class I expression, particularly HLA-A expression, in tumor cells unexpectedly correlates with longer disease-free survival. They further showed that CD8+ and CD57+ cells along the invasive margin cells are independent prognostic factors for a longer disease-free survival by multivariate analysis, and that intraepithelial CD8+ and CD57+ cells inversely correlated with HLA-A and HLA-B/C expression by cancer cells. The same group also confirmed that there were fewer CD56+ intraepithelial cells than CD8+ intraepithelial cells (they confirmed that these CD8+ cells are CD3+ but CD56-), and that loss of MHC class I molecules in cancer cells is associated with a higher number of intraepithelial T cells, but not CD56+ NK cells (24). The interpretation of these findings is unclear. It is possible that MHC class I-negative variants are selected due to killing of MHC class I-positive tumor cells by intraepithelial T cells, and these tumor cells are able to maintain the capacity to attract T cells (25). Taken together, intraepithelial CD8+ T cell infiltration may occur despite a decrease in MHC class I expression by colon cancer cells, and these CD8+ T cells, as well as NK cells, play a significant role in immune surveillance in colorectal cancer.
Dendritic cells and TILs
Dendritic cells (DCs) are the most potent antigen-presenting cells, and as such are now one of the many important tools for tumor immunotherapy (8). Their main functioning sites are the T cell zone in secondary lymphoid organs, for example, regional lymph nodes (Figure 5). However, DCs are also distributed in peripheral inflamed tissues. In colorectal cancer, mature DCs are distributed along the invasive margin together with T cells (26) (Figure 2). The prognostic significance of these DCs is also important. Dadabayev et al. (27) described that MHC class II positive cells are distributed in tumor stroma, and cases with abundant class II positive cells are associated with shorter survival. However, it is possible that these class II positive cells may not be mature DCs, since mature DCs and T cells are not usually abundant in tumor stroma (26), and an abundant expression of intercellular adhesion molecule-1 (ICAM-1) by fibroblasts in the stroma (28) may interfere with the function of DCs, if present. Sandel et al. (29) from the same group described that intraepithelial DC-lamp (CD208+) mature DCs and DC1a+ DCs in the invasive margin are associated with a shorter survival. Unfortunately, the figure illustrating this observation is difficult to interpret in the paper (Figure 2, page 2580) and no clear data for the multivariate analysis were presented. Clearly, further clarification of the prognostic impact of DC subtypes in colorectal cancer is needed since mature DCs are distributed in colorectal cancer tissue (particularly along the invasive margin), and a certain interaction is suspected between DCs and T cells which could foster immune responses.
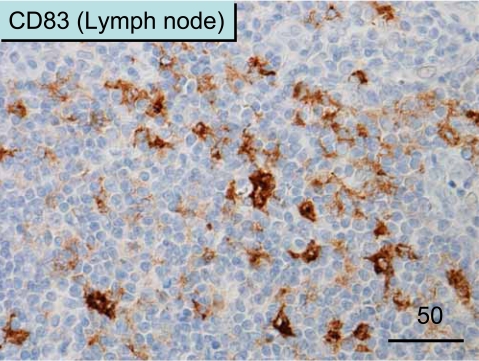
Figure 5.
CD83+ mature DCs in the T cell zone of lymph nodes. Note the abundant distribution of dendritic-shaped DCs.
Further considerations on the real effect of TILs in cancer tissue (Figure 6, Figure 7, Box 3)
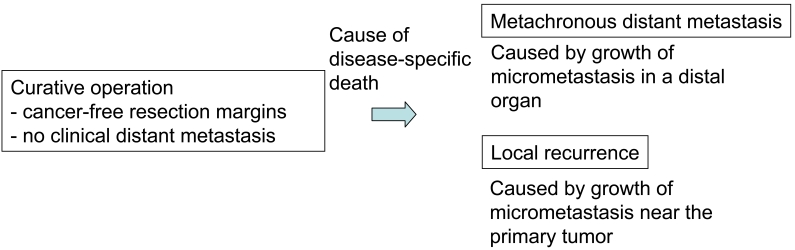
Figure 6.
Significance of a prognostic factor in patients undergoing curative operation.
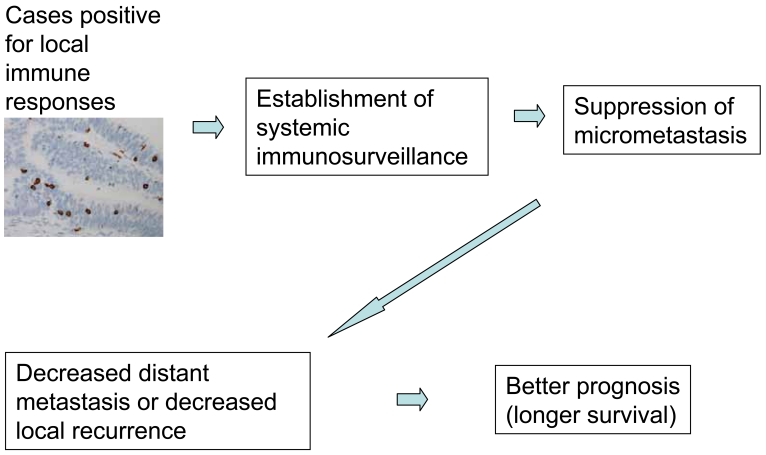
Figure 7.
Hypothesis on the effects of TILs.
Multivariate analyses clearly indicate that the prognostic impact of intraepithelial CD8+ T cells in colon cancer is more evident when the follow-up period is longer (15). Indeed, disease-specific survival curves for patients with different levels of intraepithelial CD8+ T cells (low vs. high) are similar in the first 1-2 years and then separate (15). Accordingly, immune responses in colorectal cancer appear to have a late effect. Theoretically, the patients' disease-specific death observed with a longer follow-up period is likely due to a late occurrence of distant metastasis or local recurrence. If we consider that immune responses in cancer tissue can suppress the occurrence of metastasis (or micrometastasis) in distant organs or in the connective tissue/lymph node near the primary site, we could easily understand these observations. Therefore, Chiba et al. (15) proposed the following hypothesis: the presence of intraepithelial CD8+ T cells in colorectal cancer could indicate the occurrence of systemic immunosurveillance against micrometastasis. A review of previous reports revealed similar results. For example, in an analysis of the effects of paracortical hyperplasia in lymph nodes in colorectal cancer, the two survival curves (high vs. low hyperplasia) did not show significant differences in the first 3 years of follow-up. However, the curve for high paracortical hyperplasia showed significantly longer survival in the case of a >3-year follow-up period [see Figure 3, p. 475, in Pihl et al. (7)]. This observation is in accordance with the data by Chiba et al. (15). Moreover, Pages et al. (18) also demonstrated that early metastatic invasion is negatively associated with immune responses in tumor tissues. These suggest that local immune responses observed in tumor tissues may not be confined to the local sites, but could reflect systemic anti-tumor immune responses. Further studies are required to clarify this concept which could have a significant impact on tumor immunity in human cancer.
Box 3. Example of multivariate analysis.
Curative operation group (N=260), observation period set to > 2 years (up to 10 years) after operation to check late effects.
| Co-variate (risk factor) | Hazard ratio | 95% Confidence interval | p-value |
|---|---|---|---|
| Lymph node metastasis (positive) | 4.3 | 2.6-7.4 | <0.0005 |
| Invasion pattern (infiltrating) | 2.6 | 1.6-4.4 | <0.0005 |
| Intraepithelial CD8+ cells (low) | 1.8 | 1,1-3.0 | <0.015 |
Co-variates only significant by univariate analysis are analyzed. No patients with distant metastasis at the time of operation are included by definition.
This example shows that the number of intraepithelial CD8+ T cells divided at the median value is significant for the patients’ disease-specific survival. Its power is evident when the observation period is set to a longer period. Its power is not so prominent as compared with the tumor stage (N-category). This indicates that immune response is a modulatory factor.
Further considerations on the inverse association of immune responses and tumor stage; is this cause or effect? (Figure 8)
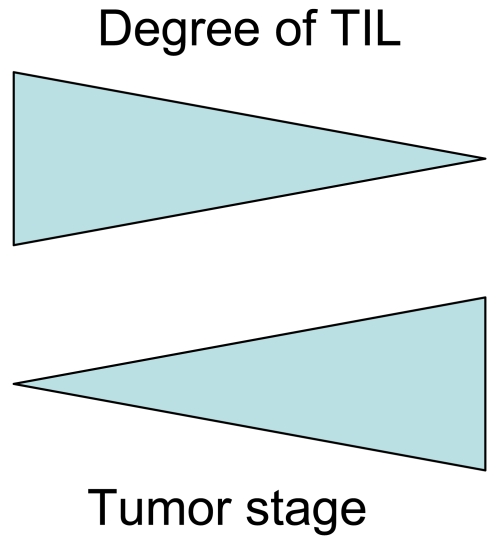
Figure 8.
Inverse association of immune responses and tumor stage. Immune responses in tumor tissues (TILs) tend to be prominent in early stages and decrease in advanced stages. Both the tumor stage and the presence/absence of TILs (mutually correlated) contribute to determine the prognosis.
Detailed mechanistic studies are usually needed to determine a cause or an effect of correlative observations. However, it could be quite difficult to apply this to human subjects. For example, the number of peritumoral macrophages (as detected by CD68) and the number of intraepithelial CD8+ T cells is inversely associated with tumor stage, being lowest in stage IV colon cancer. This may be a consequence of systemic immune suppression in advanced stage patients; alternatively, insufficient infiltration of these cells may be a cause of metastasis. To resolve this, Saito et al. (30) and Chiba et al. (15) compared patients with synchronous distant metastasis (stage IV) and patients who underwent a curative operation and later died of the cancer. The latter should have micrometastasis at the time of resection of the primary cancer. The basic concept is as follows: if the decrease in the number of macrophages or lymphocytes is an effect of advanced cancer stage, then the number of these cells in the latter cases should be higher, because only micrometastases should be present at the time of operation, being insufficient to cause systemic immunosuppression. The results showed that there are no significant differences between the two groups. This does not support the idea that the decrease in the number of macrophages or lymphocytes in stage IV cases is an effect of systemic immunosuppression in advanced cancer. This in turn suggests that the decrease in the number of intraepithelial lymphocytes or peritumoral macrophages would be a cause of widely spread tumor. This notion could be applied to other studies.
Possible correlation between tumor grade (de-differentiation status) and immune responses in tumor tissue (Figure 9)
Figure 9.
Immune responses in tumor tissue may correlate with tumor grade (or de-differentiation status).
Cancers in most organs show histological heterogeneity; for example, histologies ranging from well differentiated to poorly differentiated, with the latter usually being associated with more aggressive behavior. We need to bear this in mind to analyze the prognostic significance of TILs, because the degree of TIL infiltration may depend on the tumor differentiation status.
In a study of renal cancer, Nakano et al. (31) clearly demonstrated that TILs, including CD8+ or CD4+ peritumoral or intraepithelial CD8+ T cells, are weakly associated with a worse prognosis, and that these TIL-factors correlated with tumor grade (de-differentiation status). Namely, more de-differentiated renal cell carcinomas have more pronounced lymphocytic infiltration. Multivariate analyses demonstrated tumor grade is significant but the presence of TILs was not. This clearly indicates that lymphocytic infiltrate does not cause worse prognosis, but positive correlation to tumor grade causes it (in renal cell carcinoma). Therefore, immune responses are not judged to be a tumor-promoting (or progressing) factor. In colorectal carcinoma, the incidence of poorly differentiated adenocarcinoma is generally low [i.e., 6.0% or 22 of 364 cases; Chiba et al. (15)], hence it does not have a significant influence. The author would like to stress this point for every study on the prognostic significance of TILs. In renal cell carcinoma, the labeling index of Ki-67 among CD8+ T cells that infiltrated into cancer cell nests (intraepithelial cells) is an independent prognostic factor (31). This indicates that patients with renal cell carcinoma infiltrated by proliferating CD8+ T cells survive longer. The magnitude of stimuli of T cells in cancer cell nests could reflect the efficacy of anti-tumor immunity.
Lymphocytes in colorectal cancer co-localize with macrophages and neutrophils
Recent reports emphasize the promoting effects of macrophages and neutrophils on cancer development and progression (32). These inflammatory cells are an important factor for cancer development when we consider cancer development based on chronic inflammation (i.e., gastric cancer in Helicobacter pylori gastritis, colitic cancer, hepatocellular carcinoma associated with viral hepatitis). However, in colorectal cancer, the situation may be different. As stated above, neutrophils are one of the predominant cell types along the invasive margin. The number of neutrophils, as detected by immunohistochemistry for neutrophil elastase, was not found to be significant for patients' survival in a univariate analysis of 200 cases of colorectal cancer (33). This suggests that neutrophils are unlikely to promote cancer progression in colorectal cancer. Considering the close in situ distribution of neutrophils and lymphocytes in colorectal cancer tissue, it is possible that there is interplay between neutrophils, dendritic cells, and/or lymphocytes (34).
Macrophages are abundant in the stroma within cancer tissue and also along the invasive margin of colorectal cancer, of which only the latter expresses the costimulatory molecule B7-2 (CD86) (12) and HLA-DR (28). As macrophages distributed along the invasive margin of colorectal cancer share some features with dendritic cells, they may be also be immunologically related to the T cells that are distributed in the same location. Indeed, immunoelectron microscopy has revealed that lymphocytes are in close cell-to-cell contact with B7-2+ macrophages that are distributed along the invasive margin of colorectal carcinoma (12). The number of macrophages along the invasive margin as detected by CD68, one of the lysosome-related antigens, was not found to be significant for patients' survival in an analysis of 200 cases of colorectal carcinoma (33). However, the number of macrophages were found to be inhibitory to distant metastasis in colorectal cancer as already mentioned (29) (see the section “Further considerations on the inverse association of immune responses and tumor stage; is this cause or effect?”). These results suggest that we need to pay much more attention to macrophages.
Regulatory T cells
To understand the balance of immune responses, it is important to analyze both effector and immunosuppressive cells. For example, CD4+ CD25+ FOXP3+ regulatory T cells (Treg) are important for tumor immune evasion (35). Sato et al. (36) pointed out that the ratio of CD8/Treg is an independent prognostic factor for a longer survival in ovarian cancer by multivariate analysis. The impact of this factor on patient survival is larger than that of the number of intraepithelial CD8+ T cell. The comprehensive analysis of immune cells with opposing functions is thus important (see the upcoming article by Sato et al. in this series of reviews on TILs).
Entry site of immune/inflammatory cells to colorectal cancer tissue
It is through the high endothelial venules (HEV) that lymphocytes enter or leave the lymphoid tissue of secondary lymphoid organs. In an analysis of colorectal cancer patients, Suzuki et al. (37) reported that venules distributed along the invasive margin express E- and P- selectins, thus expressing a similar phenotype to that observed in inflammatory bowel disease (38). This similarity, and the co-localization of lymphocytes, neutrophils and macrophages, led Suzuki et al. to speculate that immune/inflammatory cells enter cancer tissues through these venules. Therefore, analyses of vascular changes in colorectal cancer could improve the understanding of immune responses in the disease.
Chemokines and their receptors for the recruitment of T cell
Flow cytometric analysis showed that most of the T-lymphocytes isolated from colorectal cancer co-express the chemokine receptors CCR5 and CXCR3, and express interferon gamma (INF-γ) upon stimulation, suggesting that they are T helper type 1 cells (Th1) and effector T cells (39). By immunohistochemistry, IP10/CXCL10 is expressed in macrophages and part of cancer cells along the invasive margin. RANTES/CCL5 is expressed in CD8+ T cells (that also express CCR5 and CXCR3). By immunoelectron microscopy, RANTES/CCL5 is clearly localized in the cytolytic granules of lymphocytes (39). These data suggest that the mechanism of recruitment of lymphocytes to colorectal cancer tissue is analogous to that of inflammatory disease (40). The author would like to stress the importance of these comparative studies between host responses in tumor tissues and inflammatory disorders.
Conclusion
As stated above, a considerable component of immune reactivity in colon cancer could be non-specific, possibly related to intestinal bacterial flora. In addition, several mechanisms are known to prevent effective anti-tumor immune responses against cancer cells/tissues (immune evasion mechanisms) (35). Despite these, it is clear that parts of the immune/inflammatory responses are related to anti-tumor immunity, and that cases associated with more pronounced responses are not as aggressive as those with limited immune responses in colorectal carcinoma. These clinopathological and clinico-immunological data are important tools to analyze the immune response in human cancer tissues.
For data analyses, a careful collaboration between pathologists, clinicians, immunologists and statisticians is required. In the author's personal experience, one has to be cautious in planning the appropriate methods of histopathological observation (usually quantification) so that the data can be analyzed using suitable statistical methods, and one also has to carefully consider the biological significance of the results obtained by statistical analyses. The author recommends close discussion among collaborators to understand the significance of multivariate analyses.
Abbreviations
- VELIPI
vascular emboli, lymphatic invasion, and perineural invasion
Acknowledgements
The author is grateful to the participation of the following collaborators in our studies: Yuriko Saiki, Takayuki Mizoi, Yoshitaka Naito, Yukimasa Suzuki, Kazuya Saito, Satoshi Takeha, Masuko Mori, Osamu Nakano, Junichi Sugita, Hiroaki Musha, Motoji Oki, Wakana Iijima, Tomofumi Chiba and Eiichi Sato. Cordial acknowledgements are also made to Dr. Lloyd J. Old and Dr. Kunle Odunsi, for critical reading and correction of the manuscript.
References
- 1.Mamilton SR, Aaltonen LA. WHO classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. [Google Scholar]
- 2.Sobin LH, Wittekind Ch. TNM classification of malignant tumours. New York (NY): John Wiley & Sons. Inc.; 2002. 6th ed. [Google Scholar]
- 3.Ohtani H. Pathophysiologic significance of host reactions in human cancer tissue: desmoplasia and tumor immunity. Tohoku J Exp Med. 1999;187:193–202. doi: 10.1620/tjem.187.193. [DOI] [PubMed] [Google Scholar]
- 4.Jass JR, Love SB, Northover JMA. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303–1306. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 5.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Harrrison JC, Dean PJ, El-Zeky F, Vander Zwaag R. Impact of the Crohn's-like lymphoid reaction on staging of right-sided colon cancer: results of multivariate analysis. Hum Pathol. 1995;26:31–38. doi: 10.1016/0046-8177(95)90111-6. [DOI] [PubMed] [Google Scholar]
- 7.Pihl E, Nairn RC, Milne BJ, Cuthbertson AM, Hughes ESR, Rollo A. Lymphoid hyperplasia: a major prognostic feature in 519 cases of colorectal carcinoma. Am J Pathol. 1980;100:469–480. [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 9.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SPL, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6:659–670. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 10.Lertprasertsuke N, Tsutsumi Y. Gastric carcinoma with lymphoid stroma. Analysis using mucin histochemistry and immunohistochemistry. Virchows Arch A Pathol Anat Histopathol. 1989;414:231–241. doi: 10.1007/BF00822027. [DOI] [PubMed] [Google Scholar]
- 11.Saiki Y, Ohtani H, Naito Y, Miyazawa M, Nagura H. Immunophenotypical characterization of Epstein-Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest. 1996;75:67–76. [PubMed] [Google Scholar]
- 12.Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible anti-tumor immunity? Lab Invest. 1997;77:231–241. [PubMed] [Google Scholar]
- 13.Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ, Kuppen PJ. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84:493–501. doi: 10.1038/labinvest.3700055. [DOI] [PubMed] [Google Scholar]
- 14.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T-cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 15.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423–428. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 18.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 20.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 21.Tsuruyama T. NKT cell infiltration in human colorectal cancer as a prognostic factor.; 95th General Meeting of the Japanese Society of Pathology; 2006. [Google Scholar]
- 22.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macri E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa T, Fujita T, Suzuki Y, Okabe S, Yuasa Y, Iwai T, Kawakami Y. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 2003;63:5564–5572. [PubMed] [Google Scholar]
- 24.Menon AG, Morreau H, Tollenaar RA, Alphenaar E, Van Puijenbroek M, Putter H, Janssen-Van Rhijn CM, Van De Velde CJ, Fleuren GJ, Kuppen PJ. Down-regulation of HLA-A expression correlates with a better prognosis of colorectal cancer. Lab Invest. 2002;82:1725–1733. doi: 10.1097/01.lab.0000043124.75633.ed. [DOI] [PubMed] [Google Scholar]
- 25.Sandel MH, Speetjens FM, Menon AG, Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA, van de Velde CJ, Kuppen PJ. Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol. 2005;42:541–546. doi: 10.1016/j.molimm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, Kasajima T. Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol. 2002;196:37–43. doi: 10.1002/path.1018. [DOI] [PubMed] [Google Scholar]
- 27.Dadabayev AR, Sandel MH, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn C, Ensink NG, Tollenaar RA, van de Velde CJ, Kuppen PJ. Dendritic cells in colorectal cancer correlate with other tumor-infiltrating immune cells. Cancer Immunol Immunother. 2004;53:978–986. doi: 10.1007/s00262-004-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizoi T, Ohtani H, Suzuki Y, Shiiba K, Matsuno S, Nagura H. Intercellular adhesion molecule-1 expression by macrophages in human gastrointestinal carcinoma: possible roles as host immune/inflammatory reaction. Pathol Int. 1995;45:565–572. doi: 10.1111/j.1440-1827.1995.tb03504.x. [DOI] [PubMed] [Google Scholar]
- 29.Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn CM, Ensink NG, Tollenaar RA, van de Velde CJ, Kuppen PJ. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res. 2005;11:2576–2582. doi: 10.1158/1078-0432.CCR-04-1448. [DOI] [PubMed] [Google Scholar]
- 30.Saito K, Takeha S, Shiiba K, Matsuno S, Sorsa T, Nagura H, Ohtani H. Clinicopathologic significance of urokinase receptor- and MMP-9-positive stromal cells in human colorectal cancer: Functional multiplicity of matrix degradation on hematogenous metastasis. Int J Cancer. 2000;86:24–29. doi: 10.1002/(sici)1097-0215(20000401)86:1<24::aid-ijc4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of anti-tumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 32.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 33.Naito Y, Unpublished data.
- 34.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 35.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 36.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki Y, Ohtani H, Mizoi T, Takeha S, Shiiba K, Matsuno S, Nagura H. Cell adhesion molecule expression by vascular endothelial cells as an immune/inflammatory reaction in human colon carcinoma. Jpn J Cancer Res. 1995;86:585–593. doi: 10.1111/j.1349-7006.1995.tb02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura S, Ohtani H, Watanabe Y, Fukushima K, Matsumoto T, Kitano A, Kobayashi K, Nagura H. In situ expression of the cell adhesion molecules in inflammatory bowel disease: Evidence of immunologic activation of vascular endothelial cells. Lab Invest. 1993;69:77–85. [PubMed] [Google Scholar]
- 39.Musha H, Ohtani H, Mizoi T, Kinouchi M, Nakayama T, Shiiba K, Miyagawa K, Nagura H, Yoshie O, Sasaki I. Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma. Int J Cancer. 2005;116:949–956. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 40.Iijima W, Ohtani H, Nakayama T, Sugawara Y, Sato E, Nagura H, Yoshie O, Sasano T. Infiltrating CD8+ T cells in oral lichen planus predominantly express CCR5 and CXCR3 and carry respective chemokine ligands RANTES/CCL5 and IP-10/CXCL10 in their cytolytic granules: a potential self-recruiting mechanism. Am J Pathol. 2003;163:261–268. doi: 10.1016/S0002-9440(10)63649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]