Abstract
Tumors contain variable numbers of lymphocytes, referred to as tumor infiltrating lymphocytes (TILs). In melanoma, the intensity of this lymphocytic infiltrate is believed to correlate with outcome, though there is some debate about the applicability of this finding for all melanomas. Much research has gone into classifying TILs with respect to antigen receptor structure and the antigen to which melanoma-specific T cells react. However, these studies for the most part did not immunophenotype TILs, and recent data has revealed that the composition of tumoral lymphocytes is not homogenous, but rather represents varying contributions from many lymphocytic subsets. Furthermore, the function of TILs is often compromised as a result of the accumulation of immunoregulatory cells and various tumor escape mechanisms. These recent insights stress the need to collect more data on the composition and function of TIL infiltrates before definitive conclusions about the prognostic significance of TILs can be drawn. Advances in immunology have also facilitated the development of immunotherapeutic strategies, examples of which will be discussed with a special emphasis on blocking antibodies against CTLA-4, which are prototypical immunotherapeutic agents. This flurry of novel "biological" therapies will undoubtedly complicate our already incomplete understanding of TIL immunobiology as each of these agents has the potential to uniquely distort the series of immunological events which normally occur in untreated melanoma. Therefore, considerable research is needed to better elucidate the function and prognostic significance of TILs in both untreated melanoma and tumors treated with "biological" therapy.
Keywords: human, melanoma, tumor-infiltrating lymphocytes, prognosis, biological therapy, CTLA-4
Evolution of the TIL concept
More than 100 years ago, malignant tumors were first noted to contain variable numbers of lymphocytes (1), which have come to be known as tumor infiltrating lymphocytes (TILs). Initially, these TILs were thought to reflect the origin of cancer at sites of chronic inflammation (1), and later it was debated whether TILs provided a favorable environment for cancer growth or were evidence of the host's attempt to eliminate cancer (2). A relationship was first identified between the extent of immune cell infiltration and prognosis in 1949 in cases of breast cancer (3). In 1969, Clark et al. (4) first described the lymphocytic infiltration of primary cutaneous melanoma, a finding which Day et al. (5) and Tuthill et al. (6) later found to be of prognostic significance. Patients with a moderate-to-marked lymphocytic infiltrate within their primary melanoma had a significantly better prognosis and a 3-times higher 5-year survival rate than patients with a sparse or absent lymphocytic infiltrate (5). Elder et al. (7) differentiated the lymphocytic infiltrate into brisk, non-brisk, or absent, according to its intensity, and demonstrated that TILs were of prognostic significance only in vertical growth phase (VGP) melanoma. In contrast, the extent of lymphocytic infiltration had no prognostic influence in radial growth phase (RGP) melanomas, regardless of whether the melanoma was in situ or invasive (7), findings which were verified by Clemente et al. (8). The 5- and 10-year survival rates were 77% and 55% in melanomas with brisk VGP infiltrates; 53% and 45% with non-brisk VGP infiltrates; and 37% and 27% without VGP infiltrates (8). Also, the number of TILs in the primary tumor has been found to be inversely correlated with the probability for lymph node metastases (8). Patients with brisk TIL infiltrates in their primary tumors showed a 3.9% probability of a positive sentinel lymph node (SLN), compared to a 26.2% probability in patients with TILs absent from their primary melanoma (9). Furthermore, of those patients with regional lymph node metastases, patients with more marked lymphocytic responses in their metastatic melanoma showed a significantly higher 30-month disease-free survival rate (81.3% for patients with a brisk TIL infiltrate; 46.8% for patients with a non-brisk infiltrate; and, 29.3% for patients with TILs absent from their lymph node metastases) (5, 10). However, other studies could not convincingly demonstrate that brisk TIL infiltrates were associated with improved survival in melanoma patients (11-13). These discrepant results may in part be explained by differences in patient populations investigated, with particular reference to the thickness of patients' melanomas (9). The study by Clemente et al. (8) found the impact of TILs most pronounced in patients with high-risk lesions, thicker than 1.7 mm but less than 6 mm in Breslow depth (9). This suggested that the briskness of the TIL infiltrate was prognostic for T2-T4 (TMN system) (14) primary cutaneous melanoma (PCM), though the prognostic significance of TILs was lost in very thick lesions (advanced T4). In contrast, Barnhill et al. (11) did not find any survival advantage to be associated with brisk TIL infiltrates; however, patients with both RGP and VGP were included in this study (11), even though other studies did not demonstrate a prognostic significance of TILs in RGP PCM (7, 8). Furthermore, only 25.6% of patients in Barnhill's study had lesions thicker than 1.7 mm (11) while 82% and 71% of patients had lesions thicker than 1.7 mm in the studies by Clemente (8) and Tuthill (6). Taylor et al. (9) did not find an impact of TILs on survival (44% of patients had lesions thicker than 2 mm); however, they did show that TILs are an independent predictor of SLN positivity, which by itself is the most important independent predictor of recurrence and survival in malignant melanoma patients (15). Nevertheless, due to technical limitations at the time, the vast majority of these studies did not immunophenotype TILs and, therefore, did not examine the difference in composition or function of tumoral lymphocytes.
TIL targets and their mechanism of recognition
The antigens which T cells recognize are comprised of peptides and a population of polymorphic cell-surface proteins called major histocompatibility complex (MHC) Ags, which associate with peptides via their peptide binding groove (16). CD4 "helper" T cells (TH) recognize peptides of at least 13 amino acids in length presented by MHC class II (HLA DR, DP, DQ) Ag, whereas CD8 "cytotoxic" T lymphocytes (CTLs) recognize 8-10 amino acid peptides presented by MHC class I (HLA A, B, C) Ags (16). MHC class I Ags are essentially expressed on all nucleated cells (16) and hence have a broad distribution, including tumor cells; however, as will be discussed later, these may be lost during tumor progression (17). In contrast, MHC class II Ag expression is normally limited to "professional" Ag-presenting cells (APC); however, these Ags may be expressed aberrantly on tumor cells as a result of peritumoral inflammation (18) or as a direct result of neoplastic transformation (19). In fact, a significant percentage of melanomas express cell surface MHC class II molecules (20), and treatment with interferon-gamma (IFN-γ) can induce class II expression on the majority of melanomas (21). MHC Ag expression in malignant melanoma has also been shown to have prognostic relevance as both expression of HLA-DR in melanoma lesions and a decreased expression of HLA-A, -B, -C Ags in loco-regional metastases are associated with an unfavorable prognosis (19). Although all nucleated cells normally express MHC class I molecules and have the capability to upregulate MHC class II molecules in an inflammatory milieu, professional APCs (macrophages, dendritic cells and B cells) are best equipped for the priming of a T cell response given their constitutional expression of MHC II Ags and their ability to express numerous T cell co-stimulatory molecules (16). Thus numerous studies are targeting the function of professional APCs, particularly the dendritic cell (DC) subset of these cells, to bolster anti-tumor immunity.
The majority of melanoma peptide Ags have been identified by: screening cDNA expression libraries against melanoma-reactive T cells (22); mass spectrometry following their elution from purified HLA molecules (23); or, prediction from the genomic sequence based upon the need for specific anchor residues (16) to fit into respective "pockets" of the HLA peptide binding groove (23). Recently, the study of tumor-specific T cells has been facilitated by the development of multimer technology, which allows one to track and enumerate Ag-specific T cells by flow cytometry (16, 24). Through the use of these techniques, various classes of tumor Ags have been observed in human melanoma, which include autologous tumor-specific (specific point mutations such as in the β-catenin gene), tissue-specific (e.g., MART-1/Melan-A from here on referred to as MART-1), and common cancer-specific (e.g., MAGE family) Ags (22). However, how the immune system differentially responds to these various Ag types has not yet been fully explored.
TILs derived from melanomas may lyse MHC-matched allogeneic tumors (25), suggesting that some tumor-associated Ags (TAAs) are commonly expressed by tumors from different patients (22). Given the high frequency of this phenomenon (22), it would be unusual for such cross-reactivity to involve sporadic mutations which result in novel peptide Ags (autologous tumor-specific Ags). In keeping with this hypothesis, many melanoma-specific Ags recognized to date have been non-mutated peptides derived from proteins involved in melanin synthesis (22, 26). These so-called melanosomal proteins or melanocytic differentiation proteins (tissue-specific tumor Ags) include MART-1, gp100, tyrosinase, TRP-1, and TRP-2 (22, 26). Peptides derived from these proteins have been recognized in the context of the HLA-A1, -A2, and -A3, HLA molecules that are expressed in 26%, 49%, and 25% of the Caucasian population respectively, with the HLA-A2-binding MART-1 peptides 27-35 and 26-35 being the most frequently detected peptide Ags recognized in melanoma patients (22). Melanosomal protein-derived peptide Ags have relatively low HLA binding affinities (26), due to the absence of optimal amino acid anchor residues, suggesting that they may be expressed at a low density on the melanocyte surface (22), although a subgroup of these Ags are strongly immunogenic as the therapeutic capacity of TILs often correlates with anti-gp100 and anti-TRP-2 specificity when used in adoptive immunotherapy (27). Evidence that effective anti-melanoma immunity can be directed against these melanosomal Ags includes tumor regression in some melanoma patients immunized with MART-1, gp100, or tyrosinase peptides (28, 29), as well as the different biological behavior of same-patient metastases correlating with the expression level of melanosomal Ags (22). Furthermore, a significant correlation has been observed between vitiligo development and tumor regression in patients receiving immunotherapy (22), suggesting that the T cells mediating melanoma regression also recognize Ags expressed by non-neoplastic melanocytes. Interestingly, large numbers of melanosomal-specific T cells are present not only in the blood of melanoma patients but also in healthy persons, with the frequency of MART-1/A2 tetramer positive cells being approximately 10-3 of the phenotypically naïve CD8+ T cells in the peripheral blood of healthy HLA-A2 positive donors (30). The high frequency of these cells is unusual and, to date, MART-1 is the only known tumor Ag for which Ag-specific T cells can be detected in the blood, without any prior in vitro stimulation (30). Why these cells are maintained at such a high concentration remains enigmatic; however, it has been suggested that this high frequency may be explained by the phenomenon of epitope mimicry (31). Nevertheless, melanosomal-specific cells in healthy individuals exhibit a naïve immunophenotype (CD8+ CCR7+ perforin-) (32), perhaps reflecting a low affinity interaction with low-density melanosomal peptides on the cell surface. In the vast majority of melanoma patients, however, a detectable accumulation of MART-1-specific T cells, possessing an Ag-experienced memory/effector immunophenotype (CD8+ CCR7- perforin+) (32), occurs in metastatic tumors (30). The explanation of how the same Ag can have such differential effects is unclear, but may reflect more efficient Ag presentation by mature professional APCs or the lowering of the T cell activation threshold by a rich cytokine milieu within the tumor environment.
While the melanocyte differentiation Ags are expressed constitutively by melanocytic cells, some melanoma tumor Ags are expressed as a result of malignant transformation (33), such as certain "common cancer-specific" Ags of which the MAGE family of genes is one of the best described (22). MAGE proteins are members of the cancer-testis (CT) family of TAAs, which also includes the BAGE, GAGE, and PRAME proteins (23). The biological function of these CT Ags is not yet known; however, these proteins are broadly expressed by tumors of diverse histologies (23) - for example, MAGE-6 is expressed in more than 70% of metastatic melanomas and more than 50% of carcinomas of the lung, esophagus, bladder, and head and neck (34). CT Ags are concentrated upon the X chromosome (though multiple non-X chromosome CT Ags have also been described) (35), and their expression appears to be related to hypomethylation (22). Some studies have shown that CT Ag expression is more frequently seen in more advanced melanomas (36, 37); nevertheless, preliminary studies have shown that immunization of melanoma patients with epitopes derived from MAGE proteins may result in significant tumor regression (38). Surprisingly, in these vaccinated patients with evidence of a therapeutic response, no sign of systemic immunization could be observed in the peripheral blood (38). This apparent discrepancy between the therapeutic effectiveness of MAGE-specific T cells and the ability to detect these cells in vivo may reflect a difference in the immune response to CT Ags relative to the melanocytic differentiation Ags. For example, some studies have shown that immunogenic peptides derived from MAGE proteins are presented by HLA class II molecules, such as the presentation of MAGE-6 peptides by HLA-DR4 (expressed by 15-20% of the North American population) (39). These observations highlight how our knowledge of tumor-specific lymphocytes is biased by early studies which focused on CD8+ cytotoxic T cells circulating in the peripheral blood or present within tumor. However, other lymphocyte subsets which may be equally important in the defense against melanoma, such as helper T (TH) cells, may follow alternate trafficking patterns and may be more represented in other compartments, such as the draining lymph nodes.
Another subset of "common cancer-specific" Ags is a newly described family of molecules called "stress ligands" (40). These proteins, which are best classified as "common-cancer Ags", include the non-classical MHC Ib molecules MICA/B that are expressed by a variety of cancer cell types (41). These proteins are different from the aforementioned MHC molecules in that they represent non-peptide presenting ligands, a subset of which are recognized by the NKG2D "stress ligand receptor", which is an activating/co-stimulating molecule on the surface of T cells and natural killer (NK) cells (41, 42). A subset of innate-like T cells (Vδ1 γδ T cells, see below), which are present in high numbers in various epithelial compartments (43) and which constitutively express NKG2D (44), possess a T cell receptor (TCR) that also directly recognizes MICA/B (45). These intraepithelial T cells, through the coordinate binding of the γδ TCR and NKG2D to the same stress ligand, are capable of the immediate rejection of transformed cells and thus are believed to be prototypical sentinel lymphocytes (46).
Another potential target for immunotherapy, but for which the least amount of data is available, are mutated protein Ags (autologous tumor-specific Ags), some of which are associated with tumorigenesis (22). Mutated peptides appear to be potent rejection Ags in murine tumor models (22) and some data indicates that mutated peptide Ags (22), as well as T cells directed against such Ags (47), can be found in melanoma patients with a more favorable prognosis. One of the reasons for this potency is that such Ags are "non-self" and thus T cells with high affinities for such Ags, which are capable of generating a successful immune response, are not deleted in the thymus during normal T cell ontogeny, a process which helps to ensure self-tolerance (16). Targeting these mutated Ags with immunotherapy may be a strategic approach (48), given that the altered proteins may also confer a growth advantage to the tumor that precludes the development of Ag-loss variants (17, 49) - though the loss of MHC molecules by melanoma cells may immunologically produce a functional loss of these Ags. Mutated proteins that have been isolated from human melanoma include ras, β-catenin, melanoma ubiquitous mutated 1 (MUM-1), and CDK4 (50) and, although these may serve as excellent targets for future immunotherapy, such an approach necessitates an extensive genomic understanding of each individual tumor since these Ags will not be shared between different HLA-matched melanoma patients.
TCR repertoire of melanoma TILs
Many studies have examined whether a restricted usage of TCR variable (V) genes is employed by T cells to recognize melanoma tumor Ags (25, 51); however, the results have been contradictory, perhaps reflecting the complexity of tumor immunity. In some early studies, limited TCR V gene segment usage by melanoma TILs was found (25). For example, in two representative studies, only 3 Vα gene families (Vα13, Vα15, and Vα16) were predominantly expressed by TILs from 24 melanomas examined (51), whereas TILs of uveal melanoma demonstrated a preferential expression of Vα7 genes in 7 of 8 melanoma samples (52). Using a polymerase chain reaction (PCR) approach, Strohal et al. (51) demonstrated that while lymphocytes from normal skin samples showed a heterogeneous expression of TCR Vα chains, the TILs present in or around the tumor had a restricted Vα chain repertoire, expressing only Vα13, Vα15 or Vα16. Further studies implicated a spatial organization of this TCR repertoire restriction (30, 53). For example, Clemente et al. (53) demonstrated that TILs in VGP melanoma and lymph node metastases of the same patients exhibited the same restricted repertoire of TCR Vβ chains, whereas lymphocytes present in extra-VGP areas showed no β chain restrictions. Furthermore, it was shown that TCR repertoires in the peripheral blood of melanoma patients were not restricted (30), perhaps reflecting the ability of only high affinity clones to enter and expand within the tumor microenvironment. Interestingly, even within the same individual, different T cell clones can predominate at different sites of disease, perhaps reflecting diverging subclones of melanoma exhibiting unique patterns of Ag loss that stimulates unique infiltrates of melanoma-specific T cells (54). Despite these earlier studies, it is now widely accepted that the repertoire of T cells against certain melanoma Ags (MART-1 for example) is diverse and mostly non-overlapping among different individuals (25, 30). Tetramer-based studies have revealed a highly diversified repertoire utilizing most Vβ chain families (30), with only some clones showing partial conservation of TCR structure (25, 30). Therefore, understanding how different subsets of T cells contribute to an effective anti-tumor response and assaying the functional characteristics of these cells is beginning to overshadow the significance of TCR repertoire analysis of TILs.
Immunophenotyping and subtyping TILs
An explosion of immunological data has resulted in a greater characterization of T cell subsets, which has led to an effort to immunophenotype T cells within the tumor microenvironment (9, 10, 55-61). Studies on IL-2-cultured TILs demonstrated that the T cell composition within the tumor microenvironment varies in individual patients, ranging from an infiltrate with 90% CD4+ T cells to an infiltrate with 90% CD8+ T cells (59-61), with highly specific cytolytic activity and patient outcomes correlating with the presence of tumor-specific, CD8+ T cells (9, 10, 55). Most research has focused on CD8+ cells, which are known to infiltrate tumors and, by virtue of their recognition of tumor-specific peptides presented by classical HLA class I molecules (16), are suspected to have a role in mediating the cytotoxic destruction of transformed cells (62, 63). However, recent studies that tested the function of CD8+ TILs, such as the ability of tetramer-positive cells to express IFN-γ after in vitro stimulation (64), demonstrated that many melanomas are populated by inactivated/anergic cells (65, 66). This observation stresses the importance of some form of functional assessment to best prognosticate TIL significance. Furthermore, the significance of other immunocyte populations in melanoma is uncertain despite a heterogeneous mixture of inflammatory cells often present within the tumor microenvironment (47, 57, 58, 67, 68). For example, although the role of CD4+ TILs in melanoma is not yet understood, several studies suggest that they may have an important role. For example, melanoma-specific CD4+ TILs have been shown to possess the ability to directly lyse tumor cells (58, 69) and eliminate melanoma in animal models (56, 70). Moreover, work by Rosenberg's group has demonstrated that the co-transfer of CD4+ and CD8+ T cells is more beneficial than the transfer of CD8+ T cells alone (71), and a recent report has demonstrated that the adoptive transfer of in vitro expanded autologous CD4+ T cell clones with specificity for the melanoma-associated Ag NY-ESO-1 may induce durable responses in some patients with metastatic melanoma (72). Additional work has revealed that CD4+ TILs in thinner, regressing lesions secrete a pattern of cytokines typical of TH1 CD4+ cells (IFN-γ, lymphotoxin, IL-15, IL-2) whereas thicker, non-regressing lesions contain a greater number of TGF-β- and IL-10-liberating CD4+ cells (73-75), likely belonging to the TH2 or a regulatory T cell lineage. Given that TH1 cells promote strong cell-mediated immune responses while TH2 cells promote allergic responses and/or secrete immunosuppressive factors (16, 76), the proportions of these different cell subsets will likely influence the tumor microenvironment.
As alluded to above, another subset of CD4+ T cells variably present within the tumor environment (68, 77-80) are the CD4+ CD25+ regulatory T cells (Tregs). Tregs express a CD4+/CD25+high/Foxp3+ immunophenotype and represent 5-10% of human CD4+ T cells (81). A deficiency of Tregs, either occurring naturally (82, 83) or induced experimentally (84, 85), is associated with massive T cell lymphoproliferation and multi-organ autoimmunity (82-85), illustrating how a subset of these cells are important for mediating self-tolerance (natural Tregs) (86, 87). Another subset of Tregs appear to modulate the response of immunocytes to non-self Ags (induced Tregs) (86, 87), thereby limiting the immune response to foreign Ags. Interestingly, Tregs are significantly increased in patients with epithelial malignancies (twice the number of Tregs relative to healthy volunteers) (88) and, in experimental models, depletion of Tregs evokes effective anti-tumor immunity (84, 85). Treg TILs have also been shown to be more represented in advanced human melanoma lesions, with more Treg TILs in metastatic lesions (68) and in deep VGP lesions relative to shallow VGP and RGP lesions (89). Thus the accumulation of these cells may be associated with disease progression (89, 90), a hypothesis which is supported by the finding that a higher percentage of Treg TILs is associated with a significantly higher risk of melanoma recurrence (91). It is also tempting to speculate that the increased frequency of Tregs in advanced malignant lesions (68, 78, 79) may, in part, explain the anergy of tumor-specific CD8+ T cells observed in such lesions (65, 66). Studies are on-going to investigate whether a correlation exists between melanoma survival and the frequency of Treg TILs, similar to what has been described for ovarian cancer patients (92).
Melanoma-specific B cells have also been demonstrated in limited studies (58, 93), and high levels of B cell TILs have been correlated with a favorable prognosis in certain types of cancer (93, 94). Interestingly, immunization of mice with TRP-1 protein resulted in the induction of auto-Abs and the protection against growth of TRP-1-expressing melanoma cells (95). Furthermore, human studies on the immune response to NY-ESO-1-expressing melanomas have demonstrated that CD8+ T cell responses to this Ag do not occur in patients who do not develop NY-ESO-1-specific Abs and that the titer of NY-ESO-1-specific Abs falls with the successful therapy of melanomas (96). These data suggest that melanoma-specific Abs may have a role in opsonizing tumor cells for phagocytosis and optimal Ag presentation, and that measuring a humoral immune response to vaccination can identify patients who will likely respond to therapy. However, the significance of humoral immunity in human melanoma has yet to be clarified since studies to date have shown that Ig deposits and B cells are only infrequently present within the tumor microenvironment (97).
NK cells are another lymphocyte population whose role in the melanoma immune response has not yet been closely examined. NK cells use an assortment of germline-encoded receptors, including inhibitory receptors for MHC molecules (16), that enable them to recognize cells that have aberrantly upregulated or downregulated cell surface markers as a result of cellular transformation (16, 98). Cells which have lost expression of self-MHCs are essentially marked for NK cell-mediated destruction (missing-self model) (98, 99). Similarly, activating receptors expressed by NK cells, such as NKG2D, recognize transformation-associated stress ligands, which function as another trigger for the NK cell-mediated destruction of tumor cells (98, 100). The linkage of NK receptor signaling to the release of cytotoxic granules is the basis for the immunosurveillance function of these cells (98). Although NK cells are either absent or present only infrequently within the melanoma microenvironment (97), one study demonstrated that the presence of NK TILs was seen only in responding melanoma patients but not in those with progressive disease (101).
Yet another subset of melanoma TILs which has not received much recent attention in the literature is the γδ family of T cells. While most melanoma TILs express the αβ heterodimeric TCR (102), a subset of TILs express a γδ TCR (67). γδ TCRs are assembled in a similar fashion as αβ TCRs (16); however, the γδ receptor complex is characterized by different Ag recognition properties (46). MICA/B-specific Vδ1 γδ T cells have been demonstrated within the melanoma microenvironment (67) and several studies have demonstrated a relationship between the stage of melanocytic lesions and the frequency of these cells (54, 103-106). For example, a greater number of Vδ1 γδ T cells were found to be present in dysplastic melanocytic nevi relative to invasive and metastatic melanoma (106). This observation supports an important immunosurveillance function of these cells in early lesions (46), which is in keeping with the upregulated expression of cellular stress ligands during the transformation of dysplastic nevi to invasive melanoma (107). The lesser role of γδ TILs in more advanced melanocytic disease likely reflects the tendency of invasive melanomas to downregulate the surface expression of these stress ligands (108), and it is tempting to speculate that enhancing stress ligand expression by dysplastic melanocytes or melanoma cells may prove to be an effective treatment strategy in future clinical trials.
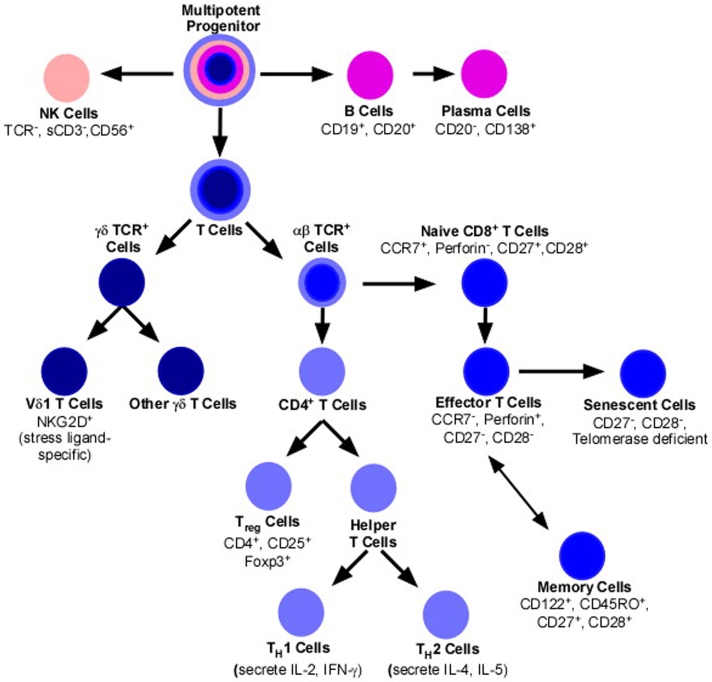
Clearly a number of different lymphocyte subsets contribute to the immunological response to melanoma. A greater understanding of the Ag specificity and immunobiology of the different lymphocyte subsets is clearly needed to better predict the prognostic significance of TILs and to more effectively modulate their immunosurveillance of melanoma. A basic subdivision of lymphoid cells is illustrated in Figure 1, and a list of markers for comprehensive TIL immunophenotyping is proposed in Table 1. Given this greater capability to immunophenotype TILs, future studies may show that the composition is as important as the "briskness" of the lymphocytic infiltrate.
Figure 1.
Stylized outline of major lymphocyte subsets.
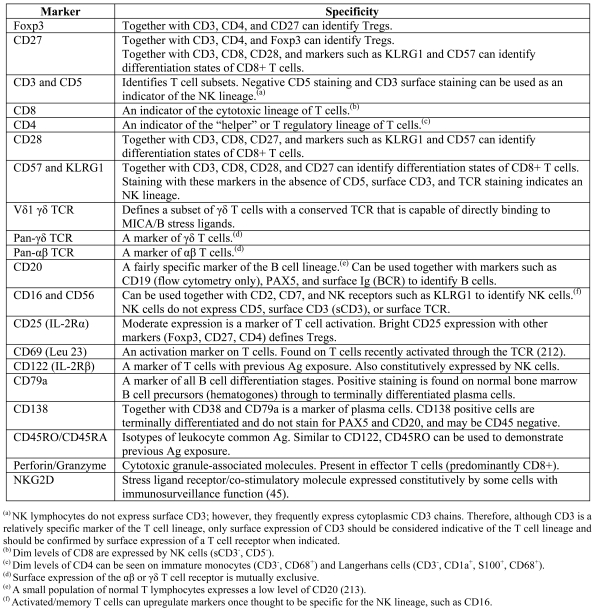
Table 1.
Proposed markers for immunophenotyping TILs.
Immunomodulating strategies to augment anti-melanoma immunity
Recently, certain forms of immunotherapy have been shown to be of potential therapeutic use in some patients with metastatic melanoma (109, 110). Unfortunately, with the implementation of such therapies, it is likely that our limited understanding of the prognostic significance of TILs will be further disadvantaged by therapy-specific immune distortions that change the usual pattern of TIL composition and infiltration. For this reason, it will be critical for those who interpret histopathological material to gain an understanding of the underlying immunobiology of such therapies.
Attempts to augment melanoma-specific immunity have involved various techniques. Vaccination of patients with melanoma Ags, or augmentation of natural Ag presentation in vivo through the use of various adjuvants to expand tumor-specific lymphocytes (22, 50, 111), has been a major focus in melanoma research. Steps taken to enhance natural Ag presentation have included the administration of a number of cytokines. IL-2, perhaps the most important of lymphocyte growth factors (16), has been investigated for more than 20 years in the therapy of melanoma (59, 109, 112), and likely works by either promoting the expansion of Ag-specific T cells or by enhancing their cytotoxicity (113, 114). Initial studies of IL-2 therapy for melanoma demonstrated that the administration of this agent resulted in substantial increases in tumoral lymphocytes (115). Subsequent prospective clinical trials demonstrated some utility of IL-2 therapy for malignant melanoma, with a 16.3% response rate, including a 6% complete response rate, observed in one trial (109). In this study, median duration of response was 6.5 months, with 60% of complete responders remaining progression-free at 5 years (109). Of 24 patients who experienced a complete regression in this trial, only five have experienced a recurrence and 19 remain in clinical remission for 46 to 137 months or more (109). These numbers, albeit small, are the first evidence for potential cure in metastatic melanoma and have led to the U.S. Food and Drug Administration’s approval of IL-2 therapy in such patients (109). IL-15, a cytokine whose receptor consists of IL-2's β and γ chain (CD122 and CD132, respectively) as well as the specificity-determining IL-15α chain, is also considered a promising agent in immunotherapy (116) as it possesses a similar effect on T cell proliferation without IL-2's effect on inducing T cell apoptosis (117). Immunotherapy with interferon-alpha (IFN-α) has also shown reproducible activity in metastatic melanoma, with 15% to 20% response rates reported in some series (118), and with clinical responders having significantly denser TIL infiltrates (119). The mechanism of IFN-α immunotherapy is not fully understood; however, it is proposed to work by both augmenting the immune response and by exerting a direct effect on melanoma cells, which may involve activation of STAT proteins (120). IL-12 has also been used as an enhancer of the immune response (121-123). IL-12 acts by inducing TH1 differentiation and, by inducing cytokine secretion, promotes the proliferation and the cytolytic activity of NK and T cells (16, 122). IL-12 has also been shown to reverse Ag-specific T cell anergy (124) and can boost the frequency of circulating tumor-specific lymphocytes (123). In trials using IL-12, these aforementioned effects were associated with brisk melanoma TIL infiltrates and encouraging treatment effects (121-123). Another cytokine found to be a useful adjuvant to tumor vaccination is granulocyte macrophage-colony stimulating factor (GM-CSF) (16). GM-CSF treatment in melanoma results in the accumulation of large numbers of professional APCs (125), an observation leading to the integration of GM-CSF in a vaccination protocol which employs irradiated autologous tumor cells that have been genetically engineered to produce large amounts of this factor (125-128). In a phase I clinical trial employing this approach, 10 out of 16 patients with stage IV melanoma developed dense lymphocytic infiltrates with extensive necrosis of metastatic lesions with one complete response, one partial response, and one mixed response (128).
Various other immunomodulating strategies have been attempted for the treatment of melanoma, including vaccination with tumor cells, tumor lysate or tumor-specific peptides, especially those derived from melanosomal Ags, with or without DCs (50, 111, 129-134). Tumor regression and tumoral lymphocytic infiltration has been observed in some melanoma patients immunized with melanosomal peptide Ags (22, 132, 135, 136) and, although some of the results have shown promise in the treatment of melanoma (50, 132, 135, 136), the great variability in the protocols used in these studies has led to a perplexing collection of data (131-138). This variability, in part, reflects different maturation states of DCs used in immunotherapy trials (133), since immature DCs are weak immunogens and can be tolerogenic, even resulting in the induction of Ag-specific Tregs (133). The collection and in vitro expansion of TILs followed by their adoptive transfer has also been used to augment anti-melanoma immunity (139, 140). This strategy is especially suitable for immunocompromised patients who may not optimally respond to a tumor vaccination approach. The administration of radio-labeled, melanoma-specific lymphocytes (as assessed with MART-1 tetramers), followed by imaging studies with a gamma camera, confirmed that transferred cells indeed localize to sites of metastatic tumor (141), which is consistent with the brisk TIL infiltrates following adoptive transfer of in vitro expanded, TAA-specific T cells (71, 142). While successful tumor eradication in murine models has been achieved by TIL transfer (143), the translation of this technique into clinical practice has been cumbersome, though it is considered by many to be the most promising immunotherapeutical strategy to date (144-146). In addition to these aforementioned approaches, novel strategies for the treatment of melanoma are being developed at a rapid rate and include immunization with recombinant viruses or plasmids encoding tumoral Ags (22) and the administration of a host of monoclonal Abs (mAbs) targeting critical regulators of immune function, such as a triggering mAb against 4-1BB (CD137) (147) and a blocking mAb against the cytotoxic T cell lymphocyte Ag-4 (CTLA-4, CD152) (111). Anti-CTLA-4 mAbs are a prototypical example of how our growing understanding of immunobiology is being translated into potentially useful immune modulating agents and thus these and related therapies will be discussed in detail.
Examples of novel biological agents in melanoma immunotherapy with a focus on CTLA-4 blockade
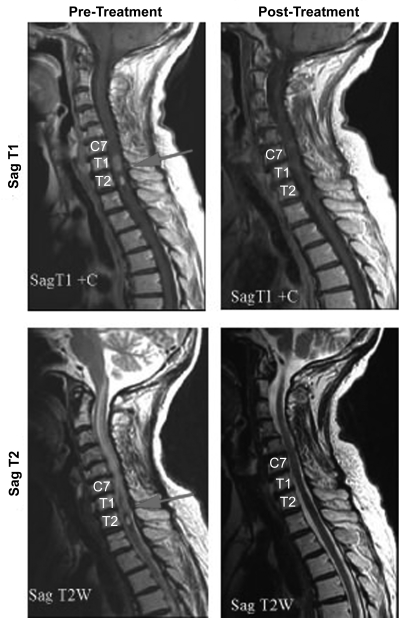
CTLA-4 is critically important for the contraction of immune responses (148), which is necessary to ensure that other T cell clones are not dangerously diluted by unopposed clonal expansions (148). CTLA-4 is not expressed on Ag-naïve T cells, but is upregulated upon the surface of T lymphocytes approximately 3 days following Ag-specific T cell activation (149, 150). CTLA-4 is a high affinity receptor for the B7.1 and B7.2 ligands (151) that are expressed on mature APCs during an immune response (16, 152) and which are critical for delivering the classically-described "co-stimulatory" signal or "signal 2" to a naïve T cell (16). This co-stimulation is necessary for the optimal activation and proliferation of responding Ag-specific T cells (16, 148), and is transduced through CD28 (16) and related cell surface molecules upon initial Ag encounter (148). CTLA-4 is believed to antagonize T cell activation/expansion by at least two possible mechanisms, the first of which involves CTLA-4's 100-to-2000-fold greater affinity for B7.1/B7.2 relative to that of CD28 (148, 151), which effectively eliminates co-stimulatory signaling by the sequestration of B7.1/B7.2 away from CD28 (151, 153). The second possible mechanism involves the recruitment of an inhibitory phosphatase (SHP-2) to the immunological synapse by the SH2-binding domain of CTLA-4 (151), leading to dephosphorylation of critical tyrosine residues and the subsequent extinguishment of downstream TCR signaling pathways (154, 155). The synergistic effect of these two processes is to halt further expansion of Ag-specific T cells and enhance the attrition of the expanded clonal population, probably by depriving T cells of survival signals which are obtained through low-level TCR signaling (156). Thus the blockade of CTLA-4 function with a mAb was proposed for use as an adjuvant to increase the frequency of tumor-reactive T cells by prolonging the clonal expansion phase following tumor vaccination or during natural tumor Ag presentation (157). Various animal models have validated the effectiveness of CTLA-4 blockade at increasing the clone size of Ag-specific T cells when used in association with tumor vaccination (158-160). Effective CTLA-4 blockade was also found to be associated with better tumor control and prolonged survival in these model systems (158, 161-163). Based on these experimental findings, two human anti-CTLA-4 IgG1 mAbs have been developed (Ipilimumab® and Ticilimumab®) that are in advanced clinical trials for the treatment of a variety of malignancies (164-169). Preliminary data from these early trials of anti-CTLA-4 mAbs alone or in association with tumor vaccination have been encouraging (164-170), resulting in better tumor control in some recipients even within immunologically privileged sites such as the brain (Figure 2) (170), which is somewhat surprising given the role of the blood-brain barrier in preventing the influx of therapeutic mAbs into the brain parenchyma (171). One study of melanoma patients treated with anti-CTLA-4 mAb therapy alone showed an overall response rate of 21%, with two complete and one partial remission in 14 treated patients (165), while another study of melanoma and ovarian cancer patients demonstrated extensive tumor necrosis in 5 out of 9 patients following CTLA-4 blockade (164).
Figure 2.
CTLA-4 blockade results in significant tumor regression in some patients. MRI images of the cervicothoracic spine from a patient with metastatic malignant melanoma which reveal enhancing intraspinal metastases with extensive cord edema prior to treatment with CTLA-4 blockade. Post-therapy images demonstrate complete resolution of the metastases and the accompanying edema. [Adapted from Hodi et al. (170)]
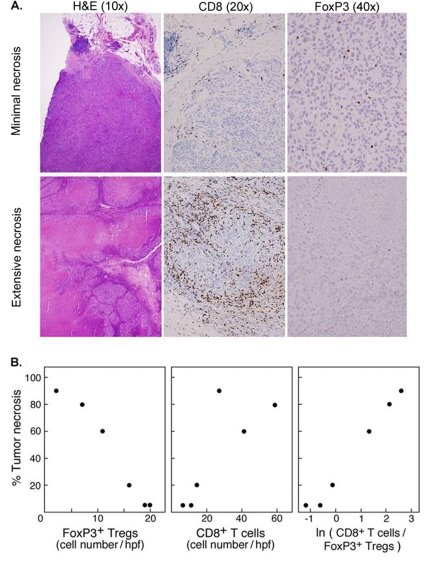
Studies of CTLA-4 blockade have also yielded information on how different TIL subsets interact within the tumor environment. For example, while marked TIL infiltrates were observed in many anti-CTLA-4 mAb studies (164, 172), in one study of advanced melanoma patients where TILs were more comprehensively immunophenotyped, the data suggested that Tregs limit the cytotoxic response to melanoma TAAs (172). In this study (172), an inverse relationship existed between the frequency of Treg TILs in metastatic melanoma and both the extent of necrosis and the frequency of cytotoxic T cells in such lesions (Figure 3). The reduction of Treg TILs noted in some of the patients in this study may have been attributable to a mAb-mediated depletion, given the constitutive expression of CTLA-4 by Tregs (173). Such Treg depletion may account for the autoimmune-like adverse effects that occur in many patients treated with CTLA-4 blockade (165, 166, 174), which are strikingly similar to the phenotypic changes in FoxP3-mutated Scurfy mice that are naturally-deficient of Tregs (175-177). Interestingly, other tumor vaccination strategies have been shown to be inexplicably accompanied by increases in Treg TILs, which may have adversely affected the clinical response in these studies (178). Based upon these observations, it has been proposed that melanoma patients may benefit from Treg depletion, either as monotherapy or in association with tumor vaccination and, therefore, a variety of strategies are being investigated for this purpose.
Figure 3.
The ratio of tumor infiltrating CD8+ T cells to Foxp3+ Treg TILs following anti-CTLA-4 treatment is tightly correlated with the extent of tumor necrosis. (A) Representative photomicrographs demonstrating CD8+ and Foxp3+ Treg TILs in melanoma metastasis exhibiting minimal (top) and extensive (bottom) necrosis. (B) Graphical demonstration of the relationship between FoxP3+ Treg TILs, CD8+ TILs, and tumor necrosis. [Adapted from Hodi et al. (172)]
Treg-depleting strategies currently being tested include agents which bind to the interleukin-2 (IL-2) receptor alpha chain (IL-2Rα, CD25) which, similar to CTLA-4, is not found on Ag-naïve T cells (16) but is expressed constitutively by Tregs (16, 179). These CD25-targeted therapies include anti-CD25 mAbs (180) and recombinant cytotoxic proteins composed of portions of bacterial toxins conjugated to either human IL-2 (181-183) or an antibody against CD25 (184, 185), that after internalization by CD25-expressing cells leads to cell death (181-183). Although CD25-directed therapies have shown some success in the depletion of Treg TILs (184), scant data exist on how these therapies immunomodulate other TIL subsets. Furthermore, these strategies to abrogate Treg function have generated mixed results in clinical trials (181-185), and it is unclear why these therapies have been less efficacious than anticipated. One possible explanation may be the unwanted depletion of tumor-specific T cells, since upregulation of CD25 is one of the earliest events in T cell activation (16, 186), and therefore, the tumor-specific population of cells one hopes to expand may in fact be depleted by CD25-directed agents.
An alternative strategy to abrogate the function of Tregs in vivo involves interrupting the migration of these regulatory cells into the tumor microenvironment by utilizing mAbs directed against chemokines and their receptors. The majority of Tregs express high levels of chemokine receptors CCR4 (receptor for CCL22) (187, 188) and CCR6 (receptor for CCL20) (189). Antagonizing these chemotactic networks with mAbs against chemokines or their receptors has proven to be effective in experimental models; for example, a mAb against CCL22 reduced Treg migration to ovarian tumors (190). Accordingly, it is anticipated that an on-going clinical trial using a mAb against CCR4 for the treatment of hematological malignancies (191, 192) will be expanded to include patients with various other malignancies, including malignant melanoma.
The effect of melanoma immunotherapy on TILs
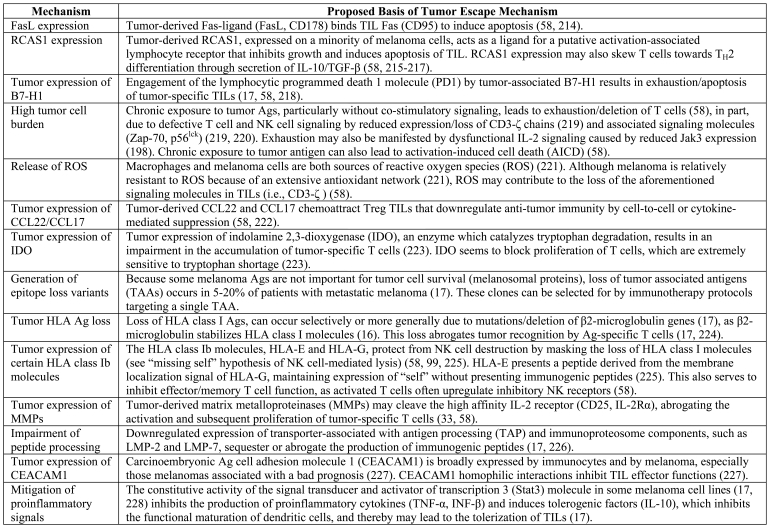
The frequent failure of melanoma immunity is highlighted by data from both human (193) and murine (194) tumor models which show that TILs are sometimes composed of quiescent and/or functionally anergic effector/memory T cells (58). For example, some TIL cell lines do not lyse but rather release GM-CSF in response to autologous tumor (195), and while normal donor lymphocytes were able to secrete IFN-γ in response to MAGE-6-derived peptides, cells from melanoma patients were unresponsive to this stimulation (39). Therefore merely demonstrating the presence of TILs may not be an entirely accurate method of predicting patient outcomes. Myriad tumor escape mechanisms, described in detail elsewhere (17) and briefly summarized in Table 2, likely work together to affect this immune compromise. Mechanisms particularly important to recognize include: loss of tumor Ags, which occurs in 5-20% of patients with metastatic melanoma in the form of selective loss of tumor Ags or the concordant loss of multiple melanosomal proteins (196); altered expression of classical and non-classical MHC molecules (17); and, the ability of tumors to liberate chemotactic factors for Tregs (197).
Table 2.
Selected tumor escape mechanisms that mitigate the anti-tumor immune response.
The frequency of these tumor escape tactics appears to increase in metastatic melanoma when compared to primary melanoma (180, 198), perhaps reflecting clonal evolution of the tumor and the selection of multiple escape mechanisms. However, while T cell incompetence is likely attributable to many of the processes listed in Table 2, it may also reflect problems invoked by current immunotherapy protocols. Although most melanoma patients treated with TIL adoptive transfer show a brisk T cell rich infiltrate (71, 142), the frequency of TAA-specific TILs does not necessarily correlate with a strong in vivo anti-tumor response (140). Similarly, vaccination protocols utilizing melanoma TAAs are frequently associated with unsatisfactory clinical responses (133). In experimental studies, one finding that correlated very strongly with positive therapeutic responses was the in vivo persistence of tumor-specific T cells (71, 139). This persistence has been defined in cell transfer techniques as the ability of at least one clonotype to remain in the peripheral blood one month after transfer at 5% or greater of the total CD8+ T cell population (199). While persistent and non-persistent TILs shared a remarkable degree of similarity in the expression of activation markers (CD69, CD25, and CD40L) and homing molecules (CCR7, CXCR4), it was shown that a greater number of CD27-expressing TILs is associated with greater persistence and better outcomes (200, 201). CD27 is stably downregulated in late effector stage T cells (200), which are terminally differentiated and have significantly shorter telomeres and extremely poor telomerase activity attributable to defective Akt phosphorylation (202). Telomere shortening has been shown to be induced by prolonged in vitro culture, which is consistent with the finding that human T cells become senescent after 20 to 30 population doublings in vitro (199). Therefore, cells cultured from a small number of harvested TILs may be functionally compromised and unable to persist or perform their immunosurveillance function after adoptive transfer (203). Immunophenotyping TILs with the panel of markers listed in Table 1 can help to estimate the frequency of senescent T cells, especially if fresh material for flow cytometry is available where multiple markers can be evaluated simultaneously on each cell (16).
Another aspect of earlier clinical trials which may have adversely affected TIL function was the selection of Ags used for in vivo or in vitro expansion of tumor-specific T cells. Although a peptide may have anchor residues necessary to bind to a particular MHC molecule (16), such a peptide may not result in the generation of useful tumor-specific T cells. For example, among a panel of 10 different MART-1 peptides containing the HLA-A2 binding motif, only one was able to induce CTL lines with specific recognition of melanoma cells (204). This inability of certain peptides to induce functional tumor-specific T cells may reflect: a low affinity of interaction between relevant T cell receptors and the Ag, which at physiological levels of Ag expression will fail to result in the recognition and/or efficient lysis of tumor cells; or, a failure to generate such peptides in vivo by the cellular machinery responsible for generating peptides for Ag presentation (proteasome) (16, 205), thereby rendering tumor cells invisible to a population of highly efficient cytotoxic cells (205, 206). With respect to this latter explanation, a similar "invisibility" may occur when Ag-specific T cells are generated from stimulation with altered peptide ligands, such as the modified high-affinity HLA-A2 binding gp100 peptide (gp100209-2M). This peptide was generated from the substitution of a threonine residue with a methionine at the anchor residue P2, which results in a 10-fold higher HLA-A2 binding affinity that more efficiently produces high frequencies of tetramer-binding CD8+ cells compared to the native epitope (207). In one study where altered peptide ligands produced Ag-specific T cells with a spectrum of functional avidities, the cells were incapable of lysing HLA-A2-expressing melanoma cells, even at 100:1 effector-to-target-cell ratios, illustrating this potential pitfall (208). The failure to generate CTLs with some natural high-affinity peptides, such as to some MART-1 peptides (204), may also be explained by the induction of T cell tolerance toward these high-affinity self-peptides, reflecting the normal immunobiology of T cell Ag recognition (16). The finding that only studies showing T cell responsiveness to physiological levels of Ag demonstrated any clinical response (64, 209, 210) exemplifies these aforementioned complications of immunotherapy. One promising avenue of research, which aims to circumvent such problems invoked by normal T cell immunobiology, involves genetically-engineered T cells that express high affinity TCR for naturally-processed peptide Ags expressed at physiological concentrations (211); nevertheless, this approach may be associated with considerable autoimmune adverse effects.
These findings emphasize the potential necessity of moving beyond enumerating TILs with routine hematoxylin and eosin (H&E) staining and simple immunohistochemistry panels, towards the use of advanced immunophenotyping and possibly functional assessments of TILs. An emphasis on cellular senescence and reactivity towards physiological levels of cognate Ags may help to best prognosticate their significance.
Concluding remarks
Since the prognostic significance of TILs was proposed, much has been learned about the immunobiology of lymphocytes and the small molecules that govern the behavior of these cells. The sometimes contradictory results of earlier studies likely reflect the great immunophenotypic and functional heterogeneity of melanoma TILs. By applying today's greater immunobiological insight to the re-evaluation of previous data and the design of future studies, the significance of TILs in melanoma will surely be elucidated allowing for definitive recommendations for the routine management of melanoma to be developed.
Abbreviations
- CT
cancer-testis
- TAA
tumor-associated antigen
- VGP
vertical growth phase
Acknowledgements
We are grateful for the contributions and review of the manuscript by Dr. Glenn Dranoff (Dana Farber Cancer Institute, USA), Dr. April Armstrong (Harvard University, USA) and Dr. Ignacio Sanchez-Carpintero (University of Madrid, Spain), as well as the financial support from the Cancer Research Institute (Grant #2000-P-0022-54/7).
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–767. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Moore OS Jr, Foote FW Jr. The relatively favourable prognosis of medullary carcinoma of the breast. Cancer. 1949;2:635–642. doi: 10.1002/1097-0142(194907)2:4<635::aid-cncr2820020411>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Clark WH Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–727. [PubMed] [Google Scholar]
- 5.Day CL Jr, Sober AJ, Kopf AW, Lew RA, Mihm MC Jr, Hennessey P, Golomb FM, Harris MN, Gumport SL, Raker JW, Malt RA, Cosimi AB, Wood WC, Roses DF, Gorstein F, Postel A, Grier WR, Mintzis MN, Fitzpatrick TB. A prognostic model for clinical stage I melanoma of the upper extremity. The importance of anatomic subsites in predicting recurrent disease. Ann Surg. 1981;193:436–440. doi: 10.1097/00000658-198104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuthill RJ, Unger JM, Liu PY, Flaherty LE, Sondak VK. Southwest Oncology Group. Risk assessment in localized primary cutaneous melanoma: a Southwest Oncology Group study evaluating nine factors and a test of the Clark logistic regression prediction model. Am J Clin Pathol. 2002;118:504–511. doi: 10.1309/WBF7-N8KH-71KT-RVQ9. [DOI] [PubMed] [Google Scholar]
- 7.Elder DE, Guerry D 4th, VanHorn M, Hurwitz S, Zehngebot L, Goldman LI, LaRossa D, Hamilton R, Bondi EE, Clark WH Jr. The role of lymph node dissection for clinical stage I malignant melanoma of intermediate thickness (1.51-3.99 mm). Cancer. 1985;56:413–418. doi: 10.1002/1097-0142(19850715)56:2<413::aid-cncr2820560234>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 10.Mihm MC Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 11.Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer. 1996;78:427–432. doi: 10.1002/(SICI)1097-0142(19960801)78:3<427::AID-CNCR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Thörn M, Pontén F, Bergström R, Sparén P, Adami HO. Clinical and histopathologic predictors of survival in patients with malignant melanoma: a population-based study in Sweden. J Natl Cancer Inst. 1994;86:761–769. doi: 10.1093/jnci/86.10.761. [DOI] [PubMed] [Google Scholar]
- 13.Larsen TE, Grude TH. A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. 3. The relation between the tumour-associated lymphocyte infiltration and age and sex, tumour cell type, pigmentation, cellular atypia, mitotic count, depth of invasion, ulceration, tumour type and prognosis. Acta Pathol Microbiol Scand [A] 1978;86A:523–530. [PubMed] [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. Chicago (IL): American Joint Committee on Cancer; 2002. 6th ed. [Google Scholar]
- 15.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, Byrd D, Desmond R, Zhang Y, Liu PY, Lyman GH, Morabito A. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. New York (NY): Garland Science; 2005. 4th ed. [Google Scholar]
- 17.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 18.Pfizenmaier K, Scheurich P, Schluter C, Krönke M. Tumor necrosis factor enhances HLA-A,B,C and HLA-DR gene expression in human tumor cells. J Immunol. 1987;138:975–980. [PubMed] [Google Scholar]
- 19.Ruiter DJ, Mattijssen V, Broecker EB, Ferrone S. MHC antigens in human melanomas. Semin Cancer Biol. 1991;2:35–45. [PubMed] [Google Scholar]
- 20.Bröcker EB, Suter L, Sorg C. HLA-DR antigen expression in primary melanomas of the skin. J Invest Dermatol. 1984;82:244–247. doi: 10.1111/1523-1747.ep12260181. [DOI] [PubMed] [Google Scholar]
- 21.Baton F, Deruyffelaere C, Chapin M, Prod'homme T, Charron D, Al-Daccak R, Alcaide-Loridan C. Class II transactivator (CIITA) isoform expression and activity in melanoma. Melanoma Res. 2004;14:453–461. doi: 10.1097/00008390-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami Y. New cancer therapy by immunomanipulation: development of immunotherapy for human melanoma as a model system. Cornea. 2000;19(3 Suppl):S2–S6. doi: 10.1097/00003226-200005001-00002. [DOI] [PubMed] [Google Scholar]
- 23.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 24.Bakker AH, Schumacher TN. MHC multimer technology: current status and future prospects. Curr Opin Immunol. 2005;17:428–433. doi: 10.1016/j.coi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Shilyansky J, Nishimura MI, Yannelli JR, Kawakami Y, Jacknin LS, Charmley P, Rosenberg SA. T-cell receptor usage by melanoma-specific clonal and highly oligoclonal tumor-infiltrating lymphocyte lines. Proc Natl Acad Sci U S A. 1994;91:2829–2833. doi: 10.1073/pnas.91.7.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakami Y, Robbins PF, Wang RF, Parkhurst M, Kang X, Rosenberg SA. The use of melanosomal proteins in the immunotherapy of melanoma. J Immunother. 1998;21:237–246. doi: 10.1097/00002371-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Castelli C, Tarsini P, Mazzocchi A, Rini F, Rivoltini L, Ravagnani F, Gallino F, Belli F, Parmiani G. Novel HLA-Cw8-restricted T cell epitopes derived from tyrosinase-related protein-2 and gp100 melanoma antigens. J Immunol. 1999;162:1739–1748. [PubMed] [Google Scholar]
- 28.Wang F, Bade E, Kuniyoshi C, Spears L, Jeffery G, Marty V, Groshen S, Weber J. Phase I trial of a MART-1 peptide vaccine with incomplete Freund's adjuvant for resected high-risk melanoma. Clin Cancer Res. 1999;5:2756–2765. [PubMed] [Google Scholar]
- 29.Linette GP, Zhang D, Hodi FS, Jonasch EP, Longerich S, Stowell CP, Webb IJ, Daley H, Soiffer RJ, Cheung AM, Eapen SG, Fee SV, Rubin KM, Sober AJ, Haluska FG. Immunization using autologous dendritic cells pulsed with the melanoma-associated antigen gp100-derived G280-9V peptide elicits CD8+ immunity. Clin Cancer Res. 2005;11:7692–7699. doi: 10.1158/1078-0432.CCR-05-1198. [DOI] [PubMed] [Google Scholar]
- 30.Mandruzzato S, Rossi E, Bernardi F, Tosello V, Macino B, Basso G, Chiarion-Sileni V, Rossi CR, Montesco C, Zanovello P. Large and dissimilar repertoire of Melan-A/MART-1-specific CTL in metastatic lesions and blood of a melanoma patient. J Immunol. 2002;169:4017–4024. doi: 10.4049/jimmunol.169.7.4017. [DOI] [PubMed] [Google Scholar]
- 31.Loftus DJ, Castelli C, Clay TM, Squarcina P, Marincola FM, Nishimura MI, Parmiani G, Appella E, Rivoltini L. Identification of epitope mimics recognized by CTL reactive to the melanoma/melanocyte-derived peptide MART-1(27-35). J Exp Med. 1996;184:647–657. doi: 10.1084/jem.184.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Liénard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, Rufer N, Lubenow N, Speiser D, Cerottini JC, Romero P, Pittet MJ. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 33.Hodi FS. Well-defined melanoma antigens as progression markers for melanoma: insights into differential expression and host response based on stage. Clin Cancer Res. 2006;12:673–678. doi: 10.1158/1078-0432.CCR-05-2616. [DOI] [PubMed] [Google Scholar]
- 34.Vantomme V, Boel P, De Plaen E, Boon T, van der Bruggen P. A new tumor-specific antigenic peptide encoded by MAGE-6 is presented to cytolytic T lymphocytes by HLA-Cw16. Cancer Immun. 2003;3:17. http://www.cancerimmunity.org/v3p17/031118.htm [PubMed] [Google Scholar]
- 35.Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A, Lehvaslaiho M, Carninci P, Hayashizaki Y, Jongeneel CV, Simpson AJ, Old LJ, Hide W. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basarab T, Picard JK, Simpson E, Russell-Jones R. Melanoma antigen-encoding gene expression in melanocytic naevi and cutaneous malignant melanomas. Br J Dermatol. 1999;140:106–108. doi: 10.1046/j.1365-2133.1999.02616.x. [DOI] [PubMed] [Google Scholar]
- 37.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 38.Rivoltini L, Loftus DJ, Squarcina P, Castelli C, Rini F, Arienti F, Belli F, Marincola FM, Geisler C, Borsatti A, Appella E, Parmiani G. Recognition of melanoma-derived antigens by CTL: possible mechanisms involved in down-regulating anti-tumor T-cell reactivity. Crit Rev Immunol. 1998;18:55–63. doi: 10.1615/critrevimmunol.v18.i1-2.70. [DOI] [PubMed] [Google Scholar]
- 39.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Brusic V, Sidney J, Sette A, Logan TF, Kasamon YL, Slingluff CL Jr, Kirkwood JM, Storkus WJ. MAGE-6 encodes HLA-DRbeta1*0401-presented epitopes recognized by CD4+ T cells from patients with melanoma or renal cell carcinoma. Clin Cancer Res. 2003;9:947–954. [PubMed] [Google Scholar]
- 40.Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv Cancer Res. 2006;95:293–322. doi: 10.1016/S0065-230X(06)95008-8. [DOI] [PubMed] [Google Scholar]
- 41.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gammadelta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinle A, Li P, Morris DL, Groh V, Lanier LL, Strong RK, Spies T. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53:279–287. doi: 10.1007/s002510100325. [DOI] [PubMed] [Google Scholar]
- 43.Xiong N, Raulet DH. Development and selection of gammadelta T cells. Immunol Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 44.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 45.Li JQ, Cui LX, He W. Distinct pattern of human Vdelta1 gammadelta T cells recognizing MICA. Cell Mol Immunol. 2005;2:253–258. [PubMed] [Google Scholar]
- 46.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 47.Kawakami Y, Suzuki Y, Shofuda T, Kiniwa Y, Inozume T, Dan K, Sakurai T, Fujita T. T cell immune responses against melanoma and melanocytes in cancer and autoimmunity. Pigment Cell Res. 2000;13 Suppl 8:163–169. doi: 10.1034/j.1600-0749.13.s8.29.x. [DOI] [PubMed] [Google Scholar]
- 48.Linard B, Bézieau S, Benlalam H, Labarrière N, Guilloux Y, Diez E, Jotereau F. A ras-mutated peptide targeted by CTL infiltrating a human melanoma lesion. J Immunol. 2002;168:4802–4808. doi: 10.4049/jimmunol.168.9.4802. [DOI] [PubMed] [Google Scholar]
- 49.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27:184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleh F, Renno W, Klepacek I, Ibrahim G, Asfar S, Dashti H, Romero P, Dashti A, Behbehani A. Melanoma immunotherapy: past, present, and future. Curr Pharm Des. 2005;11:3461–3473. doi: 10.2174/138161205774414529. [DOI] [PubMed] [Google Scholar]
- 51.Strohal R, Paucz L, Pehamberger H, Stingl G. T-cell receptor repertoire of lymphocytes infiltrating cutaneous melanoma is predominated by V alpha specificities present in T-cells of normal human skin. Cancer Res. 1994;54:4734–4739. [PubMed] [Google Scholar]
- 52.Nitta T, Oksenberg JR, Rao NA, Steinman L. Predominant expression of T cell receptor V alpha 7 in tumor-infiltrating lymphocytes of uveal melanoma. Science. 1990;249:672–674. doi: 10.1126/science.2382141. [DOI] [PubMed] [Google Scholar]
- 53.Clemente C, Rao S, Lupetti R, Tragni G, Pisarra P, Bersani I, Parmiani G, Mihm MC Jr, Sensi M. Immunohistochemical analysis of the T-cell receptor beta-chain variable regions expressed by T lymphocytes infiltrating primary human melanoma. Lab Invest. 1998;78:619–627. [PubMed] [Google Scholar]
- 54.Yazdi AS, Morstedt K, Puchta U, Ghoreschi K, Flaig MJ, Rocken M, Sander CA. Heterogeneity of T-cell clones infiltrating primary malignant melanomas. J Invest Dermatol. 2006;126:393–398. doi: 10.1038/sj.jid.5700082. [DOI] [PubMed] [Google Scholar]
- 55.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 56.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez-Montagut T, Turk MJ, Wolchok JD, Guevara-Patino JA, Houghton AN. Immunity to melanoma: unraveling the relation of tumor immunity and autoimmunity. Oncogene. 2003;22:3180–3187. doi: 10.1038/sj.onc.1206462. [DOI] [PubMed] [Google Scholar]
- 58.Chiou SH, Sheu BC, Chang WC, Huang SC, Hong-Nerng H. Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol. 2005;67:35–50. doi: 10.1016/j.jri.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Cohen PJ, Lotze MT, Roberts JR, Rosenberg SA, Jaffe ES. The immunopathology of sequential tumor biopsies in patients treated with interleukin-2. Correlation of response with T-cell infiltration and HLA-DR expression. Am J Pathol. 1987;129:208–216. [PMC free article] [PubMed] [Google Scholar]
- 60.Belldegrun A, Muul LM, Rosenberg SA. Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res. 1988;48:206–214. [PubMed] [Google Scholar]
- 61.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 62.Hussein MR. Tumour-infiltrating lymphocytes and melanoma tumorigenesis: an insight. Br J Dermatol. 2005;153:18–21. doi: 10.1111/j.1365-2133.2005.06629.x. [DOI] [PubMed] [Google Scholar]
- 63.Romero P, Valmori D, Pittet MJ, Zippelius A, Rimoldi D, Lévy F, Dutoit V, Ayyoub M, Rubio-Godoy V, Michielin O, Guillaume P, Batard P, Luescher IF, Lejeune F, Liénard D, Rufer N, Dietrich PY, Speiser DE, Cerottini JC. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol Rev. 2002;188:81–96. doi: 10.1034/j.1600-065x.2002.18808.x. [DOI] [PubMed] [Google Scholar]
- 64.Walker EB, Haley D, Miller W, Floyd K, Wisner KP, Sanjuan N, Maecker H, Romero P, Hu HM, Alvord WG, Smith JW 2nd, Fox BA, Urba WJ. gp100(209-2M) peptide immunization of human lymphocyte antigen-A2+ stage I-III melanoma patients induces significant increase in antigen-specific effector and long-term memory CD8+ T cells. Clin Cancer Res. 2004;10:668–680. doi: 10.1158/1078-0432.ccr-0095-03. [DOI] [PubMed] [Google Scholar]
- 65.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumor progression despite massive influx of activated CD8+ T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 67.Bachelez H, Flageul B, Degos L, Boumsell L, Bensussan A. TCR gamma delta bearing T lymphocytes infiltrating human primary cutaneous melanomas. J Invest Dermatol. 1992;98:369–374. doi: 10.1111/1523-1747.ep12499808. [DOI] [PubMed] [Google Scholar]
- 68.Viguier M, Lemaître F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 69.Roszkowski JJ, Lyons GE, Kast WM, Yee C, Van Besien K, Nishimura MI. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65:1570–1576. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- 70.Kahn M, Sugawara H, McGowan P, Okuno K, Nagoya S, Hellström KE, Hellström I, Greenberg P. CD4+ T cell clones specific for the human p97 melanoma-associated antigen can eradicate pulmonary metastases from a murine tumor expressing the p97 antigen. J Immunol. 1991;146:3235–3241. [PubMed] [Google Scholar]
- 71.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conrad CT, Ernst NR, Dummer W, Bröcker EB, Becker JC. Differential expression of transforming growth factor beta 1 and interleukin 10 in progressing and regressing areas of primary melanoma. J Exp Clin Cancer Res. 1999;18:225–232. [PubMed] [Google Scholar]
- 74.Lowes MA, Bishop GA, Crotty K, Barnetson RS, Halliday GM. T helper 1 cytokine mRNA is increased in spontaneously regressing primary melanomas. J Invest Dermatol. 1997;108:914–919. doi: 10.1111/1523-1747.ep12292705. [DOI] [PubMed] [Google Scholar]
- 75.Wagner SN, Schultewolter T, Wagner C, Briedigkeit L, Becker JC, Kwasnicka HM, Goos M. Immune response against human primary malignant melanoma: a distinct cytokine mRNA profile associated with spontaneous regression. Lab Invest. 1998;78:541–550. [PubMed] [Google Scholar]
- 76.Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol. 2000;85:9–18. doi: 10.1016/S1081-1206(10)62426-X. Quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- 77.Albers AE, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer Immunol Immunother. 2005;54:1072–1081. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 79.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 80.Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res. 2006;66:7301–7309. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- 81.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 82.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 83.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 84.Sakaguchi S, Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J Immunol. 1989;142:471–480. [PubMed] [Google Scholar]
- 85.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 87.Chattopadhyay S, Chakraborty NG, Mukherji B. Regulatory T cells and tumor immunity. Cancer Immunol Immunother. 2005;54:1153–1161. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 89.De Panfilis G, Campanini N, Santini M, Mori G, Tognetti E, Maestri R, Lombardi M, Froio E, Ferrari D, Ricci R. Phase- and stage-related proportions of T cells bearing the transcription factor FOXP3 infiltrate primary melanoma. J Invest Dermatol. 2008;128:676–684. doi: 10.1038/sj.jid.5701046. [DOI] [PubMed] [Google Scholar]
- 90.Hussein MR, Elsers DAH, Fadel SA, Omar AE. Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J Clin Pathol. 2006;59:316–324. doi: 10.1136/jcp.2005.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miracco C, Mourmouras V, Biagioli M, Rubegni P, Mannucci S, Monciatti I, Cosci E, Tosi P, Luzi P. Utility of tumour-infiltrating CD25+FOXP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol Rep. 2007;18:1115–1122. [PubMed] [Google Scholar]
- 92.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 93.Pavoni E, Monteriù G, Santapaola D, Petronzelli F, Anastasi AM, Pelliccia A, D'Alessio V, De Santis R, Minenkova O. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 2007;7:70. doi: 10.1186/1472-6750-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yeilding NM, Gerstner C, Kirkwood JM. Analysis of two human monoclonal antibodies against melanoma. Int J Cancer. 1992;52:967–973. doi: 10.1002/ijc.2910520623. [DOI] [PubMed] [Google Scholar]
- 95.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. J Exp Med. 1995;182:1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jäger D, Arand M, Ritter G, Cerundolo V, Dupont B, Chen YT, Old LJ, Knuth A. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci U S A. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ralfkiaer E, Hou-Jensen K, Gatter KC, Drzewiecki KT, Mason DY. Immunohistological analysis of the lymphoid infiltrate in cutaneous malignant melanomas. Virchows Arch A Pathol Anat Histopathol. 1987;410:355–361. doi: 10.1007/BF00711292. [DOI] [PubMed] [Google Scholar]
- 98.Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 99.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 100.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 101.Jørkov AS, Donskov F, Steiniche T, Ternesten-Bratel A, Naredi P, Hellstrand K, Hokland M. Immune response in blood and tumour tissue in patients with metastatic malignant melanoma treated with IL-2, IFN alpha and histamine dihydrochloride. Anticancer Res. 2003;23:537–542. [PubMed] [Google Scholar]
- 102.Schøller J, thor Straten P, Birck A, Siim E, Dahlström K, Drzewiecki KT, Zeuthen J. Analysis of T cell receptor alpha beta variability in lymphocytes infiltrating melanoma primary tumours and metastatic lesions. Cancer Immunol Immunother. 1994;39:239–248. doi: 10.1007/BF01525987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen J, Niu H, He W, Ba D. Antitumor activity of expanded human tumor-infiltrating gammadelta T lymphocytes. Int Arch Allergy Immunol. 2001;125:256–263. doi: 10.1159/000053824. [DOI] [PubMed] [Google Scholar]
- 104.Re F, Donnini A, Bartozzi B, Bernardini G, Provinciali M. Circulating gammadelta T cells in young/adult and old patients with cutaneous primary melanoma. Immun Ageing. 2005;2:2. doi: 10.1186/1742-4933-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Argentati K, Re F, Serresi S, Tucci MG, Bartozzi B, Bernardini G, Provinciali M. Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol. 2003;120:829–834. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- 106.Flageul B, Bachelez H, Boumsell L, Degos L, Bensussan A. Infiltrating lymphocytes in benign and malignant naevomelanocytic lesions. Nouv Rev Fr Hematol. 1990;32:9–11. [PubMed] [Google Scholar]
- 107.Vetter CS, Groh V, thor Straten P, Spies T, Bröcker EB, Becker JC. Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol. 2002;118:600–605. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 108.Fuertes MB, Girart MV, Molinero LL, Domaica CI, Rossi LE, Barrio MM, Mordoh J, Rabinovich GA, Zwirner NW. Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell-mediated cytotoxicity. J Immunol. 2008;180:4606–4614. doi: 10.4049/jimmunol.180.7.4606. [DOI] [PubMed] [Google Scholar]
- 109.Tarhini AA, Kirkwood JM, Gooding WE, Cai C, Agarwala SS. Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J Clin Oncol. 2007;25:3802–3807. doi: 10.1200/JCO.2006.10.2822. [DOI] [PubMed] [Google Scholar]
- 110.Buzaid AC. Management of metastatic cutaneous melanoma. Oncology (Williston Park) 2004;18:1443–1450. Discussion 1457-1459. [PubMed] [Google Scholar]
- 111.Palena C, Abrams SI, Schlom J, Hodge JW. Cancer vaccines: preclinical studies and novel strategies. Adv Cancer Res. 2006;95:115–145. doi: 10.1016/S0065-230X(06)95004-0. [DOI] [PubMed] [Google Scholar]
- 112.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 113.Itoh K, Tilden AB, Balch CM. Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating into human metastatic melanomas. Cancer Res. 1986;46:3011–3017. [PubMed] [Google Scholar]
- 114.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rubin JT, Elwood LJ, Rosenberg SA, Lotze MT. Immunohistochemical correlates of response to recombinant interleukin-2-based immunotherapy in humans. Cancer Res. 1989;49:7086–7092. [PubMed] [Google Scholar]
- 116.Liu S, Riley J, Rosenberg S, Parkhurst M. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006;29:284–293. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]
- 117.Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4 Suppl 3:S161–S167. doi: 10.1186/ar584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lens M. Cutaneous melanoma: interferon alpha adjuvant therapy for patients at high risk for recurrent disease. Dermatol Ther. 2006;19:9–18. doi: 10.1111/j.1529-8019.2005.00051.x. [DOI] [PubMed] [Google Scholar]
- 119.Moschos SJ, Edington HD, Land SR, Rao UN, Jukic D, Shipe-Spotloe J, Kirkwood JM. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 120.Carson WE. Interferon-alpha-induced activation of signal transducer and activator of transcription proteins in malignant melanoma. Clin Cancer Res. 1998;4:2219–2228. [PubMed] [Google Scholar]
- 121.Sun Y, Jurgovsky K, Möller P, Alijagic S, Dorbic T, Georgieva J, Wittig B, Schadendorf D. Vaccination with IL-12 gene-modified autologous melanoma cells: preclinical results and a first clinical phase I study. Gene Ther. 1998;5:481–490. doi: 10.1038/sj.gt.3300619. [DOI] [PubMed] [Google Scholar]
- 122.Homey B, Müller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 123.Mortarini R, Borri A, Tragni G, Bersani I, Vegetti C, Bajetta E, Pilotti S, Cerundolo V, Anichini A. Peripheral burst of tumor-specific cytotoxic T lymphocytes and infiltration of metastatic lesions by memory CD8+ T cells in melanoma patients receiving interleukin 12. Cancer Res. 2000;60:3559–3568. [PubMed] [Google Scholar]
- 124.Grohmann U, Bianchi R, Ayroldi E, Belladonna ML, Surace D, Fioretti MC, Puccetti P. A tumor-associated and self antigen peptide presented by dendritic cells may induce T cell anergy in vivo, but IL-12 can prevent or revert the anergic state. J Immunol. 1997;158:3593–3602. [PubMed] [Google Scholar]
- 125.Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22:3188–3192. doi: 10.1038/sj.onc.1206459. [DOI] [PubMed] [Google Scholar]
- 126.Mach N, Dranoff G. Cytokine-secreting tumor cell vaccines. Curr Opin Immunol. 2000;12:571–575. doi: 10.1016/s0952-7915(00)00144-8. [DOI] [PubMed] [Google Scholar]
- 127.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soiffer R, Hodi FS, Haluska F, Jung K, Gillessen S, Singer S, Tanabe K, Duda R, Mentzer S, Jaklitsch M, Bueno R, Clift S, Hardy S, Neuberg D, Mulligan R, Webb I, Mihm M, Dranoff G. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21:3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 129.Moiseyenko V, Imyanitov E, Danilova A, Danilov A, Baldueva I. Cell technologies in immunotherapy of cancer. Adv Exp Med Biol. 2007;601:387–393. doi: 10.1007/978-0-387-72005-0_42. [DOI] [PubMed] [Google Scholar]
- 130.Copier J, Ward S, Dalgleish A. Cell based cancer vaccines: regulatory and commercial development. Vaccine. 2007;25 Suppl 2:B35–B46. doi: 10.1016/j.vaccine.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 131.Lesimple T, Neidhard EM, Vignard V, Lefeuvre C, Adamski H, Labarrière N, Carsin A, Monnier D, Collet B, Clapisson G, Birebent B, Philip I, Toujas L, Chokri M, Quillien V. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–7388. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 132.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 133.Banchereau J, Ueno H, Dhodapkar M, Connolly J, Finholt JP, Klechevsky E, Blanck JP, Johnston DA, Palucka AK, Fay J. Immune and clinical outcomes in patients with stage IV melanoma vaccinated with peptide-pulsed dendritic cells derived from CD34+ progenitors and activated with type I interferon. J Immunother. 2005;28:505–516. doi: 10.1097/01.cji.0000171292.79663.cb. [DOI] [PubMed] [Google Scholar]
- 134.Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Bröcker EB, Steinman RM, Enk A, Kämpgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carrasco J, Van Pel A, Neyns B, Lethé B, Brasseur F, Renkvist N, van der Bruggen P, van Baren N, Paulus R, Thielemans K, Boon T, Godelaine D. Vaccination of a melanoma patient with mature dendritic cells pulsed with MAGE-3 peptides triggers the activity of nonvaccine anti-tumor cells. J Immunol. 2008;180:3585–3593. doi: 10.4049/jimmunol.180.5.3585. [DOI] [PubMed] [Google Scholar]
- 137.Griffioen M, Borghi M, Schrier PI, Osanto S, Schadendorf D. Analysis of T-cell responses in metastatic melanoma patients vaccinated with dendritic cells pulsed with tumor lysates. Cancer Immunol Immunother. 2004;53:715–722. doi: 10.1007/s00262-004-0514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 139.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vignard V, Lemercier B, Lim A, Pandolfino MC, Guilloux Y, Khammari A, Rabu C, Echasserieau K, Lang F, Gougeon ML, Dreno B, Jotereau F, Labarriere N. Adoptive transfer of tumor-reactive Melan-A-specific CTL clones in melanoma patients is followed by increased frequencies of additional Melan-A-specific T cells. J Immunol. 2005;175:4797–4805. doi: 10.4049/jimmunol.175.7.4797. [DOI] [PubMed] [Google Scholar]
- 141.Meidenbauer N, Marienhagen J, Laumer M, Vogl S, Heymann J, Andreesen R, Mackensen A. Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients. J Immunol. 2003;170:2161–2169. doi: 10.4049/jimmunol.170.4.2161. [DOI] [PubMed] [Google Scholar]
- 142.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.North RJ. The murine antitumor immune response and its therapeutic manipulation. Adv Immunol. 1984;35:89–155. doi: 10.1016/s0065-2776(08)60575-1. [DOI] [PubMed] [Google Scholar]
- 144.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–294. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ju SA, Lee SC, Kwon TH, Heo SK, Park SM, Paek HN, Suh JH, Cho HR, Kwon B, Kwon BS, Kim BS. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol Cell Biol. 2005;83:344–351. doi: 10.1111/j.1440-1711.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 148.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 149.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily - CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 150.Jago CB, Yates J, Camara NO, Lechler RI, Lombardi G. Differential expression of CTLA-4 among T cell subsets. Clin Exp Immunol. 2004;136:463–471. doi: 10.1111/j.1365-2249.2004.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 152.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 153.Schrama D, Becker JC. Biologics in Targeted Cancer Therapy. In: Radeke HH, editor. Biologics in Targeted Cancer Therapy. Berlin, Germany: Springer; 2007. (Ed.) [Google Scholar]
- 154.Siu E, Carreno BM, Madrenas J. TCR subunit specificity of CTLA-4-mediated signaling. J Leukoc Biol. 2003;74:1102–1107. doi: 10.1189/jlb.0503198. [DOI] [PubMed] [Google Scholar]
- 155.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 156.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kapadia D, Fong L. CTLA-4 blockade: autoimmunity as treatment. J Clin Oncol. 2005;23:8926–8928. doi: 10.1200/JCO.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 158.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patiño JA, Perales MA, Mortazavi F, Bacich D, Heston W, Latouche JB, Sadelain M, Allison JP, Scher HI, Houghton AN. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 159.Sotomayor EM, Borrello I, Tubb E, Allison JP, Levitsky HI. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96:11476–11481. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen Y, Ma Y, Chen Y. Roles of cytotoxic T-lymphocyte-associated antigen-4 in the inductive phase of oral tolerance. Immunology. 2002;105:171–180. doi: 10.1046/j.1365-2567.2002.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 162.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63:3281–3288. [PubMed] [Google Scholar]
- 164.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, Kleiner D, Mavroukakis SA, Yellin M, Rosenberg SA. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 168.O'Mahony D, Morris JC, Quinn C, Gao W, Wilson WH, Gause B, Pittaluga S, Neelapu S, Brown M, Fleisher TA, Gulley JL, Schlom J, Nussenblatt R, Albert P, Davis TA, Lowy I, Petrus M, Waldmann TA, Janik JE. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958–964. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 169.Reuben JM, Lee BN, Li C, Gomez-Navarro J, Bozon VA, Parker CA, Hernandez IM, Gutierrez C, Lopez-Berestein G, Camacho LH. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006;106:2437–2444. doi: 10.1002/cncr.21854. [DOI] [PubMed] [Google Scholar]
- 170.Hodi FS, Oble DA, Drappatz J, Velazquez EF, Ramaiya N, Ramakrishna N, Day AL, Kruse A, Mac Rae S, Hoos A, Mihm M. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5:557–561. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 171.Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D, Grillo-Lopez A, Shuman MA. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 172.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oble DA, Mino-Kenudson M, Goldsmith J, Hodi FS, Seliem RM, Dranoff G, Mihm M, Hasserjian R, Lauwers GY. Anti-CTLA-4 mAb associated panenteritis: A histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;2:1130–1137. doi: 10.1097/PAS.0b013e31817150e3. [DOI] [PubMed] [Google Scholar]
- 175.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scufy mouse mutant. J Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 176.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mouse. Am J Pathol. 1991;138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 177.Fontenot JD, Gavin MA, Rudinsky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 178.Appay V, Jandus C, Voelter V, Reynard S, Coupland SE, Rimoldi D, Lienard D, Guillaume P, Krieg AM, Cerottini JC, Romero P, Leyvraz S, Rufer N, Speiser DE. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177:1670–1678. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 179.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 181.Wong BY, Gregory SA, Dang NH. Denileukin diftitox as novel targeted therapy for lymphoid malignancies. Cancer Invest. 2007;25:495–501. doi: 10.1080/07357900701360096. [DOI] [PubMed] [Google Scholar]
- 182.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Powell DJ Jr, Felipe-Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C, White DE, Mavroukakis S, Kreitman RJ, Rosenberg SA, Pastan I. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919–4928. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S, Waldmann TA, Pastan I. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 186.Minami J, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 187.Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 188.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97:1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 190.Shevach EM. Fatal attraction: tumors beckon regulatory T cells. Nat Med. 2004;10:900–901. doi: 10.1038/nm0904-900. [DOI] [PubMed] [Google Scholar]
- 191.Yano H, Ishida T, Inagaki A, Ishii T, Ding J, Kusumoto S, Komatsu H, Iida S, Inagaki H, Ueda R. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: a novel immunotherapy for aggressive/refractory Mycosis fungoides and Sezary syndrome. Clin Cancer Res. 2007;13:6494–6500. doi: 10.1158/1078-0432.CCR-07-1324. [DOI] [PubMed] [Google Scholar]
- 192.Alfonso-Perez M, Lopez-Giral S, Quintana NE, Loscertales J, Martin-Jimenez P, Munoz C. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol. 2006;79:1157–1165. doi: 10.1189/jlb.1105623. [DOI] [PubMed] [Google Scholar]
- 193.Agrawal S, Marquet J, Delfau-Larue MH, Copie-Bergman C, Jouault H, Reyes F, Bensussan A, Farcet JP. CD3 hyporesponsiveness and in vitro apoptosis are features of T cells from both malignant and nonmalignant secondary lymphoid organs. J Clin Invest. 1998;102:1715–1723. doi: 10.1172/JCI3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Radoja S, Saio M, Schaer D, Koneru M, Vukmanovic S, Frey AB. CD8(+) tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001;167:5042–5051. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- 195.Robbins PF, El-Gamil M, Li YF, Zeng G, Dudley M, Rosenberg SA. Multiple HLA class II-restricted melanocyte differentiation antigens are recognized by tumor-infiltrating lymphocytes from a patient with melanoma. J Immunol. 2002;169:6036–6047. doi: 10.4049/jimmunol.169.10.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Slingluff CL Jr, Colella TA, Thompson L, Graham DD, Skipper JC, Caldwell J, Brinckerhoff L, Kittlesen DJ, Deacon DH, Oei C, Harthun NL, Huczko EL, Hunt DF, Darrow TL, Engelhard VH. Melanomas with concordant loss of multiple melanocytic differentiation proteins: immune escape that may be overcome by targeting unique or undefined antigens. Cancer Immunol Immunother. 2000;48:661–672. doi: 10.1007/s002620050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim J, Mori T, Chen SL, Amersi FF, Martinez SR, Kuo C, Turner RR, Ye X, Bilchik AJ, Morton DL, Hoon DS. Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg. 2006;244:113–120. doi: 10.1097/01.sla.0000217690.65909.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjöberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48:353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ Jr, Rosenberg SA, Hodes RJ. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007;30:123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, Robbins PF. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, Dokal I, Webster D, Lawson AD, Akbar AN. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 203.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rivoltini L, Kawakami Y, Sakaguchi K, Southwood S, Sette A, Robbins PF, Marincola FM, Salgaller ML, Yannelli JR, Appella E, Rosenberg SA. Induction of tumor-reactive CTL from peripheral blood and tumor-infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J Immunol. 1995;154:2257–2265. [PubMed] [Google Scholar]
- 205.Lindauer M, Stanislawski T, Häussler A, Antunes E, Cellary A, Huber C, Theobald M. The molecular basis of cancer immunotherapy by cytotoxic T lymphocytes. J Mol Med. 1998;76:32–47. doi: 10.1007/s001090050188. [DOI] [PubMed] [Google Scholar]
- 206.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 208.Powell DJ Jr, Rosenberg SA. Phenotypic and functional maturation of tumor antigen-reactive CD8+ T lymphocytes in patients undergoing multiple course peptide vaccination. J Immunother. 2004;27:36–47. doi: 10.1097/00002371-200401000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen DS, Soen Y, Stuge TB, Lee PP, Weber JS, Brown PO, Davis MM. Marked differences in human melanoma antigen-specific T cell responsiveness after vaccination using a functional microarray. PLoS Med. 2005;2:e265. doi: 10.1371/journal.pmed.0020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yee C. Adoptive T cell therapy: Addressing challenges in cancer immunotherapy. J Transl Med. 2005;3:17. doi: 10.1186/1479-5876-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 213.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 214.Kim R, Emi M, Tanabe K, Uchida Y, Toge T. The role of Fas ligand and transforming growth factor beta in tumor progression: molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy. Cancer. 2004;100:2281–2291. doi: 10.1002/cncr.20270. [DOI] [PubMed] [Google Scholar]
- 215.Fukuda K, Tsujitani S, Maeta Y, Yamaguchi K, Ikeguchi M, Kaibara N. The expression of RCAS1 and tumor infiltrating lymphocytes in patients with T3 gastric carcinoma. Gastric Cancer. 2002;5:220–227. doi: 10.1007/s101200200038. [DOI] [PubMed] [Google Scholar]
- 216.Takahashi H, Iizuka H, Nakashima M, Wada T, Asano K, Ishida-Yamamoto A, Watanabe T. RCAS1 antigen is highly expressed in extramammary Paget's disease and in advanced stage squamous cell carcinoma of the skin. J Dermatol Sci. 2001;26:140–144. doi: 10.1016/s0923-1811(00)00170-5. [DOI] [PubMed] [Google Scholar]
- 217.Nakashima M, Sonoda K, Watanabe T. Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med. 1999;5:938–942. doi: 10.1038/11383. [DOI] [PubMed] [Google Scholar]
- 218.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 219.Zea AH, Curti BD, Longo DL, Alvord WG, Strobl SL, Mizoguchi H, Creekmore SP, O'Shea JJ, Powers GC, Urba WJ, Ochoa AC. Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin Cancer Res. 1995;1:1327–1335. [PubMed] [Google Scholar]
- 220.Maccalli C, Pisarra P, Vegetti C, Sensi M, Parmiani G, Anichini A. Differential loss of T cell signaling molecules in metastatic melanoma patients' T lymphocyte subsets expressing distinct TCR variable regions. J Immunol. 1999;163:6912–6923. [PubMed] [Google Scholar]
- 221.Wittgen HG, van Kempen LC. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007;17:400–409. doi: 10.1097/CMR.0b013e3282f1d312. [DOI] [PubMed] [Google Scholar]
- 222.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 224.Natali PG, Nicotra MR, Bigotti A, Venturo I, Marcenaro L, Giacomini P, Russo C. Selective changes in expression of HLA class I polymorphic determinants in human solid tumors. Proc Natl Acad Sci U S A. 1989;86:6719–6723. doi: 10.1073/pnas.86.17.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wischhusen J, Waschbisch A, Wiendl H. Immune-refractory cancers and their little helpers--an extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin Cancer Biol. 2007;17:459–468. doi: 10.1016/j.semcancer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 226.Maeurer MJ, Martin D, Elder E, Storkus WJ, Lotze MT. Detection of naturally processed and HLA-A1-presented melanoma T-cell epitopes defined by CD8(+) T-cells' release of granulocyte-macrophage colony-stimulating factor but not by cytolysis. Clin Cancer Res. 1996;2:87–95. [PubMed] [Google Scholar]
- 227.Markel G, Seidman R, Stern N, Cohen-Sinai T, Izhaki O, Katz G, Besser M, Treves AJ, Blumberg RS, Loewenthal R, Mandelboim O, Orenstein A, Schachter J. Inhibition of human tumor-infiltrating lymphocyte effector functions by the homophilic carcinoembryonic cell adhesion molecule 1 interactions. J Immunol. 2006;177:6062–6071. doi: 10.4049/jimmunol.177.9.6062. [DOI] [PubMed] [Google Scholar]
- 228.Kreis S, Munz GA, Haan S, Heinrich PC, Behrmann I. Cell density dependent increase of constitutive signal transducers and activators of transcription 3 activity in melanoma cells is mediated by Janus kinases. Mol Cancer Res. 2007;5:1331–1341. doi: 10.1158/1541-7786.MCR-07-0317. [DOI] [PubMed] [Google Scholar]