Abstract
Breast tumours consist of phenotypically diverse populations of breast cancer cells of which only a minority has the ability to form new tumours. The capacity for breast tumour development has been shown to be restricted to breast cancer stem cells with the CD44+/CD24(-/low) phenotype. These cells can resist apoptosis through mechanisms such as the regulation of Bcl-2. Identification of this population of cells is important because of its implication in the development of new therapeutic strategies. One hundred and forty-six primary operable breast cancer patients were investigated in order to identify the population of CD44+ and Bcl-2+ cells in paraffin-embedded tissues by immunohistochemistry. The prevalence of these phenotypes was then correlated with clinicopathological features. CD44 and Bcl-2 expression was detected in 86% and 82% of breast tumours, respectively. There was no significant correlation between CD44+ tumour cell prevalence and tumour characteristics, whereas the prevalence of CD44+ cells was associated with higher levels of Bcl-2 expression (P = 0.004). In univariate analysis, Bcl-2 expression was correlated with breast tumours of lower grade (P < 0.001) and fewer lymphatic metastases (P < 0.05). Our findings suggest that the prevalence of CD44+ tumour cells as a subpopulation of breast cancer stem cells was of no clinicopathological significance, but was correlated with higher Bcl-2 expression. This population of tumour cells may thus be more resistant to apoptosis. Targeting these cells in combination with current treatments may be more effective in treating breast cancer patients.
Keywords: human, breast cancer, CD44, Bcl-2, immunohistochemistry
Introduction
Breast cancer is the most common type of cancer and the second leading cause of cancer deaths among women in Western countries. The common phenotypes of cancer cells and stem cells suggest that breast cancers arise from stem cells (SCs). Breast cancer stem cells are a small population of cells which have the capacity to become tumourigenic through the accumulation of mutations that share classic features of stem cells, such as self-renewal and differentiation (1). Indeed, only cancer stem cells (CSCs) or tumour-initiating cells can repopulate the bulk of a tumour in the long term so as to maintain its ability of renewing continuously, thereby mimicking their normal counterparts albeit abnormally (2, 3).
The cancer stem cell concept has been supported by findings in haematopoietic malignancies and also some solid tumours, including breast, lung, ovarian, prostate, gastric, colorectal and brain tumours, based on the presence of specific surface markers (4). Breast cancer stem cells have been identified to strongly express adhesion molecule CD44, together with no or very low levels of adhesion molecule CD24. Only the population of CD44+/CD24(-/low) cells was able to initiate the process of carcinogenesis in immunodeficient mice (5). However, the prevalence of CD44+/CD24(-/low) tumour cells in breast sections does not show any association with clinical outcome or survival (6).
It has been proven that stem cells can resist apoptosis through a variety of mechanisms, including the regulation of Bcl-2, whereas non-tumourigenic cells in tumours are more susceptible to the induction of apoptosis or chemotherapy (7-9). The resistance of CSCs to chemotherapy may be caused through increased expression of the Bcl-2 family proteins, which leads to increased expression of membrane proteins responsible for drug resistance. The increased expression of the BCL2 oncogene in haemapoietic SCs has an anti-apoptotic effect and leads to an increased number of haemapoietic SCs (1, 10-12). The expression of such proteins in tumourigenic breast cancer cells may make them resistant to current therapies (5, 13). Therefore, the prospective identification of the tumourigenic population of cancer cells expressing these molecules could provide a means to target and eliminate this population of cancer cells. Targeting this population may result in more effective therapies since current treatments only eliminate non-tumourigenic cells (14). It is hypothesized that potential cancer stem cell markers can be screened from the plethora of cell surface molecules using other properties of CSCs. Therefore some molecules, such as CD44, can be empirically chosen as prospective markers.
It is therefore important to examine if increased expression of CD44 as a prospective marker of CSCs in breast tumour cells is associated with the expression of Bcl-2 as an anti-apoptotic protein. To date, no data is available regarding the relationship between Bcl-2 and CSC markers in breast tumour tissues. The aim of this study was to identify CD44+ tumour cells in breast tumour sections by immunohistochemistry and to determine whether expression of CD44 correlated with the expression of the Bcl-2 oncoprotein. We also sought to determine whether the expression level of CD44 and Bcl-2 correlated with clinicopathological parameters. Our findings may help researchers design therapeutic models aimed at the selective elimination of cells that have the properties of stem cells and are more resistant to apoptosis.
Results
Demographics of patient and tumour characteristics
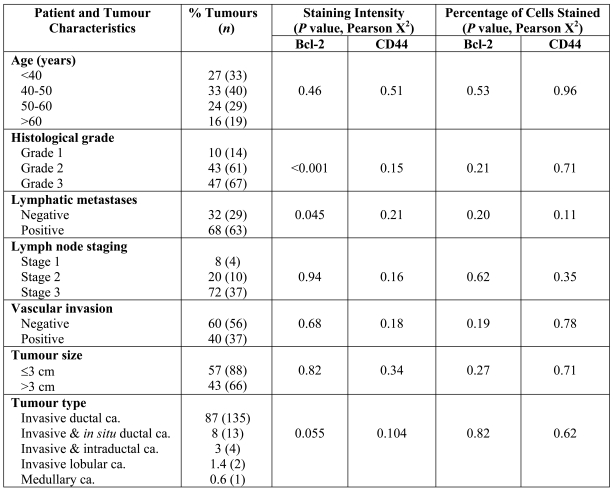
Patient and tumour characteristics are summarized in Table 1. At the time of diagnosis, the patients ranged in age from 25 to 82 years old (mean of 46 years). Of these patients, 27% were younger than 40, 33% were 40-50 years old, 24% were 50-60 years old and only 16% were over 60 years of age.
Table 1.
Association between Bcl-2 and CD44 expression with clinicopathological parameters in invasive breast carcinoma.
The most common histological type was invasive ductal carcinoma, comprising 87% of the cases. The majority of tumours were grade 3 (47%) or grade 2 (43%), and only 10% of cases were grade 1 (10%). Fifty-seven percent of the tumours were 3 cm or less in size. Of the patients with known lymph node status, 32% were node negative while 68% had one or two auxiliary nodes involved. Vascular invasion was seen in 40% of tumours while 60% were without any vascular invasion.
Expression of CD44 in breast tumour cells
CD44 expression was determined by immunohistochemistry. From the series of 146 patients, 10 cases were excluded from the CD44 analysis due to lack of sufficient tumour area. An external positive control (human tonsil) and infiltrating lymphocytes within the tumour (internal positive control) showed strong and uniform staining of CD44.
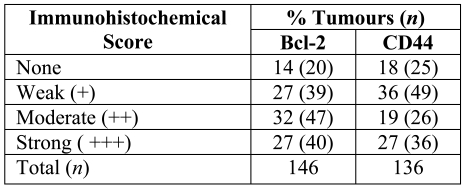
Analysis of CD44 expression revealed that 82% (111/136) of the breast tumour samples showed positive staining for CD44, with weak staining in 36% of samples, moderate staining in 19% of samples, and strong immunoreactivity in 27% of the breast tumour samples examined (Table 2). The immunohistochemical pattern of CD44 expression in breast carcinoma was mostly cell membrane staining, with no background staining of stroma or nuclei (Figure 1).
Table 2.
Intensity of Bcl-2 and CD44 expression.
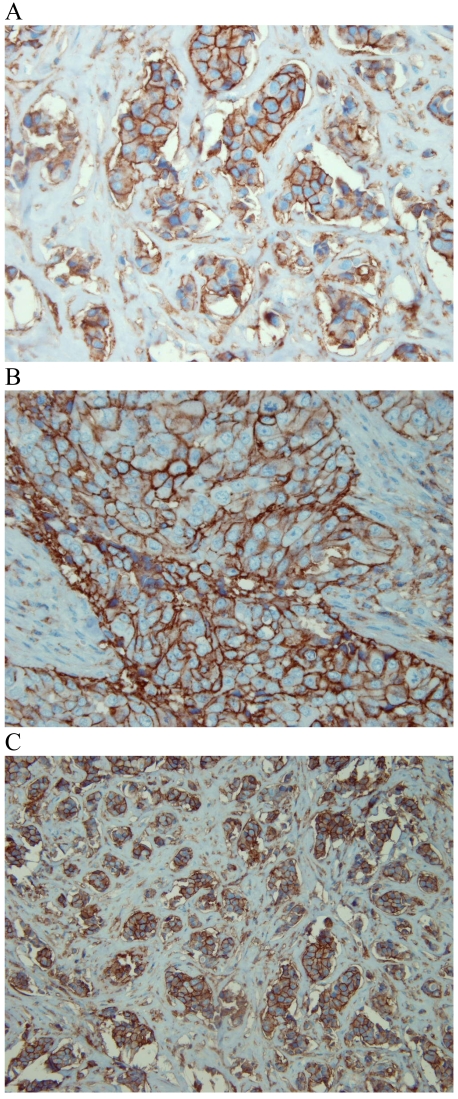
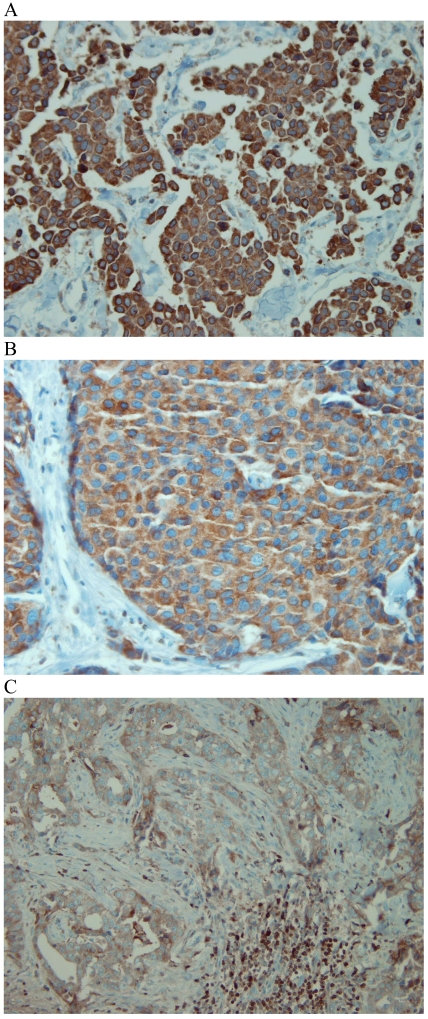
Figure 1.
CD44 expression in breast tumour cells. Moderate (A) and strong (B, C) expression of CD44 was observed on the cell membrane of invasive breast carcinomas, with no staining of stroma.
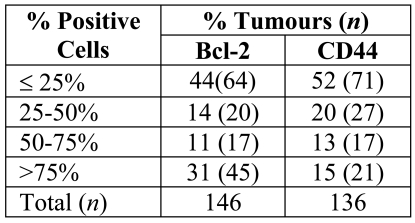
The proportion of CD44 positive cells was observed to be variable: 15% of the tumours showed extensive expression of CD44 (>75% positive cells), whereas 52% of the samples demonstrated CD44 immunoreactivity in less than 25% of the tumour cells (Table 3).
Table 3.
Percentage of Bcl-2 and CD44 positive tumour cells.
Expression of Bcl-2 in breast tumour cells
Immunohistochemistry was used to evaluate Bcl-2 expression. The pattern of Bcl-2 staining in breast tumour cells was mostly cytoplasmic with no staining of nuclei (Figure 2). A positive control (human tonsil) and tumour infiltrating leukocytes showed strong and uniform staining of Bcl-2.
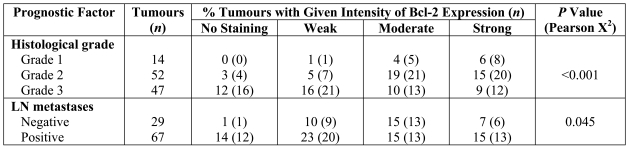
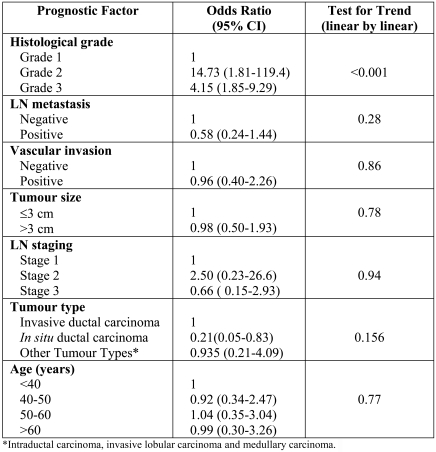
Figure 2.
Bcl-2 expression in breast tumour cells. Strong (A), moderate (B) and weak (C) cytoplasmic expression of Bcl-2 was observed in invasive breast carcinomas. Infiltrating lymphocytes that express high levels of Bcl-2 were used as internal positive control (C).
Bcl-2 was expressed variably within breast tumour samples in terms of the staining intensity and the percentage of tumour cells stained. Analysis of Bcl-2 expression revealed 86% of the breast cancer samples showed positive staining for Bcl-2. Staining intensity varied greatly (Table 2): strong staining was seen in 27% of samples, moderate staining in 32% of samples and weak staining was observed in 27% of samples; 14% of the tumours were completely negative. The proportion of Bcl-2 positive cells showed a discrepancy: 31% of the tumours showed extensive expression of Bcl-2 (>75% positive cells), whereas 44% of the samples demonstrated Bcl-2 immunoreactivity in less than 25% of tumour cells and 25% of the tumours were in between (25%-50% positive cells) (Table 3).
A significant association was found between the intensity of expression of the anti-apoptotic protein Bcl-2 and that of the adhesion molecule CD44 (P = 0.004, Table 2).
Comparison with known prognostic factors
The level of expression of two markers, CD44 and Bcl-2, was assessed by two scoring methods, namely the intensity of the staining and the percentage of positively staining cells. The association between expression of these markers and different prognostic parameters is shown in Table 1. In univariate analysis, there was no significant correlation between the prevalence of CD44+ tumour cells and the patients' or tumours' characteristics including age (P = 0.51), tumour grade (P = 0.15), lymph node stage (P = 0.16), vascular invasion (P = 0.18), tumour size (P = 0.34) or tumour type (P = 0.10) (Table 1).
On the contrary, we found a significant inverse association between the intensity of Bcl-2 expression (categorised into four groups: no staining, weak, moderate and strong staining) and histological grading (P < 0.001) and lymph node metastases (P = 0.045), i.e. higher levels of Bcl-2 were more often seen in breast carcinomas of lower grade and tumours with less auxiliary lymph node involvement (Table 4).
Table 4.
Association between the intensity of Bcl-2 expression and histological grading and lymph node metastases.
In multiple logistic regression, Bcl-2 intensity was reclassified as a binary outcome as low (0 or 1) and high (2 or 3) expression. The odds ratio for high intensity of Bcl-2 expression in those tumours with a poor histological grade compared with that of well-differentiated tumours was 4.15 (95% CI, 1.85-2.297) (Table 5).
Table 5.
Logistic regression analysis of Bcl-2 intensity re-categorized into two groups, high and low intensity.
However, no association was detected between the expression of Bcl-2 in these invasive breast carcinomas and other prognostic factors, including the absence or presence of vascular invasion (P = 0.68), tumour size (P = 0.82), tumour type (P = 0.055) and patient's age at the time of diagnosis (P = 0.46).
Similarly, no correlation was found between the percentage of Bcl-2 or CD44 positive cells and pathological prognostic factors or patient characteristics. In contrast, a significant association was found between the expression level of the adhesion molecule CD44 and the anti-apoptotic protein Bcl-2 (P = 0.004); i.e. CD44+ cells had higher levels of Bcl-2 expression.
Discussion
The aim of the present study was to determine the expression of Bcl-2 and CD44 in paraffin-embedded sections of breast tumours, as well as their association with clinicopathological parameters. To our knowledge, this is the first study on the expression of Bcl-2 protein in tumourigenic breast cancer cells to look at both properties of the cancer stem cells and resistance to apoptosis.
This retrospective analysis of 146 breast tumours investigated the prevalence of CD44+ breast tumours, as a marker of cancer stem cells, and its association with the presence of the anti-apoptotic protein Bcl-2. We observed a wide variation in the percentage of cells positive for Bcl-2 and CD44 expression, as well as in the intensity of staining, in this series of breast tumours. Fifteen percent of breast carcinomas showed a high expression of CD44 (>75% positive cells), whereas 52% of the tumour samples expressed CD44 in <25% of tumour cells. It has previously been shown that in breast cancer tissues, CD44+/CD24(-/low) tumour cells range from 0% to 80% and that the majority of tumours (78%) display <10% CD44+/CD24(-/low) cells while the remainder contain >10%.
In this study, we could not find any correlation between the prevalence of CD44+ cells and tumour prognosis. This finding confirms previous results from other studies which indicate that the percentage of CD44+/CD24(-/low) cells does not increase over the course of tumour progression from in situ carcinoma to carcinoma in all cases examined (6). The lack of association between the prevalence of CD44+/CD24- cells and clinical outcome and tumour characteristics warrants further investigation with a larger number of samples.
Of the 146 samples examined in this study, 86% expressed Bcl-2 with a variable intensity ranging from strong (27%) to weak (27%) in the cytoplasmic side. Similarly, 31% of the tumours showed a high percentage of Bcl-2+ tumour cells (>75% positive cells), whereas 44% of the cases showed less than 25% of cells staining for Bcl-2, with no staining of stroma or nuclei. The variable expression of Bcl-2 in the cytoplasmic side of breast tumours has been noted in other immunohistochemical studies (15). We found a higher level of Bcl-2 expression in breast carcinomas of lower grade and tumours with fewer lymph node metastases, which was in accordance with Rolland's study that also showed a significant correlation between Bcl-2 expression and lower grade and earlier lymph node stage.
Breast tumours that lack the anti-apoptotic protein Bcl-2 appear to have high rates of both cell proliferation and cell death which indicates rapid cell turnover, and they are often associated with poorer clinical outcomes (16-19). This contradiction may be clarified by the finding that while Bcl-2 inhibits apoptosis, it also decelerates cell growth via an anti-proliferative power distinct from those regulating its anti-apoptotic capacities (20, 21).
Moreover, a meta-analysis (using a search of PubMed) conducted on 18 different series including 5,892 cases and investigating the role of Bcl-2 expression by immunohistochemistry confirmed that Bcl-2 is an independent prognostic marker in breast cancer (15, 18).
In contrast, our evaluation did not show a significant association between the prevalence of CD44+ tumour cells and any tumour or patient characteristic. This finding is in-line with other works showing that the prevalence of CD44+/CD24(-/low) cells is not significantly associated with breast tumour progression or patient survival except for those with distant metastases (6) or lymph node metastases (22). Due to the lack of information on distant metastases, we could not investigate its correlation with the prevalence of CD44+ tumour cells. Since no relationship has been found between CD44+/CD24(-/low) tumour cells and most of the clinicopathological parameters in different studies, this population cannot fully illustrate the tumourigenicity of breast tumour epithelial cells and they may represent a subclass of tumourigenic cells (6). On the other hand, recent studies stress the role of CD44 as a homing receptor for distant tissue compartments, i.e. CD44 expression increases the risk of metastasis in breast tumours (6, 23).
In summary, further to previous studies we found a significant association between the two phenotypes under investigation, Bcl-2+ and CD44+, in breast tumour cells suggesting that CD44+/Bcl-2+ tumour cells may also be a sub-population of tumourigenic cells. Therefore, identification of this subpopulation which has both the property of tending to metastasize and that of resistance to apoptosis may help target this crucial sub-population of breast CSCs, yielding more effective treatments.
Abbreviations
- CSC
cancer stem cell
Acknowledgements
This work was supported by a grant from the Cellular Molecular Research Centre, Iran University of Medical Sciences (grant number 123). We are grateful to Dr. Ali Sadeghipour, head of Rasool Akram laboratory, and also to Mrs. Sharzavi for her technical assistance and expert advice.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Ponti D, Zaffaroni N, Capelli C, Daidone MG. Breast cancer stem cells: an overview. Eur J Cancer. 2006;42:1219–1224. doi: 10.1016/j.ejca.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Chang CC. Recent translational research: stem cells as the roots of breast cancer. Breast Cancer Res. 2006;8:103. doi: 10.1186/bcr1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa FF, Le Blanc K, Brodin B. Concise review: cancer/testis antigens, stem cells, and cancer. Stem Cells. 2007;25:707–711. doi: 10.1634/stemcells.2006-0469. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- 7.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 8.van Stijn A, van der Pol MA, Kok A, Bontje PM, Roemen GM, Beelen RH, Ossenkoppele GJ, Schuurhuis GJ. Differences between the CD34+ and CD34- blast compartments in apoptosis resistance in acute myeloid leukemia. Haematologica. 2003;88:497–508. [PubMed] [Google Scholar]
- 9.Wei C, Guo-min W, Yu-jun L. Apoptosis resistance can be used in screening the markers of cancer stem cells. Med Hypotheses. 2006;67:1381–1383. doi: 10.1016/j.mehy.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Lagasse E, Weissman IL. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell. 1997;89:1021–1031. doi: 10.1016/s0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]
- 11.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 13.Gil J, Stembalska A, Pesz KA, Sasiadek MM. Cancer stem cells: the theory and perspectives in cancer therapy. J Appl Genet. 2008;49:193–199. doi: 10.1007/BF03195612. [DOI] [PubMed] [Google Scholar]
- 14.Behbod F, Rosen JM. Will cancer stem cells provide new therapeutic targets? Carcinogenesis. 2005;26:703–711. doi: 10.1093/carcin/bgh293. [DOI] [PubMed] [Google Scholar]
- 15.Rolland P, Spendlove I, Madjd Z, Rakha EA, Patel P, Ellis IO, Durrant L. The p53 positive Bcl-2 negative phenotype is an independent marker of prognosis in breast cancer. Int J Cancer. 2007;120:1311–1317. doi: 10.1002/ijc.22430. [DOI] [PubMed] [Google Scholar]
- 16.van Slooten HJ, van de Vijver MJ, van de Velde CJ, van Dierendonck JH. Loss of Bcl-2 in invasive breast cancer is associated with high rates of cell death, but also with increased proliferative activity. Br J Cancer. 1998;77:789–796. doi: 10.1038/bjc.1998.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipponen P. Apoptosis in breast cancer: relationship with other pathological parameters. Endocr Relat Cancer. 1999;6:13–16. doi: 10.1677/erc.0.0060013. [DOI] [PubMed] [Google Scholar]
- 18.Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, Ellis IO, Huntsman D, Caldas C. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12:2468–2475. doi: 10.1158/1078-0432.CCR-05-2719. [DOI] [PubMed] [Google Scholar]
- 19.Charpin C, Garcia S, Bonnier P, Martini F, Andrac L, Horschowski N, Lavaut MN, Allasia C. bcl-2 automated and quantitative immunocytochemical assays in breast carcinomas: correlation with 10-year follow-up. J Clin Oncol. 1998;16:2025–2031. doi: 10.1200/JCO.1998.16.6.2025. [DOI] [PubMed] [Google Scholar]
- 20.Pietenpol JA, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 1994;54:3714–3717. [PubMed] [Google Scholar]
- 21.Theodorakis P, D'Sa-Eipper C, Subramanian T, Chinnadurai G. Unmasking of a proliferation-restraining activity of the anti-apoptosis protein EBV BHRF1. Oncogene. 1996;12:1707–1713. [PubMed] [Google Scholar]
- 22.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L. The clinicopathologic and prognostic significance of CD44+/CD24(-/low) and CD44-/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39:1096–1102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Looi LM, Cheah PL, Zhao W, Ng MH, Yip CH. CD44 expression and axillary lymph node metastasis in infiltrating ductal carcinoma of the breast. Malays J Pathol. 2006;28:83–86. [PubMed] [Google Scholar]
- 24.Madjd Z, Pinder SE, Paish C, Ellis IO, Carmichael J, Durrant LG. Loss of CD59 expression in breast tumours correlates with poor survival. J Pathol. 2003;200:633–639. doi: 10.1002/path.1357. [DOI] [PubMed] [Google Scholar]
- 25.Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF, Nicholson RI, Ellis IO. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004;203:661–671. doi: 10.1002/path.1559. [DOI] [PubMed] [Google Scholar]
- 26.Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, Ellis IO. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol. 2005;18:26–35. doi: 10.1038/modpathol.3800255. [DOI] [PubMed] [Google Scholar]
- 27.Madjd Z, Spendlove I, Moss R, Bevin S, Pinder SE, Watson NF, Ellis I, Durrant LG. Upregulation of MICA on high-grade invasive operable breast carcinoma. Cancer Immun. 2007;7:17. http://www.cancerimmunity.org/v7p17/070817.htm [PMC free article] [PubMed] [Google Scholar]
- 28.Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer. 2005;117:248–255. doi: 10.1002/ijc.21163. [DOI] [PubMed] [Google Scholar]
Materials and methods
Patients and tumour samples
Paraffin-embedded tumour tissues from 146 patients with breast cancer who underwent breast surgery or biopsy between 2006-2007 at Milad and Toos Hospitals, two major public and private referral centers in Tehran, Iran, were used in this study. Surgical specimens were obtained before systemic treatment and paraffin-embedding was performed within the framework of diagnostic procedures. Patient characteristics (age) and tumour characteristics, including histological grade, vascular invasion, tumour size, lymph node metastasis, tumour stage and type were collected and recorded in a database. Patients' data were kept fully anonymous. This research was approved by the IUMS Research Ethics Committee.
Immunohistochemistry
Following breast cancer diagnosis and confirmation of tissue types by H&E staining, immunohistochemical staining was performed as described previously (24) on 4 µm tissue sections with primary monoclonal antibodies against CD44 (clone DF185, Novocastra) and Bcl-2 (clone 124, Dako). After deparaffinization, sections were immersed in methanol containing 0.3% hydrogen peroxide for 15 minutes to block endogenous peroxidase activity. Antigens were retrieved by autoclaving for 10 minutes in citrate buffer (pH 6.0) and EDTA (pH 8.0) for CD44 and Bcl-2, respectively. The sections were then incubated with primary antibodies for 1 hour at room temperature; the optimal dilution was found to be 1/40 for the anti-CD44 mAb and 1/20 for the anti-Bcl-2 mAb.
Tissues were then incubated in biotinylated goat anti-mouse/rabbit IgG (Dako) for 30 minutes followed by HRP-labelled streptavidin complex (Dako) for 1 hour at room temperature with the addition of 3, 3'-diaminobenzidine (DAB, Dako) to achieve visualization of the antigen. In the final step, sections were lightly counterstained with haematoxylin (Dako), dehydrated in alcohol, cleared in xylene (Dako) and mounted for examination.
The primary antibody was omitted from the negative control and human tonsil sections were used as positive controls for both antibodies, while infiltrating lymphocytes within the tumour were used as internal positive controls.
Evaluation of immunohistochemical staining
The staining of tissue sections was interpreted using a semi-quantitative scoring system by one observer (ZM) in a coded manner without previous knowledge of clinical and pathological parameters on two separate occasions and a consensus was achieved between the two scorings. Initially, the slides were scanned at 10x magnification to obtain a general impression of the overall distribution of the tumour cells and the positive cells were then assessed semi-quantitatively at higher magnifications and the final scores were given. Semi-quantitative scoring systems are the historical standard for the assessment of immunostaining of tissue sections. These systems usually rely on the subjective assessment of multiple independent observers or by one observer on two separate occasions, blinded to the patient outcomes and clinicopathological data (25, 26).
Two scoring methods (27, 28) were used to assess CD44 and Bcl-2 levels in breast tumours:
The intensity of the immunostaining was classified into four categories: 0 (no immunostaining present), 1 (weak staining), 2 (moderate staining) and 3 (strong staining).
The percentage of positive cells was assessed semi-quantitatively and classified into four groups: 1 (<25% positive cells), 2 (25-50% positive cells), 3 (51-75% positive cells) and 4 (>75% positive cells).
Statistical analysis
Statistical analysis of data was performed using SPSS software version 15 (Chicago, IL). Pearson’s chi-square and Pearson’s r tests were used for univariate analysis to determine the significance of associations between the prevalence of CD44+, Bcl-2+ tumour cells and each clinicopathological parameter. To obtain effect sizes and to look at the independence of effects, Bcl-2 intensity was reclassified as a binary outcome as low (0 or 1) and high (2 or 3) expression and effects of clinicopathological parameters were assessed using multiple logistic regression to give adjusted odds ratios and 95% confidence intervals. P values less than 0.05 were identified as statistically significant.