Abstract
Cancer-embryo antigens or developmentally restricted differentiation antigens (DRDAGs), such as PLAC1 (CT92) and developmental pluripotency associated-2 (DPPA2/CT100), are expressed in pluripotent embryonic cells. They are also recognized as cancer-testis antigens (CT) which are proteins normally expressed only in the human germ line but that are also present in a significant subset of malignant tumors. These antigens may prove to be markers of 'repopulating' cells with stem cell-like characteristics and could be critical targets for immunotherapy in epithelial ovarian cancer (EOC). Our objective was to define the frequency of expression and immunogenicity of PLAC1 and DPPA2 in EOC and correlate expression with clinical outcome. One-step reverse transcriptase PCR was performed on 101 EOC samples and a panel of normal tissues. Expression of PLAC1 and DPPA2 in the EOC specimens was 21/101 (21%) and 31/101 (31%) respectively. In normal tissues, PLAC1 expression was restricted to the placenta while DPPA2 expression was restricted to the placenta and testis. Immunohistochemistry (IHC) and enzyme-linked immunosorbent assay (ELISA) were also performed on a subset of specimens. Humoral immunity was demonstrable in 2/12 serum samples from patients whose tumors expressed DPPA2. There was no demonstrable antibody response to PLAC1 in patients with PLAC1 positive tumors. The presence of PLAC1 and DPPA2 did not have a statistically significant effect on recurrence-free and overall survival. The tissue-restricted expression of PLAC1 and DPPA2, their expression in a significant proportion of EOC patients, and their potential to represent markers of stem cells make DRDAGs attractive targets for antigen-specific immunotherapy in EOC.
Keywords: human, ovarian cancer, PLAC1, DPPA2, RT-PCR, immunohistochemistry, humoral immunity
Introduction
There has been much speculation that cancers acquire characteristic traits by reactivating genes normally expressed in embryonic and fetal stages (1). The discovery of a growing number of proteins that appear to be expressed only in germ cells, trophoblasts and tumors − the cancer/testis (CT) antigens (2-4) − has led to the theory that aberrant expression of germline genes in cancer reflects the activation of the silenced gametogenic program in somatic cells, and that this event is important for tumor progression (5). In previous studies, CT antigens such as NY-ESO-1 and LAGE-1 have been examined in a large panel of EOC tissues and cell lines using RT-PCR and IHC (6). Expression of NY-ESO-1 in EOC was demonstrated by RT-PCR and/or IHC in 82 of 190 (43%) specimens. LAGE-1 mRNA expression was present in 22 of 107 (21%) tumor tissues. These findings have led to the development of NY-ESO-1-based immune therapies for EOC (7, 8).
Due to the similarities between embryo implantation and the growth of cancer cells, there has been further discovery of new CT antigens. Two of these CT antigens are PLAC1 (CT92) (9), a human X-linked gene with placenta-specific expression, and developmental pluripotency associated-2 (DPPA2/CT100) (10), a non-X-linked gene which is expressed in pluripotent embryonic cells. These antigens may prove to be markers of 'repopulating' cells with stem cell-like characteristics that could be critical targets for immunotherapy in EOC. Our objective was to define the frequency of expression and immunogenicity of PLAC1 and DPPA2 in EOC patients and to examine the relationship between their expression and clinical outcome.
Results
Study population
The characteristics of the study population are presented in Table 1. The median age of the study population was 65 years (range 32-89 years) and the median duration of follow-up was 34 months. The majority of patients presented at stage IIIC (70%) with grade 3 tumors (71%) of serous differentiation (58%). A complete response to therapy was achieved in 44 patients (44%) while the remaining patients achieved a partial response.
Table 1.
Patient characteristics.
Expression of PLAC1 and DPPA2 mRNA in normal tissues and EOC specimens
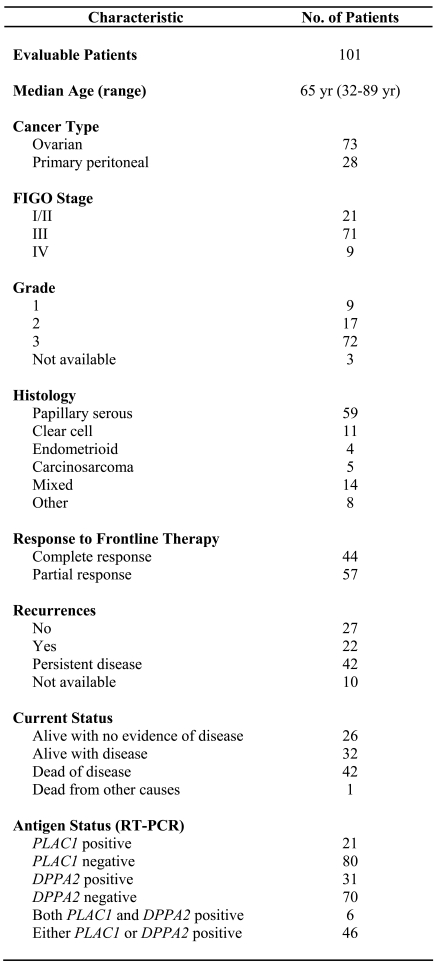
Expression of PLAC1 and DPPA2 mRNA in a panel of human tissues and epithelial ovarian tumor specimens was investigated using RT-PCR (Figure 1, panels A and B). PLAC1 expression was restricted to the placenta and DPPA2 expression was restricted to the placenta and testis. The amplification products for PLAC1 and DPPA2 were confirmed by sequencing. Together, these results confirm the tissue-restricted expression of these two DRDAGs/CT antigens.
Figure 1.
RT-PCR analysis for PLAC1 and DPPA2 expression in a normal tissue panel. (A) PLAC1 mRNA expression yielded a 328 bp product. (B) DPPA2 mRNA expression yielded a 430 bp product. (C) GAPDH (glyceraldehyde-3-phosphate dehydrogenase) served as an endogenous control and yielded a 204 bp product. Lanes: M, marker; 1, blank; 2, placenta; 3, adrenal gland; 4, bone marrow; 5, brain; 6, cerebellum; 7, fetal liver; 8, fetal brain; 9, heart; 10, placenta; 11, prostate; 12, testis.
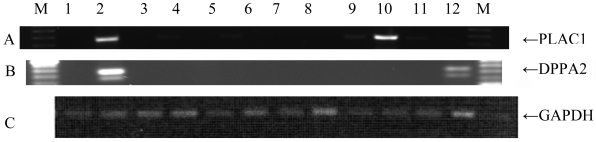
PLAC1 and DPPA2 mRNA expression was tested in 101 epithelial ovarian tumor specimens. Twenty-one EOC samples were positive for PLAC1 (Figure 2) and 31 were positive for DPPA2. Coordinate expression of PLAC1 and DPPA2 was found in 6 patients while 46 patient specimens were positive for either PLAC1 or DPPA2.
Figure 2.
RT-PCR analysis for PLAC1 expression in a subset of ovarian cancer specimens. PLAC1 mRNA expression yielded a 328 bp product. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) served as an endogenous control and yielded a 204 bp product. Shown is mRNA from placenta (lane 1). Lanes 2, 4, 6, and 7 are positive for PLAC1 mRNA expression whereas lanes 3, 8, and 9 are negative. Lane 5 has a negative GAPDH control and hence PLAC1 mRNA expression in this specimen is inconclusive.
Expression of DPPA2 examined by immunohistochemistry
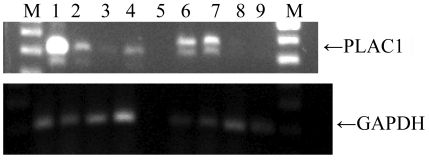
DPPA2 exhibited intense nuclear immunostaining in the spermatocytes of the testis (Figure 3A). Of the 31 tumors that tested positive for DPPA2 by RT-PCR, 18 tumors were subjected to IHC and 3 (17%) tested positive. Of 25 tumors which were negative for DPPA2 by RT-PCR, 4 (16%) tested positive by IHC (Figure 3B).
Figure 3.
DPPA2 expression in testis and an ovarian tumor specimen. Immunohistochemical staining was carried out with a polyclonal rabbit anti-DPPA2 antibody. (A) The spermatocytes of the normal testis show a nuclear pattern of staining of strong intensity. (B) Staining of an ovarian tumor specimen.
Antibody response to PLAC1 and DPPA2 in ovarian cancer patients
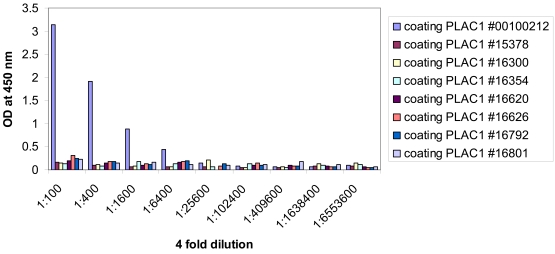
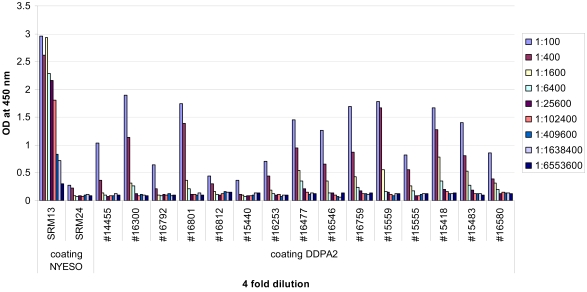
Archived sera were obtained from 20 patients with PLAC1 positive tumors (determined by RT-PCR). A healthy donor serum specimen was included as a positive control. This healthy donor serum specimen was used as a positive control in a previous study by Silva et al. (11). There were no demonstrable antibody responses to PLAC1 in these patients. Archived sera were also obtained from a subset of patients with DPPA2 positive and negative tumors (determined by RT-PCR). A well-known cancer-testis antigen, NY-ESO-1, was used as a positive control. Two of the 12 patients who expressed DPPA2 by RT-PCR showed demonstrable antibody responses at a serum dilution of 1:400. In addition, 2 of the 3 patients with DPPA2 negative tumors also showed this same seropositivity. Figure 4 and Figure 5 illustrate PLAC1 and DPPA2 ELISA titration curves with sera from selected ovarian cancer patients.
Figure 4.
Serological reactivity of the recombinant PLAC1 protein in a subset of ovarian cancer patients. The presence of anti-PLAC1 antibodies in serum samples was assessed by ELISA. Serum obtained from a healthy donor (#00100212) was used as a known positive control.
Figure 5.
Serological reactivity of the recombinant DPPA2 protein in a subset of ovarian cancer patients. The presence of anti-DPPA2 antibodies in serum samples was assessed by ELISA. Serum obtained from a seropositive NY-ESO-1 patient (SRM13) was used as a positive control and that obtained from a seronegative NY-ESO-1 patient (SRM24) was used as a negative control. Sera #15559, #15555, and #15418 tested negative for DPPA2 expression by RT-PCR.
Relationship between PLAC1, DPPA2, NY-ESO-1 and BORIS expression
It has been reported that Brother of the Regulator of Imprinted Sites (BORIS/CTCFL), an autosomal CT antigen, functions as an oncogene in human cancer via dysregulation of the cancer epigenome (12). This raises the possibility that expression of BORIS may regulate the expression of developmentally restricted differentiation antigens/CT antigens. Therefore, we determined the expression of BORIS and NY-ESO-1 by RT-PCR in the same panel of tissue specimens we previously described (6, 13). The pairwise relationships between PLAC1 (CT92), DPPA2 (CT100), NY-ESO-1, and BORIS expression were not statistically significant (all P values were greater than 0.3 by the Pearson chi-square test), suggesting that PLAC1 and DPPA2 are not co-expressed with other classical cancer-testis genes.
Correlation of PLAC1 and DPPA2 expression with clinical outcome
The median estimated overall survival (OS) for all patients was 40.3 months [95% confidence interval (CI) 27.7-48.6] while the recurrence-free survival (RFS) excluding patients with persistent/progressive disease after initial therapy was 19.7 months (95% CI 16.1-Inf). DPPA2 positivity was associated with older age at diagnosis (Wilcoxon-Mann-Whitney rank sum test, P = 0.0024) and larger residual tumor burden at initial surgery (Fisher's exact test, P = 0.030). The presence of PLAC1 and DPPA2 did not have a statistically significant effect on RFS or OS. Median RFS was 40.8 months in the PLAC1 positive patients vs. 19.7 months in the PLAC1 negative patients (log rank test, P = 0.37). Median OS was 49.9 months in the PLAC1 positive patients vs. 40 months in the PLAC1 negative patients (log rank test, P = 0.52). Median RFS was 36.1 months in the DPPA2 positive patients vs. 19.1 months in the DPPA2 negative patients (log rank test, P = 0.46). Median OS was 46.1 months in the DPPA2 positive patients vs. 39.7 months in the DPPA2 negative patients (log rank test, P = 0.27).
Discussion
The identification of tumor-specific antigens is a prerequisite for the development of efficient immunotherapeutic approaches to cancer. In this study, we sought to explore genes that are highly expressed in placenta and ovarian cancer but show relatively restricted expression in normal tissues. These genes could be involved in processes that are similar between embryo implantation and growth of cancer cells and therefore represent new potential therapeutic targets (vaccines as well as monoclonal antibodies) for ovarian cancer. In the present study, we have demonstrated the expression of two developmentally restricted differentiation antigens/CT antigens, PLAC1 (CT92) and DPPA2 (CT100), in 46% of ovarian cancer specimens. Independent frequencies of expression were 21% and 31% respectively for PLAC1 and DPPA2. Coordinate expression of PLAC1 and DPPA2 was found in only 6% of patients suggesting that some aspects of the regulation of the expression of both genes are different. However, the low frequencies of concordance between RT-PCR and IHC for DPPA2 suggest shared features with regards to antigenic and tumor heterogeneity. It is unclear why there is a discrepancy in the detection of DPPA2 expression by PCR and IHC. Since the antibody used in our study stained normal human testes (positive control) appropriately and had previously been shown to stain tumor tissues (10), our results are unlikely to be due to issues relating to sensitivity or specificity of the antibody. It is possible that, at least in ovarian cancer, there is a post-translational modification of the DPPA2 protein such that the DPPA2 antibody cannot recognize changes in the epitope. Similar conclusions were drawn by Tammela et al. (14) who investigated SCP-1, a cancer-testis antigen, in epithelial ovarian cancer and found no correlation between SCP-1 expression at the mRNA and protein levels. Clearly, additional studies would be needed to clarify the mechanisms of DPPA2 expression in epithelial ovarian cancer.
Although PLAC1 (CT92) and DPPA2 (CT100) are considered part of the cancer-testis antigen family, they appear to have a different expression pattern from that of other classic CT antigens, such as NY-ESO-1 and BORIS. These varied relationships suggest that PLAC1 expression is probably regulated by independent mechanisms and questions whether DDPA2, a transcriptional regulator, is involved in the transcriptional regulation of the X chromosome CT antigens or is activated by the same mechanisms that activate the X chromosome CT antigens.
There are shared features between these cancer-placental antigens/DRDAGs and cancer-testis antigens with regards to tissue restriction and expression pattern. These features have led to the hypothesis that cancer is a somatic cell pregnancy and commandeers aspects of the gametogenic program for its own purposes (1). Based on this hypothesis, there may be signals and functions associated with the initiation and/or progress of deprogramming and/or maintenance of the undifferentiated proliferative stem cell state common to both human embryogenesis and cancer.
ECSA/DPPA2 has a shared function in embryos and cancers and is expressed in lung, liver, and colon cancers (10, 15). This indicates that some function in embryogenesis specifically confined to this stage of the emergence and/or maintenance of the embryonic stem cell is commonly involved in the cancer stem cell phenotype (15). Further evidence to support the shared regulatory mechanisms of human embryogenesis and cancer was presented by Monk et al. (15) who examined the real-time PCR expression profiles in human early development of the embryo cancer gene, ECSA/DPPA2, and the testis deprogramming gene BORIS, compared with the expression of the well-known pluripotency gene, OCT4. They concluded that, unlike OCT4 which is expressed throughout preimplantation development, BORIS and ECSA/DPPA2 are predominantly expressed at early and late stages of preimplantation development, respectively, consistent with different roles in reprogramming during embryogenesis (15).
DPPA2 has been studied in non-small cell lung carcinoma (10). Expression of DPPA2 was demonstrated in 30% of 110 non-small cell lung carcinomas by RT-PCR and/or IHC, as well as in a small percentage of melanomas, colorectal cancers, and lymphomas. On immunohistochemical analysis, the subpopulation of cells which stained positively for DPPA2 were large basally located cells adjacent to the stroma, an area reported to be a niche of cancer stem cells, thereby suggesting that these DPPA2 positive cells could be putative lung cancer stem cells.
Expression of PLAC1 has also been found in many cancer cell lines (11), lung carcinoma (11), gastric carcinoma (16), and hepatocellular carcinoma specimens (10). Possible functions of PLAC1 include involvement in early developmental events (11), cell motility, migration, and invasion (17, 18), all of which are normal physiological characteristics of the human trophoblast.
To be considered for antigen-specific immunotherapy of any tumor type, including EOC, an ideal candidate antigen should not only demonstrate high frequency expression in tumor tissues and restricted expression in normal tissues, but also evidence for inherent immunogenicity (6). In the study by Silva et al. (11), 14/226 plasma samples from NSCLC patients were found to have detectable titers to PLAC1, ranging from 1/100 to 1/1200. While our 20 ovarian cancer patients did not show PLAC1 seropositivity, further testing of a larger panel of patients is warranted. In contrast, DPPA2 ELISA testing revealed seropositivity in both RT-PCR positive and negative patients. This could represent differences in assay sensitivity as well as tumor heterogeneity. In addition, reactivity to DPPA2 could suggest a possible gender-specific link with the immunogenicity of the antigen (11). The demonstration of DPPA2 seropositivity in healthy female donors and patients whose tumors did not express the antigen suggest previous exposure to DPPA2. Thus, spontaneous immunity to DPPA2 could be evoked as a consequence of pregnancy and may persist in the long term. Since the presence of antibody response to a tumor antigen could reflect evidence of a T cell response, it is also critical to determine PLAC1- and DPPA2-specific CD4+ and CD8+ T cell responses in future studies.
Although the lack of correlation between PLAC1 and DPPA2 expression and clinico-pathologic characteristics, such as recurrence and survival, may reflect the fact that the majority of the patients (Table 1) had advanced stage disease, it may also imply that the antigens are not solely responsible for tumor progression. The lack of correlation between PLAC1 and DPPA2 expression and clinical outcome is not unique. The expression of other CT antigens, such as NY-ESO-1 and LAGE-1, has also not been correlated with pathologic variables or survival outcomes (6). Despite this lack of correlation between CT antigen expression and clinical outcome, NY-ESO-1 has proven to be an attractive target for immunotherapy due to its tissue-restricted expression and immunogenicity (7, 8). Since PLAC1 and DPPA2 share these CT antigen characteristics, they also represent potential immunotherapeutic targets. Further discovery regarding the role of CT antigens in tumorigenesis, invasion, and metastasis in epithelial ovarian cancer is critically needed in order to further define the functional and therapeutic significance of CT antigens.
In summary, we have shown expression of two cancer-testis antigens in a significant proportion of ovarian cancer patients. We have also demonstrated that DPPA2 evokes a spontaneous immune response in a subset of patients. Together with the potential of these antigens to be markers of 'repopulating' cells with stem cell-like characteristics, our findings indicate that PLAC1 (CT92) and DPPA2 (CT100) could be potential targets for immunotherapy in epithelial ovarian cancer. Further studies regarding the immunogenicity of PLAC1 (CT92)- and DPPA2 (CT100)-expressing tumors and characterization of DRDAG/CT antigen-specific T cells are warranted.
Abbreviations
- CT
cancer-testis
- EOC
epithelial ovarian cancer
- IHC
immunohistochemistry
Acknowledgements
The PLAC1 recombinant protein and rabbit polyclonal antibody were generously provided by the Ludwig Institute for Cancer Research at Memorial Sloan Kettering. The DPPA2 recombinant protein and rabbit polyclonal antibody were generously provided by Dr. Jonathan Cebon of the Ludwig Institute for Cancer Research Centre for Clinical Sciences, Austin Health, Heidelberg, Victoria, Australia. This work was supported by a Cancer Research Institute (CRI) Ovarian Cancer Working Group Grant and an Anna-Marie Kellen Clinical Investigator Award to KO from the CRI.
References
- 1.Old LJ. Cancer is a somatic cell pregnancy. Cancer Immun. 2007;7:19. http://www.cancerimmunity.org/v7p19/071019.htm [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida LG, Sakabe NJ, deOliveira AR, Silva MC, Mundstein AS, Cohen T, Chen YT, Chua R, Gurung S, Gnjatic S, Jungbluth AA, Caballero OL, Bairoch A, Kiesler E, White SL, Simpson AJ, Old LJ, Camargo AA, Vasconcelos AT. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37 (Database issue):D816–D819. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. http://www.cancerimmunity.org/v4p1/031220.htm [PubMed] [Google Scholar]
- 4.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 5.Old LJ. Cancer/testis (CT) antigens - a new link between gametogenesis and cancer. Cancer Immun. 2001;1:1. http://www.cancerimmunity.org/v1p1/010304.htm [PubMed] [Google Scholar]
- 6.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D, Williamson B, Scanlan MJ, Ritter G, Chen YT, Driscoll D, Sood A, Lele S, Old LJ. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 7.Diefenbach CS, Gnjatic S, Sabbatini P, Aghajanian C, Hensley ML, Spriggs DR, Iasonos A, Lee H, Dupont B, Pezzulli S, Jungbluth AA, Old LJ, Dupont J. Safety and immunogenicity study of NY-ESO-1b peptide and montanide ISA-51 vaccination of patients with epithelial ovarian cancer in high-risk first remission. Clin Cancer Res. 2008;14:2740–2748. doi: 10.1158/1078-0432.CCR-07-4619. [DOI] [PubMed] [Google Scholar]
- 8.Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman EW, Pan L, Ritter G, Villella J, Thomas B, Rodabaugh K, Lele S, Shrikant P, Old LJ, Gnjatic S. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocchia M, Huber R, Pantano S, Chen EY, Ma P, Forabosco A, Ko MS, Schlessinger D. PLAC1, an Xq26 gene with placenta-specific expression. Genomics. 2000;68:305–312. doi: 10.1006/geno.2000.6302. [DOI] [PubMed] [Google Scholar]
- 10.John T, Caballero OL, Svobodova SJ, Kong A, Chua R, Browning J, Fortunato S, Deb S, Hsu M, Gedye CA, Davis ID, Altorki N, Simpson AJ, Chen YT, Monk M, Cebon JS. ECSA/DPPA2 is an embryo-cancer antigen that is coexpressed with cancer-testis antigens in non-small cell lung cancer. Clin Cancer Res. 2008;14:3291–3298. doi: 10.1158/1078-0432.CCR-07-1322. [DOI] [PubMed] [Google Scholar]
- 11.Silva WA Jr, Gnjatic S, Ritter E, Chua R, Cohen T, Hsu M, Jungbluth AA, Altorki NK, Chen YT, Old LJ, Simpson AJ, Caballero OL. PLAC1, a trophoblast-specific cell surface protein, is expressed in a range of human tumors and elicits spontaneous antibody responses. Cancer Immun. 2007;7:18. http://www.cancerimmunity.org/v7p18/071020.htm [PMC free article] [PubMed] [Google Scholar]
- 12.Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI, Pugacheva E, Hong JA, Morse H 3rd, Schrump DS, Risinger JI, Barrett JC, Lobanenkov VV. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 2005;65:7751–7762. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- 13.Woloszynska-Read A, James SR, Link PA, Yu J, Odunsi K, Karpf AR. DNA methylation-dependent regulation of BORIS/CTCFL expression in ovarian cancer. Cancer Immun. 2007;7:21. http://www.cancerimmunity.org/v7p21/071122.htm [PMC free article] [PubMed] [Google Scholar]
- 14.Tammela J, Jungbluth AA, Qian F, Santiago D, Scanlan MJ, Keitz B, Driscoll D, Rodabaugh K, Lele S, Old LJ, Odunsi K. SCP-1 cancer/testis antigen is a prognostic indicator and a candidate target for immunotherapy in epithelial ovarian cancer. Cancer Immun. 2004;4:10. http://www.cancerimmunity.org/v4p10/040811.htm [PubMed] [Google Scholar]
- 15.Monk M, Hitchins M, Hawes S. Differential expression of the embryo/cancer gene ECSA(DPPA2), the cancer/testis gene BORIS and the pluripotency structural gene OCT4, in human preimplantation development. Mol Hum Reprod. 2008;14:347–355. doi: 10.1093/molehr/gan025. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Pang XW, Liu FF, Dong XY, Wang HC, Wang S, Zhang Y, Chen WF. [PLAC1/CP1 gene expression and autologous humoral immunity in gastric cancer patients]. Beijing Da Xue Xue Bao. 2006;38:124–127. [PubMed] [Google Scholar]
- 17.Dong XY, Peng JR, Ye YJ, Chen HS, Zhang LJ, Pang XW, Li Y, Zhang Y, Wang S, Fant ME, Yin YH, Chen WF. Plac1 is a tumor-specific antigen capable of eliciting spontaneous antibody responses in human cancer patients. Int J Cancer. 2008;122:2038–2043. doi: 10.1002/ijc.23341. [DOI] [PubMed] [Google Scholar]
- 18.Koslowski M, Sahin U, Mitnacht-Kraus R, Seitz G, Huber C, Tureci O. A placenta-specific gene ectopically activated in many human cancers is essentially involved in malignant cell processes. Cancer Res. 2007;67:9528–9534. doi: 10.1158/0008-5472.CAN-07-1350. [DOI] [PubMed] [Google Scholar]
- 19.Serov SF, Scully RE, Sobin LJ. International histological classification of tumors No. 9. Geneva: World Health Organization; 1973. Histological typing of ovarian tumors. pp. 51–53. [Google Scholar]
- 20.R Core Development Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. ISBN 3-900051-07-0. [Google Scholar]
Materials and methods
Patients and specimens
Formalin-fixed and paraffin-embedded tissue specimens were obtained from patients undergoing debulking surgery for epithelial ovarian cancer, primary peritoneal cancer, and fallopian tube cancers at the Roswell Park Cancer Institute, Buffalo, NY between 1998 and 2007. All tissue specimens were collected under an approved protocol from the Institutional Review Board (IRB). All pathology specimens were reviewed in our institution and tumors were classified according to WHO criteria (19). For patients whose tumors tested positive for PLAC1 and DPPA2 by RT-PCR and/or IHC, a subset of serum samples were also available for ELISA analysis. The medical records of patients were also retrospectively reviewed under an IRB approved protocol. The review comprised outpatient and inpatient treatment, including surgery and chemotherapy. Study outcomes included overall survival (OS) and recurrence-free survival (RFS). Both survival criteria were measured from time of definitive surgery. Recurrence was defined as objective evidence of progression as all therapy was given in an adjuvant setting. The duration of OS was the interval between diagnosis and death. Observation time was the interval between diagnosis and last contact (death or last follow-up). Data were censored at the last follow-up for patients with no evidence of recurrence or progression.
Total tissue RNA isolation
Total tissue RNA was isolated from frozen tumor tissues using the TRI Reagent (Molecular Research Center Inc., Cincinnati, OH) according to the manufacturer's protocol. After isopropanol, 75% ethanol treatment and drying, the RNA was dissolved in RNAse-free water. The resulting RNA concentration was measured spectrophotometrically (DU Series 500 Spectrophotometer, Beckman Coulter, Fulleron, CA) and the quality of the RNA was checked by electrophoresis on a 1.5% agarose/ethidium bromide gel.
RT-PCR analysis of PLAC1 and DPPA2 expression
Two micrograms of each RNA sample was subjected to cDNA synthesis using the Ready-To-Go™ RT-PCR beads (GE Healthcare, Buckinghamshire, UK). PCR was subsequently performed to analyze expression of PLAC1 (CT92) and DPPA2 (CT100) in a panel of human tissues (Human Total RNA Master Panel II; Clontech, Mountain View, CA) and 101 epithelial ovarian tumor specimens. A 328 bp PCR product was amplified using PLAC1 (CT92)-specific sense 5'-CCC CTC TTC AGT TCC AGT GA-3' and antisense 5'-CCA GTC TAT GGA GCA CAG CA-3' primers. A 430 bp PCR product was amplified using DPPA2 (CT100)-specific sense 5'-GCC CTT TGT TTA TGG CCT GA-3' and antisense 5'-ACG CTT GGT TCC ATT TGT TC-3' primers. Glyceraldehyde-3-phosphodehydrogenase (GAPDH)-specific sense 5'- TCT TCA CCA CCA TGG AGA AG-3' and antisense 5'-CAA AGT TGT CAT GGA TGA CCT TGG-3' primers were used to obtain a 204 bp PCR product as a control. PCR was performed using 35 amplification cycles at an annealing temperature of 60˚C. PCR products were then visualized on a 1.5% agarose/ethidium bromide gel.
Immunohistochemistry (IHC)
For detection of DPPA2 proteins, five micron thick sections were deparaffinized and pretreated in EDTA buffer for 20 minutes using an ordinary vegetable steamer. Sections were cooled for 20 minutes and were then incubated for 10 minutes with 3% H2O2 to quench endogenous peroxidase activity. Blocking was performed using serum-free protein block (Dakocytomation, Carpenteria, CA) for 30 minutes. Anti-DPPA2 antibody was added at a 1:150 dilution to each section and incubated for 60 minutes at room temperature. Rabbit IgG from Sigma (St. Louis, MO) was used as our negative isotype matched control. Labeled streptavidin biotin (LSAB+) reagents (Dakocytomation, Carpenteria, CA) were used according to the manufacturer's instructions followed by a 5 minute incubation with 3,3'-diaminobenzidine (DAB) (Dakocytomation, Carpenteria, CA). Sections were counterstained with hematoxylin and coverslipped. DPPA2 IHC was scored based on staining intensity as negative, weakly positive, moderately positive and strongly positive. For statistical analysis, cases were classified into two groups: Group 1 (negative) included the cases with negative staining and group 2 (positive) included cases staining with weak, moderate, and strong intensity.
ELISA
Recombinant PLAC1 and DPPA2 (1 µg/ml) in 100 µl PBS/well were absorbed to 96-well plates at 4˚C overnight. Plates were washed three times with PBS and blocked with 5% NF milk/PBS and again incubated at 4˚C overnight. Plates were washed three times with PBS. Four-fold serum dilutions in blocking buffer were prepared in 96-well dilution trays. After blot drying, 100 µl/well of serum dilution was added and incubated at 4˚C overnight. Plates were washed three times with PBS/0.1% Tween and three times with PBS. After blot drying, plates were incubated with secondary antibody (HRP goat anti-human, Jackson Immunoresearch Lab, Inc., West Grove, PA) at 1:5000 dilution for 1 hour at room temperature. Plates were washed and incubated in the dark with 100 µl tetramethylbenzidine (TMB) substrate (KPL, Gaithersburg, MD) for 10 minutes. After addition of 0.2 N H2SO4 (50 µl), the absorbance was determined with a microplate reader (Dynex Technologies, Inc., Chantilly, VA).
Statistical analysis
All statistical analyses were performed with R software (20). Estimated survival distributions were calculated by the method of Kaplan and Meier and tests of significance with respect to survival distributions were based on the log rank test. The Pearson chi-square is the most common statistic used to test the significance of the relationship between categorical variables. In this study, the Pearson chi-square test assessed the significance of the relationship between PLAC1 and DPPA2 expression with the expression of two other well-known CT antigens, NY-ESO-1 and BORIS, which we previously reported to be frequently expressed in ovarian cancer (6, 13).