Abstract
In situ immunohistochemical staining of tumor-infiltrating immune cells in large cohorts of human colorectal cancers has recently supported the hypothesis that the adaptive immune response influences the behavior of human tumors. Tumor-infiltrating immune cells therefore represent a valuable prognostic marker for patients with colorectal cancer, with a high density of immune cells being associated with a good outcome independently of other established prognostic markers. The aim of the present study was to investigate the correlation between infiltrates of immune cells, in either the primary tumor or (where available) the corresponding liver metastases, with the response to chemotherapy in patients with metastatic colorectal cancer. The analysis consisted of 32 samples from 22 patients with metastasized colorectal cancer, including ten pairs of primary tumors and corresponding liver metastases. In primary tumors the ratio of stained immune cells in the epithelial portion of the tumor as compared to the total number of immune cells staining for CD3, CD8 and Granzyme B showed a relationship to the response to chemotherapy and the time to progression under chemotherapy. The primary tumors showed marked intra-tumoral heterogeneity with respect to immune cell densities. Infiltrate densities differed significantly between corresponding primary tumors and liver metastases, a variability that was also observed at the invasive margin of liver metastases. This suggests that immune infiltrates at the invasive margin of liver metastases could be predictive with respect to response to treatment. This is currently being evaluated in a larger patient cohort.
Keywords: human, colorectal cancer, metastases, immunohistochemistry, cellular immunity
Introduction
In the United States alone, colorectal cancer (CRC) is one of the major causes of cancer death, causing more than 55 000 deaths per year (1). Almost 50% of the patients die because of problems related to disease progression (2). Prognostic estimations are based on the standard TNM classification, whereas it has recently been shown that outcome is governed predominantly by immune responses at the primary tumor site (3).
Not only genetic changes in cancer cells promote tumor progression, but there is also increasing evidence for epigenetic and environmental factors playing a role (4). Inflammation can promote tumor progression in colorectal cancer, e.g. through inflammatory mediators like TNF-α (5, 6). On the other hand, immune surveillance can not only eliminate tumors but can also select tumor cell variants resistant to immune surveillance mechanisms (7), a process called "immunoediting" (8, 9, 10). In humans, a detailed evaluation of immune responses at the tumor site can be used to predict a prognosis for patients with CRC (3, 11, 12, 13).
Tumor-infiltrating immune cells can be understood as the anti-tumor immune response of the host. It has been shown that patients with a prominent lymphocyte infiltrate have improved survival rates (3, 13), whereas the overall density of the lymphoid infiltrate is not the critical parameter. Intra-tumoral immune cells are frequently functionally defective, incompletely activated (14), and include regulatory subtypes which vary with the type of cancer (15, 16, 17). Therefore, detailed distinctions with respect to lymphocyte types, their location, functional status, and their interactions have been shown to have a more profound impact on prognosis (3).
Another largely unknown aspect of the adaptive immune response is the synergistic or antagonistic interaction with chemotherapy. It is known that certain chemotherapies can induce a more "immunogenic" apoptosis of malignant cells and thereby stimulate an immune response (18, 19). Anti-tumoral CD8+ T cell function can be reconstituted (e.g. in ovarian carcinomas) through a successful systemic chemotherapy (20), supporting the idea that anti-tumor chemotherapies with limited immunosuppressive side effects can indeed restore anti-tumoral immune responses. Furthermore, low dose chemotherapy was successful in enhancing the anti-tumor responses of adoptively-transferred antigen-specific CD8 T cells in metastatic melanoma (21). If tumor-infiltrating immune cells have an impact on the efficacy of chemotherapy, it is of great interest to understand where these infiltrates are localized and the composition of these infiltrates.
Results
Immunohistochemical characteristics of primary colorectal tumors
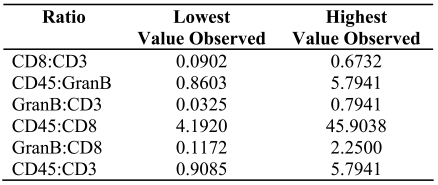
Primary colorectal tumors were stained with anti-CD3, CD8, Granzyme B and CD45RO antibodies. Intra-tumoral heterogeneity with respect to infiltrate densities was observed (Figure 1). On each slide we randomly selected up to eight windows, 1 mm2 in size, of the primary tumor. Positively stained cells were counted manually, as well as assessed automatically. Automatic counting was performed by a two-step approach. The median number of positively stained cells for each antibody staining (for each patient) was then used to calculate the minimum and maximum cell counts observed in the patients; these values are expressed as multiples of the median. The following ranges of values were determined for the samples from all 22 patients: The number of cells staining for CD3 ranged from 1 cell/mm2 to 1116 cells/mm2. A minimum of 0 and a maximum of 570 cells/mm2 stained positively for CD8. The number of cells staining for CD45RO ranged from 12 cells/mm2 to 1560 cells/mm2. For Granzyme B, a minimum of 0 and a maximum of 95 cells/mm2 stained positively. As an additional sub-analysis, the proportion of each subgroup of immune cells was calculated for each antibody staining on identical regions on the slide. Ratios for CD8:CD3, GranB:CD3, CD45:CD3, GranB:CD8, CD45:CD8 and GranB:CD45 positively stained cells were calculated and are presented in Table 1. Surprisingly, analyses of single stainings of CD3, CD8, Granzyme B and CD45RO did not reveal any interrelationships in clustering analyses.
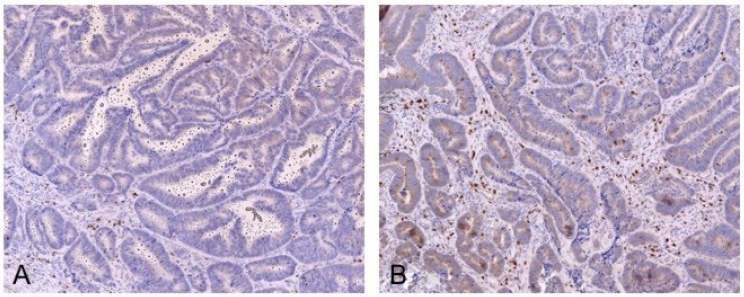
Figure 1.
Heterogeneity in the density of infiltrating lymphocytes in primary tumors. Two different regions of the same primary tumor are shown. (A) Low density infiltrate. (B) High density infiltrate. CD3 staining in dark red, with a hematoxylin counterstain.
Table 1.
Range of values seen for the ratios of CD3, CD8, Granzyme B and CD45RO positively stained cells in primary colorectal tumor samples.
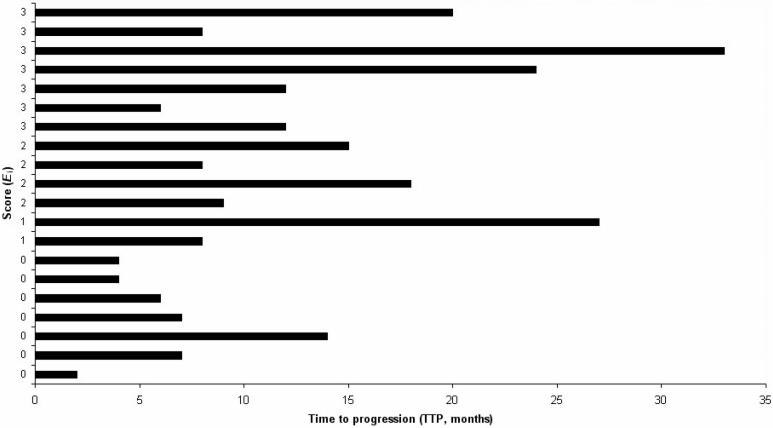
Since differentiated colorectal tumors consist of two compartments, the tumor stroma (usually bearing higher numbers of immune cells) and the epithelial part (or "adenomatous" region), these two well defined areas were further investigated. Infiltrating cells were counted differentially as epithelial or stromal. A new index named the "Epithelial index" (Ei) was defined which has the following formula: Ei = (Cepithelial/Ctotal) x 100, where C is the number of positively stained cells per mm2 and Ctotal = Cepithelial + Cstromal. Using this index for all primary colorectal tumors analyzed, we could identify a clustering of Ei values for CD3, CD8 and Granzyme B stainings. Clustering revealed single cut-off values for each staining (ECD3 7%, ECD8 15%, EGranB 12%) (see Materials and methods; for details of the number of positively stained cells, refer to Supplementary Table 1). Combining these three cut-off values to generate a score (equal weight, range 0-3) resulted in two patient groups, a group with the score of 0 versus a group with a score 1 or more (Figure 2). Statistical analysis with respect to time to progression under chemotherapy showed a significant difference between these two groups (Mann-Whitney U-test, P = 0.004; two-tailed, z = -2.861, n = 20). The median time to progression in the group with a score of 0 was 6 months, while that of the group with a score of 1 or more was 12 months.
Figure 2.
Clustering of patients according to the epithelial index (Ei) score. The center of each primary tumor was analyzed and the epithelial index determined. Each row corresponds to a single patient, with the length of the column representing the time to progression (in months) from the start of chemotherapy.
As an additional sub-analysis, the ratios of each subgroup of immune cells (see above) were analyzed with respect to time to progression. However these data were not informative and did not show any clustering (data not shown).
Immunohistochemical characteristics of liver metastases
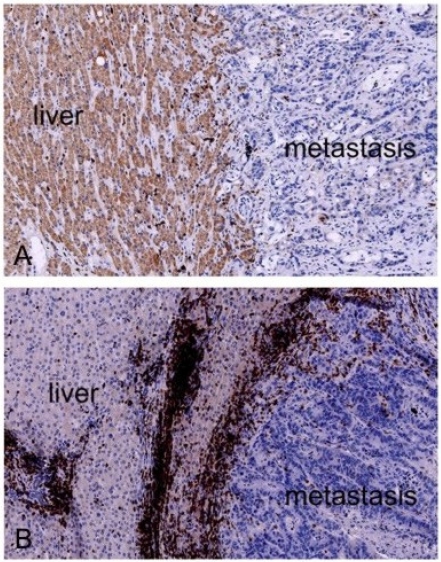
Evaluation of the center of the metastases by hematoxylin staining (and antibody staining) revealed a heterogeneous immune cell infiltration dependent on the presence (and dimension) of necrotic, hemorrhagic, mucinous and fibrotic compartments. However, the invasive margin (the boundary between normal liver tissue and the metastasis) showed marked variations: the difference in infiltrate density at this boundary between different patients was striking (Figure 3). The cell count revealed a broad range of values for CD3, CD8 and Granzyme B positively staining cells. However the number of liver metastases investigated was much too small for a detailed statistical analysis to be carried out to determine whether these were related to the response to chemotherapy. Further analyses are currently being conducted.
Figure 3.
Invasive margin of liver metastases with different densities in infiltrates. Images from two different patients are shown. (A) Low density infiltrate. (B) High density infiltrate. CD3 staining in dark red, with a hematoxylin counterstain.
Immunohistochemical characteristics of primary tumors and of the respective liver metastases
The relationship between infiltrates in primary tumors and in the corresponding liver metastases was analyzed in ten patients. For each pair of samples, the number of positively staining cells was expressed as various ratios and showed great heterogeneity. There was no clear association between the density of infiltrates in the primary tumor and the infiltrate density at the corresponding liver metastasis. However the number of samples was small and this evaluation could have been influenced by the heterogeneity of the primary tumor. Great variations in infiltrate density were seen in primary tumors making comparisons difficult. We will try to evaluate this aspect in a larger group of patients, using different statistical models to single out possible informative infiltrate densities (e.g. "one-out-of-ten" system).
Discussion
For metastatic colorectal carcinoma, the association between tumor-infiltrating lymphocyte densities and overall survival was reported previously (3, 11), demonstrating the importance of infiltrating immune cells in shaping the course of the disease. Still unknown is the correlation of infiltrate densities between primary tumors and metastases and the influence of the presence of infiltrates on chemotherapy. We observed an enormous intra-tumoral variation in infiltrate densities within some of the primary tumors. This observation required careful evaluation of the overall densities by elaborate quantification methods. Given this heterogeneity, it appears difficult to base predictions only on single fields 1 mm2 in size. Virtual microscopy allows large sections of tumors (e.g. 12 mm2) to be evaluated and therefore provides a superior estimation of densities, especially with heterogeneous tumors or markers.
By applying robust statistical methods we were not able to detect a relationship between the number of CD3, CD8, CD45RO or Granzyme B positively staining cells and the time to progression under chemotherapy. There was, however, an association between the epithelial index and time to progression. The importance of intraepithelial immune cells for the overall prognosis of primary colorectal cancer specimens has been described before (12), and these intraepithelial immune cells also appear to play a role in chemotherapy efficacy. However, the association between epithelial index (Ei) and time to progression can only be seen as a surrogate marker. Positively stained epithelial effector immune cells have apparently migrated into the epithelial compartment and the index value reflects the percentage of this subset of positively stained cells in the region of interest. This could be understood as a specific immune reaction against tumor tissue, where the immune cells are able to migrate into the epithelium of the tumor tissue. It is, however, pure speculation as to why these cells are present in the epithelium. Taking the heterogeneity of the primary tumors into account, this could be attributed to a different immunological environment with a different antigen and cytokine expression. Nevertheless we could observe a statistically significant association between the proportion of positively stained immune cells in the epithelium, as expressed by the epithelial index (Ei), and the time to progression.
Of course, only tumors which were clearly differentiated into an epithelial and a stromal component could be analyzed. One sample had to be excluded from this analysis due to this constraint (being a mucinous carcinoma). This observation is generally limited to a smaller number of colorectal carcinomas. As hypothesized, the ratio of epithelial to total (stromal and epithelial) cell counts reflects a specific effective anti-tumor reaction of the effector cells against the tumor, while absolute numbers reflect the general activation. A clear limitation of the detected relationship is the small number of patient samples, but the differences observed were statistically significant and should spark further investigations in larger cohorts of patients. In clinical routine, no marker for the prediction of chemotherapy efficacy is available. Thus many patients receive chemotherapies without benefits and with marked side effects. The observed association with the epithelial index (Ei) might be a possible future tool to guide clinical and therapeutic decisions for patients with metastasized colorectal cancer. No direct association was observed between infiltrates in the primary tumor and that in liver metastases. The difference in immune cell densities between primary tumors and liver metastases may point to the immunoediting function of the immune system, whereby circulating tumor cells are subject to selection by the adaptive immune response.
Another interesting observation was the marked difference in cell densities in different patients in the invasive margin of liver metastases. This suggests that the immune infiltrates at the invasive margin of liver metastases may be informative. To address this observation, larger cohorts of patient samples will be analyzed in further immunological studies. These studies may also shed some light on how tumor-infiltrating immune effector cells actually influence the time to progression under chemotherapy.
Acknowledgements
We thank all patients who participated in this study. NH and IZ are financially supported through a grant by the medical faculty of the University of Heidelberg.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Gill S, Thomas RR, Goldberg RM. Review article: colorectal cancer chemotherapy. Aliment Pharmacol Ther. 2003;18:683–692. doi: 10.1046/j.1365-2036.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc P, Trajanoski Z, Fridman W, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 5.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 6.Greten FR, Eckmann L, Greten TF, Park JM, Li Z, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 9.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc P, Trajanoski Z, Fridman W, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 12.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–816. doi: 10.1016/j.humpath.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmiani G. Tumor-infiltrating T cells--friend or foe of neoplastic cells? N Engl J Med. 2005;353:2640–2641. doi: 10.1056/NEJMp058236. [DOI] [PubMed] [Google Scholar]
- 16.Yu P, Fu Y. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 17.Chiou S, Sheu B, Chang W, Huang S, Hong-Nerng H. Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol. 2005;67:35–50. doi: 10.1016/j.jri.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravi R, Fuchs EJ, Jain A, Pham V, Yoshimura K, Prouser T, Jalla S, Zhou X, Garrett-Mayer E, Kaufmann SH, Schulick RD, Pardoll DM, Bedi A. Resistance of cancers to immunologic cytotoxicity and adoptive immunotherapy via X-linked inhibitor of apoptosis protein expression and coexisting defects in mitochondrial death signaling. Cancer Res. 2006;66:1730–1739. doi: 10.1158/0008-5472.CAN-05-3377. [DOI] [PubMed] [Google Scholar]
- 20.Coleman S, Clayton A, Mason MD, Jasani B, Adams M, Tabi Z. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res. 2005;65:7000–7006. doi: 10.1158/0008-5472.CAN-04-3792. [DOI] [PubMed] [Google Scholar]
- 21.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ImageJ: Image processing and analysis in Java software package. http://rsb.info.nih.gov/ij/
Materials and methods
Samples and patients
Archival specimens were obtained from 22 patients at the University Clinic Heidelberg. Samples were used after receiving a signed informed consent from patients. The study was approved by the Medical Ethical Committee of the University of Heidelberg. A total of 32 samples were analyzed, 22 primary tumors and 10 liver metastases, allowing the analysis of 10 pairs of primary tumor and corresponding liver metastasis.
For patients' characteristics see Supplementary Table 2. Patients were included in this analysis when diagnosed with stage UICC IV colorectal cancer and treated at our facilities. Mean age was 64 years, with 38% of the patients being women. Two out of the 22 patients had metachrone metastases. These two patients received adjuvant chemotherapy consisting of 5-FU- and oxaliplatin-based regimens which were administered for six months. When investigating the association between infiltrate densities and the response to chemotherapy in a homogeneous patient group, these two patients were omitted from the statistical analysis. All other patients received either an irinotecan- or an oxaliplatin-based first line therapy plus additional antibody treatment with either bevacizumab or cetuximab. Patient characteristics of the subgroup with low time to progression (Ei score of 0) were similar to the overall distribution of patient characteristics (mean age 58 versus 64 years old, 40% female versus 38% female, chemotherapy regimen used). Time to progression was calculated from the beginning of palliative chemotherapy onwards.
Immunohistochemical staining
Sections were cut from paraffin-embedded blocks. The following mouse monoclonal antibodies were used: anti-human CD3 (DAKO, Carpinteria, CA), CD8, Granzyme B (Novocastra, Newcastle, United Kingdom) and CD45RO (1:100 dilution, clone UCHL-1, Biologo, Germany). Immunohistochemistry of paraffin sections was carried out using a two-step protocol (Novolink Polymer Detection System, Novocastra) according to the manufacturer's instructions. Paraffin sections were deparaffinized and then hydrated. Following microwave antigen retrieval, endogenous peroxidase activity was blocked by incubating the slides in 0.3% H2O2 and nonspecific binding sites were blocked with Protein Block (RE7102; Novocastra), as required. After serial incubation with primary antibodies, Post Primary Block (RE7111; Novocastra), and secondary antibody (Novolink Polymer RE7112), the sections were developed in diaminobenzidine solution under a microscope and counterstained with hematoxylin. Negative control slides in which primary antibodies were omitted were included in all assays. All slides were counterstained using hematoxylin.
Evaluation of immunohistochemical variables
The number of stained immune cells was counted using a computerized image analysis system consisting of an NDP Nanozoomer from Hamamatsu Photonics attached to a personal computer. This system is unique in the world and was installed in the Hamamatsu Tissue Imaging and Analysis Center (TIGA) in the BIOQUANT Center at the University of Heidelberg. Using the NDP Nanozoomer, complete microscopic images of full tissue sections can be automatically obtained for later automatic or visual analysis (virtual microscopy). Such gigabyte-size images require special algorithms for effective image processing. As opposed to conventional digital microscopy systems, the NDP Nanozoomer generates digital images of full tissue sections, allowing large scale histological evaluations with high precision across the complete section. Thus ambiguities due to varying cell densities across the tissue can be avoided. Moreover, a sophisticated digital imaging system allows the precise comparison of regions of interest between different slides from the same tissue.
To manually evaluate immune cell numbers in the invasive margin of the metastasis, 5 to 8 regions of interest (each 1 mm2) were selected. The manual evaluation of stained immune cells was performed (in duplicate) without knowledge of the clinicopathologic data by two independent observers. Variations in the enumeration within a range of 5% were re-evaluated and a consensus decision was made. The results were expressed as the mean number of positively stained cells per mm2 for one computerized microscopic field (1 mm2 in size).For the center of the tumor (and that of the corresponding metastasis), a grid of tiles (each 1 mm2) was generated and each tile was numbered sequentially. Five tiles were then selected randomly by a computer program for further evaluation.
To reassess the manual cell counts we developed a software program to measure cell densities across a given region of interest (12 mm2). The resulting ratios were then compared and generally yielded a high coherence to the number of cells counted manually. To further evaluate the manual counting we used the ImageJ software package (22) to count cells directly from digital images. Again the resulting numbers supported the manually counted cell numbers, with a tendency to underestimate cell numbers in regions with very high cell densities (due to misinterpreted cell agglomerates).
Granzyme B, constitutively expressed by natural killer cells, is only expressed on activated CD8+ CTLs. Both Granzyme B positive cells with a sparsely granulated pattern and densely positive natural killer cells were evaluated.
Statistical analyses
Statistical analyses were performed using the SPSS 15.0 software (SPSS, Chicago, IL). Clustering analyses were performed using either the hierarchical clustering analysis tool or visual inspection. "Cut-off" values were defined as the lowest number of cells separating a subgroup of patients, e.g. with no response to chemotherapy. In the first analysis, the samples were clustered according to "marked" chemotherapy response (i.e. partial or complete remission, not stable disease). A secondary analysis was performed to assess the relationship between immune cell stainings (clustering results) and clinicopathologic characteristics using the time to progression interval under chemotherapy as a surrogate for therapeutic efficacy. For the comparison of individual variables, Mann-Whitney U-tests and, where possible, t-tests were carried out as appropriate. Two-tailed P < 0.05 was judged to be significant.
Supplemental data
Download from http://www.cancerimmunity.org/v9p1/090102_suppl_tab1.txt (2 KB tab-delimited TXT file).
Download from http://www.cancerimmunity.org/v9p1/090102_suppl_tab2.txt (2 KB tab-delimited TXT file).
Download from http://www.cancerimmunity.org/v9p1/090102_suppl_data.zip (2 KB WinZip file).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Download from http://www.cancerimmunity.org/v9p1/090102_suppl_tab1.txt (2 KB tab-delimited TXT file).
Download from http://www.cancerimmunity.org/v9p1/090102_suppl_tab2.txt (2 KB tab-delimited TXT file).
Download from http://www.cancerimmunity.org/v9p1/090102_suppl_data.zip (2 KB WinZip file).