Abstract
We show correlation between strong and decreased NY-ESO-1-specific immunity with spontaneous regression and subsequent recurrence, respectively, in a long-surviving patient with an NY-ESO-1-expressing lung adenocarcinoma. An integrated immune response consisting of IgG antibody, as well as CD4 and CD8 T cells, against NY-ESO-1 was observed at the time of spontaneous regression of multiple pleural metastases. After tumor dormancy for 3 years, the tumor started to progress. IgG antibody levels and the number of CD4 and CD8 T cells against NY-ESO-1 decreased, but were still detectable. On the other hand, the number of Foxp3+ CD25 high T regulatory cells gradually increased. The findings suggest the relevance of the NY-ESO-1 immune response and its regulation by Foxp3+ CD25 high T regulatory cells in the clinical course of this lung cancer patient.
Keywords: human, lung cancer, NY-ESO-1, humoral immunity, cellular immunity
Introduction
The NY-ESO-1 antigen was originally found in an esophageal cancer by serological recombinant cDNA expression cloning (SEREX) and is in the category of cancer/testis (CT) antigens (1-3). Its expression is restricted to germ cells in the testes in normal adult tissues, but is observed in various cancer types at different frequencies (4). A characteristic of the NY-ESO-1 antigen is its extremely high immunogenicity (5). Patients with NY-ESO-1-expressing tumors frequently show a spontaneous immune response to the NY-ESO-1 antigen. An antibody response against the NY-ESO-1 antigen was frequently observed in patients with NY-ESO-1-expressing tumors (6, 7). Most patients who showed an antibody response also showed CD4 and CD8 T cell responses (8, 9). Such strong immunogenicity indicates the NY-ESO-1 antigen to be a very promising candidate target molecule for a cancer vaccine.
We recently reported a lung adenocarcinoma patient who showed spontaneous regression of multiple pleural metastases without any treatment (10). Analysis of the immune response using the patient's peripheral blood revealed extremely strong immune responses to the NY-ESO-1 antigen, but not to other CT antigens commonly expressed in lung cancer in the Japanese population. Recently, the patient showed tumor recurrence. In this paper, we show a correlation between high and decreased NY-ESO-1-specific immunity to spontaneous regression and subsequent recurrence. A gradual decrease in NY-ESO-1 immunity was associated with a gradual increase in regulatory T cells (Tregs), suggesting the relevance of Tregs for recurrent tumor progression.
Results
Spontaneous regression and recurrence of the lung adenocarcinoma in patient GO
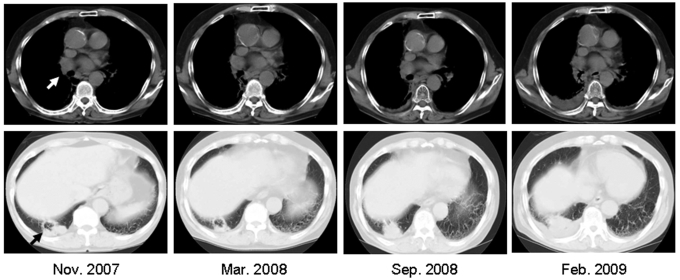
Patient GO is a 71-year old Japanese man. A right hilar tumor (3 x 3 cm) and right multiple pleural tumors were found by chest computed tomography in November 2004 (10). The hilar tumor was unchanged, but the pleural metastases regressed spontaneously by March 2005. Histopathological examination of pleural metastases biopsy specimens showed that the tumor was a poorly differentiated adenocarcinoma. Immunohistochemical analyses showed that approximately 50%-60% of the tumor cells expressed NY-ESO-1 and 30%-40% expressed HLA class I antigens. Infiltration by many CD8 T cells was observed in the stromal tissue surrounding the tumor and in the tumor tissue. Subsequently, a tumor was noticed in the right lower lobe in November 2007 (Figure 1 and Figure 2A) that was probably the primary lesion which had disappeared and left a scar by his first visit to the hospital in November 2004. The tumors gradually increased in size until April 2009.
Figure 1.
Chest computed tomography. The white arrow indicates a right hilar tumor of the lung and the black arrow indicates a new lesion in the right lower lobe.
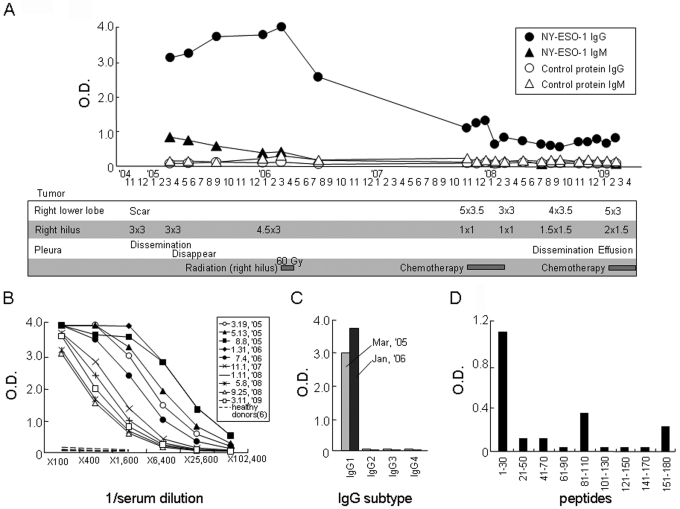
Figure 2.
Serum antibody response against NY-ESO-1 in patient GO. (A) The IgG and IgM responses against recombinant NY-ESO-1 protein at a serum dilution of 1:1,600 were plotted during the course of the disease. The control recombinant protein used was RL-Akt. The clinical course and treatments shown in the box correspond to the time points. (B) Titration of serum obtained at different time points is shown. Sera from 6 healthy donors were included as a control. (C) The IgG subtype was determined using specific secondary mAbs for detection. (D) The peptide regions recognized by the antibody were determined using 30-mer NY-ESO-1 overlapping peptides.
Serum antibody response
The serum antibody response against CT antigens, whose expression is frequently observed in lung cancer, was investigated in patient GO using recombinant proteins by ELISA. An antibody against NY-ESO-1, but not against XAGE-1b, SSX2 or SSX4, was observed (10). An extremely strong serum IgG response against NY-ESO-1 was observed from March 2005 to July 2006 during the course of the disease (Figure 2). Thereafter, the response decreased gradually. The serum IgM response decreased gradually from March 2005 onwards and was undetectable in July 2006. The dominant IgG subtype was IgG1 (Figure 2C).
The peptide regions recognized by the antibody were defined using 30-mer overlapping peptides spanning the entire NY-ESO-1 protein. A peptide corresponding to amino acids 1-30 was the region dominantly recognized by the antibody (Figure 2D), while peptides corresponding to amino acids 81-110 and 151-180 were weakly recognized.
CD4 and CD8 T cell responses and their recognition of NY-ESO-1 peptides
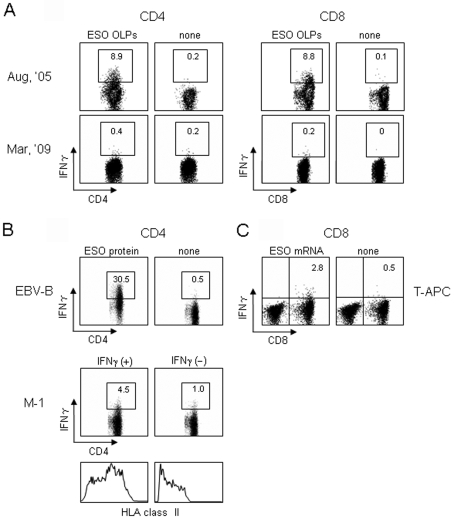
CD4 and CD8 T cell responses against NY-ESO-1 in patient GO were investigated by an IFNγ secretion assay using 28 18-mer overlapping peptides and a 30-mer C-terminal peptide (OLPs) spanning the entire NY-ESO-1. MACS beads-purified CD4 and CD8 T cells from PBMCs were cultured with irradiated (40 Gy) autologous CD4- and CD8-depleted PBMCs as APCs in the presence of a mixture of OLPs for 12 days. The cells were then assayed for IFNγ secretion against paraformaldehyde (PFA)-fixed autologous CD4- and CD8-depleted PBMCs pre-pulsed with OLPs. As shown in Figure 3, extremely strong CD4 and CD8 T cell responses were observed in PBMCs obtained in August 2005 when the strong IgG response was observed. On the other hand, decreased CD4 and CD8 T cell responses were observed in PBMCs obtained in March 2009, when a decreased IgG response was observed. After two in vitro stimulations, however, significantly amplified CD4 and CD8 T cell responses were detected in PBMCs obtained in March 2009 (data not shown), confirming the presence of specific CD4 (0.2%) and CD8 (0.2%) T cells in the assay stimulated once in vitro.
Figure 3.
IFNγ secretion assays. MACS beads-purified CD4 and CD8 T cells (2 x 106) from PBMCs were cultured with irradiated (40 Gy) autologous CD4- and CD8-depleted PBMCs (2 x 106) as APCs in the presence of 28 overlapping 18-mer peptides and a 30-mer C-terminal peptide (OLPs) spanning the entire NY-ESO-1 protein (1 µg of each peptide/ml) in 24-well culture plates for 12 days. (A) IFNγ secretion by CD4 and CD8 T cells (1 x 105) was assayed against PFA-treated CD4- and CD8-depleted PBMCs (1 x 105) pre-pulsed with NY-ESO-1 OLPs for 30 min. (B and C) CD4 and CD8 T cells obtained in August 2005 were used on the twenty-sixth day following two stimulations. (B) IFNγ secretion by CD4 T cells (1 x 105) was assayed against the patient's EBV-transformed B cells (1 x 105) pretreated with NY-ESO-1 protein (20 µg/ml) for 24 h, and NY-ESO-1-expressing melanoma (M-1) cells (1 x 105) pretreated with IFNγ (100 U/ml) for 48 h by stimulation for 4 h. HLA class II expression on M-1 cells after IFNγ treatment is also shown. (C) IFNγ secretion by CD8 T cells (1 x 105) was assayed against the patient's PHA-stimulated CD4 T cells (T-APC) (1 x 105) transfected with NY-ESO-1 mRNA (20 µg).
The response of CD4 T cells was observed against autologous Epstein-Barr virus-transformed human B lymphocytes (EBV-B) pretreated with NY-ESO-1 protein and allogeneic NY-ESO-1-expressing melanoma cells (M-1) with compatible HLA class II expression (Figure 3B). The response of CD8 T cells was observed against NY-ESO-1 mRNA-transfected autologous phytohemagglutinin (PHA)-stimulated CD4 T cells (Figure 3C). The findings indicate that the CD4 and CD8 T cell responses detected by the stimulation with OLPs were directed against naturally processed CD4 and CD8 T cell epitopes, respectively.
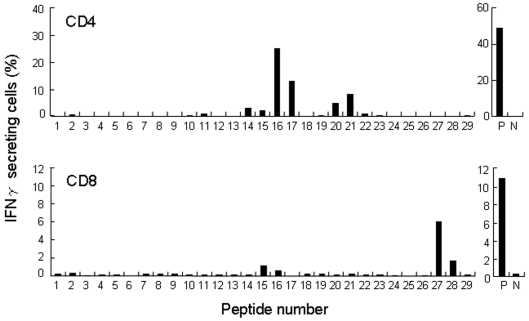
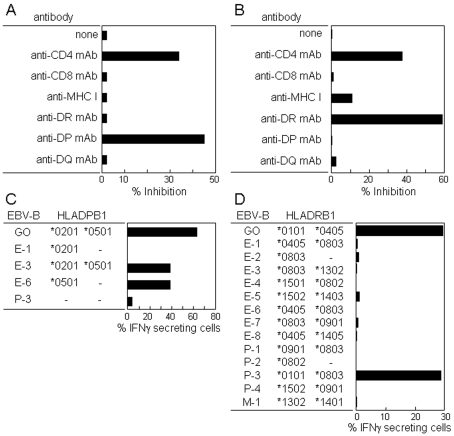
Peptide regions recognized by the CD4 and CD8 T cells were investigated using PFA-fixed autologous CD4- and CD8-depleted PBMCs pre-pulsed with individual overlapping peptides in the assays. As shown in Figure 4, peptides 16 (aa 91-108) and 17 (aa 97-114), and peptides 20 (aa 115-132) and 21 (aa 121-138) were the two dominant regions recognized by CD4 T cells. On the other hand, peptides 27 (aa 153-170) and 28 (aa 156-173) made up the dominant region recognized by CD8 T cells.
Figure 4.
NY-ESO-1 regions recognized by CD4 and CD8 T cells in patient GO. MACS beads-purified CD4 and CD8 T cells (2 x 106) from PBMCs obtained in August 2005 were cultured with irradiated (40 Gy) autologous CD4- and CD8-depleted PBMCs (2 x 106) as APCs in the presence of 28 overlapping 18-mer peptides and a 30-mer C-terminal peptide (OLPs) at a concentration of 1 µg/ml of each peptide in a 24-well culture plate for 14 days. On the twenty-sixth day after two stimulations, CD4 and CD8 T cells (3 x 104) were assayed for IFNγ secretion against PFA-treated autologous CD4- and CD8-depleted PBMCs (3 x 104) pre-pulsed with the individual peptide after stimulation for 4 h. The peptide number corresponds to the individual overlapping peptides. Abbreviations: P, positive control stimulated with a mixture of OLPs; N, negative control without peptides.
Restriction molecules involved in the CD4 T cell recognition of peptides 16 (aa 91-108) and 21 (aa 121-138) were investigated. As shown in Figure 5, CD4 T cell recognition of peptides 16 (aa 91-108) and 21 (aa 121-138) was blocked by an anti-DP mAb and an anti-DR mAb, respectively. Analyses using EBV-B cells for which HLA genotypes have been determined showed restriction by DPB1*0501 for the recognition of peptide 16 (aa 91-108) and restriction by DRB1*0101 for the recognition of peptide 21 (aa 121-138).
Figure 5.
Restriction molecules involved in the CD4 T cell recognition of NY-ESO-1. CD4 T cell recognition of peptide 16 (aa 91-108) (A and C) and peptide 21 (aa 121-138) (B and D) was analyzed by antibody blocking (A and B) and using various EBV-B cells as APCs (C and D). CD4 T cells cultured with irradiated (40 Gy) autologous CD4- and CD8-depleted PBMCs in the presence of a mixture of OLPs for 14 days, as described in the legend of Figure 4, were assayed for IFNγ secretion against PFA-treated autologous CD4- and CD8-depleted PBMCs pre-pulsed with peptide 16 (aa 91-108) (A) and peptide 21 (aa 121-138) (B) in the presence of various mAbs (2 µg/ml) during the assay, and against PFA-treated EBV-B cells as APCs pre-pulsed with peptide 16 (aa 91-108) (1 µg/ml) (C) and peptide 21 (aa 121-138) (1 µg/ml) (D) for which HLA genotypes have been determined. IFNγ production was determined by ELISA in A and B using the supernatant after culture for 18 h and by an IFNγ secretion assay in C and D after stimulation for 4 h.
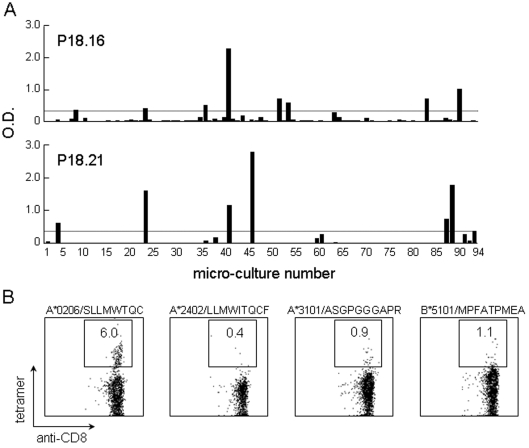
CD8 T cell recognition of peptide 27 (aa 153-170) was analyzed using tetramers. The sequence SLLMWTQC (aa 157-165) used for preparing A*0206-tetramers lies in peptide 27. As shown in Figure 6B, a significant fraction of A*0206-tetramer positive CD8 T cells were detected in the culture stimulated with OLPs. A*2402-tetramer positive CD8 T cells were at background level.
Figure 6.
NY-ESO-1-reactive CD4 and CD8 T cell frequencies. Frequency analysis of peptide-specific CD4 T cells (A) and tetramer staining of CD8 T cells (B) are shown. (A) A limited number (2 x 104) of CD4 T cells were seeded in duplicate in 96-well plates and cultured with irradiated (40 Gy) autologous CD4- and CD8-depleted PBMCs (2 x 104) as APCs in the presence of peptides 16 (aa 91-108) (1 µg/ml) (top) and 21 (aa 121-138) (1 µg/ml) (bottom) for 14 days. On the twenty-sixth day after stimulating twice, IFNγ production by the cells in each well was determined against each peptide (1 µg/ml) using autologous EBV-B cells (1 x 104) as APCs by ELISA after incubation for 18 h. An O.D. value exceeding 0.3 after subtraction of the background (without peptide) was taken as positive. The number of positive wells was 6 in both cultures. The peptide-specific CD4 T cell frequency was calculated to be 3.2 x 10-6 for both peptides. (B) Four tetramers were prepared. The patient HLA class I genotype was HLA-A*0206, A*2402, B27, B54, and Cw1. The sequence SLLMWTQC (aa 157-165) used to prepare the A*0206-tetramers lies in peptide 27 (aa 153-170) recognized by the patient's CD8 T cells, as shown in Figure 4.
NY-ESO-1-reactive CD4 and CD8 T cell frequencies
The frequency of CD4 T cells specific for peptides 16 and 21 was determined by limiting dilution. As shown in Figure 6A, the CD4 T cell frequency was calculated as 3.2 x 10-6 with either peptide. The frequency of CD8 T cells positive for A*0206 tetramer staining was 6% (Figure 6B). Multiplication during culture for 12 days, assuming a doubling time of 24 h, was estimated at 1.4 x 10-5.
Foxp3+ CD25 high T regulatory cells (Tregs) in PBMCs from the patient during the course of the disease
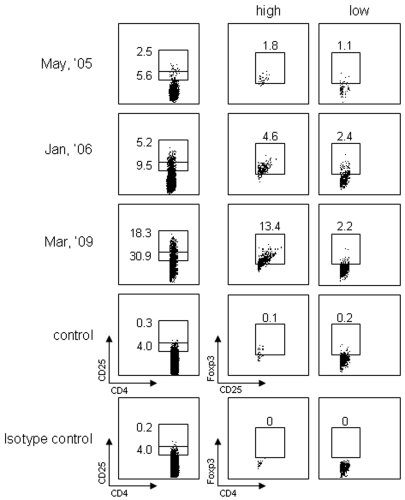
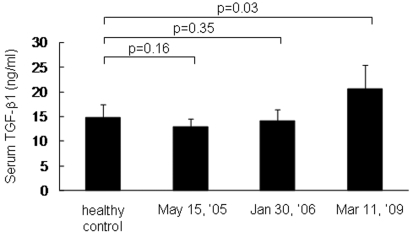
Foxp3+ CD25 high Tregs in PBMCs were examined at three different time points simultaneously by intracellular staining using FACS. As shown in Figure 7, Foxp3+ CD25 high Tregs in normal individuals were in the range of 0% to 2%. In the patient, these cells were detected at frequencies of 1.8% and 4.6% in May 2005 and January 2006, respectively. In March 2009, at the time when obvious tumor progression and a decreased immune response were observed, the number of Foxp3+ CD25 high Tregs went up to 13.4%. Cytokines in the serum were determined by ELISA using specific mAbs. As shown in Figure 8, a gradual increase in TGF-β1 was detected in sera from May 2005 to March 2009. No IL-4 or IL-10 was detected.
Figure 7.
Flow cytometry of Foxp3+ CD25+ CD4 T regulatory cells at different time points. CD25 high and low CD4 T cells were analyzed for Foxp3 expression by intracellular staining using FACS Calibur. A normal individual was used as control.
Figure 8.
Serum TGF-β1 determined by ELISA.
Discussion
In this study, we show a correlation between a strong NY-ESO-1-specific immune response and its decreasing levels to spontaneous tumor regression of multiple pleural metastases and recurrence, respectively, during the course of the disease in a long-surviving patient with an NY-ESO-1-expressing lung adenocarcinoma. An integrated immune response consisting of IgG antibody and CD4 and CD8 T cells against NY-ESO-1 was observed at the time of spontaneous tumor regression. A tumor mass (3 x 3 cm) in the right hilar lymph node remained the same size for more than a year. After 3 years, a tumor recurred at the primary lesion site, which had disappeared spontaneously before the time of his first visit to the hospital and had been noticed as interstitial thickening on computed tomography. IgG antibody levels and CD4 and CD8 T cells against NY-ESO-1 decreased, but were still detectable. On the other hand, Foxp3+ CD25 high Tregs gradually increased in association with tumor progression. Thus, the decrease in the initially strong immune response against NY-ESO-1 was associated with a gradual increase in Foxp3+ CD25 high Tregs, suggesting a relationship between the NY-ESO-1 immune response and the clinical course of the disease in this lung cancer patient.
Suppression of tumor immune responses by tumors has been well documented in experimental tumors and human cancers (11). Depletion of Tregs in the tumor host by injection of CD25 mAb caused regression in various murine tumors (12). In vitro depletion of Tregs from PBMCs in healthy donors and cancer patients unmasked NY-ESO-1 CD4 T cell responses which were otherwise undetectable (13). In human ovarian cancer, the frequency of Tregs in the tumor has been shown to negatively correlate with survival (14) and, in malignant melanoma, a high proportion of Tregs was observed in metastatic tumors (15). We have recently shown a high proportion of Tregs in the blister fluid of local tumor sites in a malignant melanoma patient vaccinated with a complex of cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein (16). In the peripheral blood, the number of Tregs has also been shown to have increased in various cancers (17). Recently, it was shown that patients with advanced melanoma had a high number of circulating Tregs, and that the number of Tregs in the blood was correlated with the stage of disease and the clinical and immunologic efficacy of the NY-ESO-1 ISCOMATRIX™ cancer vaccine (18).
Immune epitopes of NY-ESO-1 recognized by the IgG antibody and the CD4 and CD8 T cells were determined in the patient. The antibody recognized the amino acid region 1-30 dominantly, and the amino acid regions 81-110 and 151-180 weakly. Recognition of the NY-ESO-1 amino acid region 1-30 is not common in the immune response of Japanese patients with NY-ESO-1-expressing tumors (19). The most common amino acid region recognized by the antibodies corresponds to amino acids 91-108. Eight of 9 patients who had antibodies against NY-ESO-1 responded to the amino acid region 91-108 predominantly (19). Six of 9 patients who were immunized with a complex of cholesterol-hydrophobized pullulan and NY-ESO-1 protein responded to the same region (19). The IgG antibody response to the rather rare epitope region may have been related to the favorable clinical course in this patient. In this regard, we are now trying to produce a human monoclonal antibody using CD19+ B cells from PBMCs of this patient.
Recent analyses of the immune responses of the patients vaccinated with the NY-ESO-1 protein show that most of the CD4 or CD8 T cell responses were directed against two dominant regions in the NY-ESO-1 molecule (20-23). One was the region corresponding to aa 73-114 and the other corresponds to aa 115-144 (22). In this study, we determined that the two epitope regions recognized by CD4 T cells lie in the two previously described dominant regions and that the epitope region recognized by CD8 T cells is in the C-terminus. One of the epitopes recognized by the CD4 T cells was peptide 91-114, for which recognition was restricted to DPB1*0501. This peptide is in a region frequently recognized by the antibody (as described above) and also by CD4 and CD8 T cells, for which restriction molecules have not been fully elucidated. The other was peptide 115-138, for which recognition was restricted to DRB1*0101. Zarour et al. (24) originally identified the peptide 119-143 as a promiscuous HLA class II epitope that binds to multiple DR molecules such as DRB1*0101, DRB1*0401, DRB1*0701, DRB1*1101, DRB1*1501, DRB3*0101, DRB4*0101 and DRB5*0101 expressed at a high frequency in Caucasian populations. Recently, the shorter peptide 122-138 was shown to bind to DRB1*0802, DRB1*0901, DRB1*1502, and DRB1*0405/*0410, which are common DR alleles in the Japanese population (25). The promiscuous peptide 119-143 was also shown to contain multiple HLA class I epitopes that bound to A66, A68, Cw3 and Cw15 (26). The epitope recognized by CD8 T cells in the patient was restricted to *A0206, as demonstrated by tetramer staining. The peptide epitope 157-165 was identified as the immunodominant *A0201 epitope and was also shown to bind to *A0206 (27). The strong immunogenicity of these peptide epitopes may have contributed to the rather high frequency of NY-ESO-1-specific CD4 and CD8 T cells in this patient.
Abbreviations
- OLPs
overlapping peptides
- PFA
paraformaldehyde
Acknowledgements
We thank Ms. K. Nishida for tetramer production and Ms. J. Mizuuchi for preparation of the manuscript. We also thank Dr. L. J. Old for continuous encouragement during this study. This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Chen YT, Scanlan MJ, Sahin U, Türeci O, Güre AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scanlan MJ, Güre AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 3.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 4.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 5.Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, Knuth A, Chen YT, Old LJ. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 6.Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jäger E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jäger D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against "Cancer-Testis" antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Gnjatic S, Atanackovic D, Jäger E, Matsuo M, Selvakumar A, Altorki NK, Maki RG, Dupont B, Ritter G, Chen YT, Knuth A, Old LJ. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci USA. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jäger D, Arand M, Ritter G, Cerundolo V, Dupont B, Chen YT, Old LJ, Knuth A. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura Y, Noguchi Y, Sato E, Uenaka A, Sato S, Kitazaki T, Kanda T, Soda H, Nakayama E, Kohno S. Spontaneous remission of a non-small cell lung cancer possibly caused by anti-NY-ESO-1 immunity. Lung Cancer. 2009;65:119–122. doi: 10.1016/j.lungcan.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 12.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 13.Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 14.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Méndez R, Ruiz-Cabello F, Rodríguez T, Del Campo A, Paschen A, Schadendorf D, Garrido F. Identification of different tumor escape mechanisms in several metastases from a melanoma patient undergoing immunotherapy. Cancer Immunol Immunother. 2007;56:88–94. doi: 10.1007/s00262-006-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji K, Hamada T, Uenaka A, Wada H, Sato E, Isobe M, Asagoe K, Yamasaki O, Shiku H, Ritter G, Murphy R, Hoffman EW, Old LJ, Nakayama E, Iwatsuki K. Induction of immune response against NY-ESO-1 by CHP-NY-ESO-1 vaccination and immune regulation in a melanoma patient. Cancer Immunol Immunother. 2008;57:1429–1437. doi: 10.1007/s00262-008-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 18.Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N, Tan B, Maraskovsky E, Miloradovic L, Hopkins W, Pan L, Venhaus R, Hoffman EW, Chen W, Cebon J. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15:2166–2173. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata R, Wada H, Isobe M, Saika T, Sato S, Uenaka A, Miyata H, Yasuda T, Doki Y, Noguchi Y, Kumon H, Tsuji K, Iwatsuki K, Shiku H, Ritter G, Murphy R, Hoffman E, Old LJ, Monden M, Nakayama E. Antibody response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int J Cancer. 2007;120:2178–2184. doi: 10.1002/ijc.22583. [DOI] [PubMed] [Google Scholar]
- 20.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jäger E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, Ayyoub M, Ritter E, Ritter G, Jäger D, Panicali D, Hoffman E, Pan L, Oettgen H, Old LJ, Knuth A. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci USA. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uenaka A, Wada H, Isobe M, Saika T, Tsuji K, Sato E, Sato S, Noguchi Y, Kawabata R, Yasuda T, Doki Y, Kumon H, Iwatsuki K, Shiku H, Monden M, Jungbluth AA, Ritter G, Murphy R, Hoffman E, Old LJ, Nakayama E. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immun. 2007;7:9. http://www.cancerimmunity.org/v7p9/070309.htm [PMC free article] [PubMed] [Google Scholar]
- 23.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O'Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S, Castelli F, Land S, Bennouna J, Logan T, Kirkwood JM. NY-ESO-1 119-143 is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor-reactive CD4+ T cells. Cancer Res. 2002;62:213–218. [PubMed] [Google Scholar]
- 25.Ohkuri T, Sato M, Abe H, Tsuji K, Yamagishi Y, Ikeda H, Matsubara N, Kitamura H, Nishimura T. Identification of a novel NY-ESO-1 promiscuous helper epitope presented by multiple MHC class II molecules found frequently in the Japanese population. Cancer Sci. 2007;98:1092–1098. doi: 10.1111/j.1349-7006.2007.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuzaki J, Qian F, Luescher I, Lele S, Ritter G, Shrikant PA, Gnjatic S, Old LJ, Odunsi K. Recognition of naturally processed and ovarian cancer reactive CD8+ T cell epitopes within a promiscuous HLA class II T-helper region of NY-ESO-1. Cancer Immunol Immunother. 2008;57:1185–1195. doi: 10.1007/s00262-008-0450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen WF. Identification and characterization of human hepatocellular carcinoma-associated antigens [abstract].; Cancer Immun; 2005. 21. [Google Scholar]
- 28.Uenaka A, Ono T, Akisawa T, Wada H, Yasuda T, Nakayama E. Identification of a unique antigen peptide pRL1 on BALB/c RL male 1 leukemia recognized by cytotoxic T lymphocytes and its relation to the Akt oncogene. J Exp Med. 1994;180:1599–1607. doi: 10.1084/jem.180.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 30.Morishima S, Akatsuka Y, Nawa A, Kondo E, Kiyono T, Torikai H, Nakanishi T, Ito Y, Tsujimura K, Iwata K, Ito K, Kodera Y, Morishima Y, Kuzushima K, Takahashi T. Identification of an HLA-A24-restricted cytotoxic T lymphocyte epitope from human papillomavirus type-16 E6: the combined effects of bortezomib and interferon-gamma on the presentation of a cryptic epitope. Int J Cancer. 2007;120:594–604. doi: 10.1002/ijc.22312. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi H, Tanaka F, Ohta M, Inoue H, Mori M. Identification of HLA-A24-restricted CTL epitope from cancer-testis antigen, NY-ESO-1, and induction of a specific antitumor immune response. Clin Cancer Res. 2004;10:890–896. doi: 10.1158/1078-0432.ccr-1086-3. [DOI] [PubMed] [Google Scholar]
- 32.Wang RF, Johnston SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA. A breast and melanoma-shared tumor antigen: T cell responses to antigenic peptides translated from different open reading frames. J Immunol. 1998;161:3598–3606. [PubMed] [Google Scholar]
- 33.Jäger E, Karbach J, Gnjatic S, Jäger D, Maeurer M, Atmaca A, Arand M, Skipper J, Stokert E, Chen YT, Old LJ, Knuth A. Identification of a naturally processed NY-ESO-1 peptide recognized by CD8+ T cells in the context of HLA-B51. Cancer Immun. 2002;2:12. http://www.cancerimmunity.org/v2p12/020812.htm [PubMed] [Google Scholar]
- 34.Tsukahara T, Kawaguchi S, Torigoe T, Asanuma H, Nakazawa E, Shimozawa K, Nabeta Y, Kimura S, Kaya M, Nagoya S, Wada T, Yamashita T, Sato N. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006;97:1374–1380. doi: 10.1111/j.1349-7006.2006.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Materials and methods
Patient clinical course
Patient GO is a 71-year-old Japanese man with a lung adenocarcinoma. His clinical course from November 2004 to September 2007 was reported previously (10). Briefly, in November 2004, a right hilar mass and multiple pleural dissemination of the tumor were found. Pathological diagnosis based on specimens of the pleural tumors was poorly differentiated adenocarcinoma. Surprisingly, by March 2005, the pleural dissemination disappeared without any treatment. In February 2006, the right hilar mass increased in size. In November 2007, a new lesion appeared in the right lower lobe which was noticed as interstitial thickening by chest computed tomography. It appeared to be a recurrence of the primary tumor which had disappeared before his first visit to the hospital. Chemotherapy (carboplatin and gemcitabine, 4 cycles) was started in November 2007, but the tumor gradually increased in size and pleural effusion appeared. Second line chemotherapy (gemcitabine, 2 cycles) was started in March 2009. No other metastatic lesion was observed as of April 2009.
Blood samples
Peripheral blood was drawn from the patient with informed consent. Peripheral blood mononuclear cells (PBMCs) and plasma were isolated by density gradient centrifugation using Histo-Paque 1077 (Sigma-Aldrich, St. Louis, MO). CD4 and CD8 T cells were purified from PBMCs using CD4 and CD8 microbeads, respectively, with a large scale column and a magnetic device (Miltenyi Biotec, Auburn, CA). The residual cells were used as CD4- and CD8-depleted cells. The cells were stored in liquid N2 until use. HLA typing of PBMCs was done using a sequence-specific oligonucleotide probe and sequence-specific priming of genomic DNA using standard procedures.
Cell lines
EBV-B cells were generated from CD19+ peripheral blood B cells using the culture supernatant from the EBV-producing B95-8 cells. A melanoma cell line M-1 was established from the surgically resected primary tumor.
Peptides
The following series of 28 overlapping 18-mer peptides spanning the NY-ESO-1 protein were synthesized: 18.1 (1-18), 18.2 (7-24), 18.3 (13-30), 18.4 (19-36), 18.5 (25-42), 18.6 (31-48), 18.7 (37-54), 18.8 (43-60), 18.9 (49-66), 18.10 (55-72), 18.11 (61-78), 18.12 (67-84), 18.13 (73-90), 18.14 (79-96), 18.15 (85-102), 18.16 (91-108), 18.17 (97-114), 18.18 (103-120), 18.19 (109-126), 18.20 (115-132), 18.21 (121-138), 18.22 (127-144), 18.23 (133-150), 18.24 (139-156), 18.25 (145-162), 18.26 (149-166), 18.27 (153-170) and 18.28 (156-173). Nine 30-mer peptides spanning the protein were also synthesized: 30.1 (1-30), 30.2 (21-50), 30.3 (41-70), 30.4 (61-90), 30.5 (81-110), 30.6 (101-130), 30.7 (121-150), 30.8 (141-170) and 30.9 (151-180). These peptides were synthesized using standard solid-phase methods based on N-(9-fluorenyl)-methoxycarbonyl (Fmoc) chemistry on an ABIMED Multiple Peptide Synthesizer (AMS422, ABIMED, Langenfeld, Germany) at Okayama University (Okayama, Japan).
Recombinant proteins
Recombinant NY-ESO-1 (1) and RL-Akt (28) proteins were prepared as described previously. cDNAs for NY-ESO-1 and RL-Akt were cloned into the SphI/SalI and BamHI/SphI sites of the pQE-30 vector. N-His tagged protein was purified by nickel-ion affinity chromatography under denaturing conditions.
Preparation of mRNA
The NY-ESO-1 plasmid was linearized with the restriction enzyme NdeI, transcribed in vitro using T7 polymerase (mMESSAGE mMACHINE™ T7 Kit, Ambion, Austin, TX) and polyadenylated using poly (A) polymerase [Poly (A) Tailing kit, Ambion] according to the manufacturer's instructions. The capped and tailed RNA was resuspended in water and stored at -80˚C before use.
Electroporation of mRNA
The cells (approx. 5 x 106) in X-VIVO 20 medium (100 µl) and mRNA (20 µg) were mixed and transferred to a 2-mm gap cuvette (BTX Genetronics, San Diego, CA) and electroporated using a BTX 830 square wave electroporator. The cells were immediately suspended in X-VIVO 20 medium (2 ml) and cultured for 18 h to 24 h in 24-well plates at 37˚C in a 5% CO2 atmosphere.
Antibody ELISA
Antibody responses to NY-ESO-1 protein or synthetic peptides were evaluated by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (19). Recombinant protein (1 µg/ml) or peptide (5 µg/ml) in a coating buffer (15 mM Na2CO3, 30 mM NaHCO3, pH 9.6) was adsorbed onto 96-well Polysorp immunoplates (Nunc, Roskilde, Denmark) and incubated overnight at 4˚C. Plates were washed with PBS and blocked with 200 µl/well of 5% FCS/PBS for 1 h at room temperature. After washing, 100 µl of serially diluted serum was added to each well and the plates incubated for 2 h at room temperature. After extensive washing, goat anti-human IgG (Medical & Biological Laboratories, Nagoya, Japan) or mouse anti-human IgM, IgG1, IgG2, IgG3 or IgG4-HRP (Southern Biotechnologies, Birmingham, AL) was added to the wells as a second antibody, and the plates were incubated for 1 h at room temperature. After washing, signals were developed with 100 µl per well of 0.03% o-phenylene diamine dihydrochloride (OPDA, Wako, Osaka, Japan), 0.02% hydrogen peroxide and 0.15 M citrate buffer, and the absorbance at 490 nm was read using an ELISA reader (Benchmark Microplate Reader; Bio-Rad, Hercules, CA). Recombinant RL-Akt (28) was used as control protein.
IFNγ ELISA
CD4 T cells (1 x 104) and paraformaldehyde (PFA) (0.2%)-treated EBV-B cells (1 x 104) pre-pulsed with the peptides or pretreated with the protein were cultured in a 96-well round-bottomed culture plate at 37ºC in a 5% CO2 atmosphere. After 18 h, culture supernatants were collected and the amount of IFNγ was measured by sandwich ELISA.
TGF-β1 ELISA
Serum TGF-β1 was estimated by DuoSet Sandwich ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The serum was treated with activation reagent for 10 min at room temperature, followed by addition of a neutralization reagent. Treated samples were transferred to ELISA plates coated with a capture antibody. Recombinant human TGF-β1 was used as a standard.
In vitro stimulation of CD4 and CD8 T cells
Frozen cells were thawed and resuspended in AIM-V medium (Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated pooled human serum (CM), and kept at 37˚C in a 5% CO2 atmosphere for 2 h. CD4 and CD8 T cells (2 x 106) were cultured with irradiated (40 Gy) autologous CD4- and CD8-depleted PBMCs (2 x 106) as antigen-presenting cells (APCs) in the presence of 28 overlapping 18-mer peptides and a 30-mer C-terminal peptide (OLPs) spanning the entire NY-ESO-1 protein (1 µg /ml for each peptide) in 2 ml of CM supplemented with 10 units/ml rIL-2 (Takeda Chemical Industries, Osaka, Japan) and 10 ng/ml rIL-7 (Peprotech, London, UK) in a 24-well culture plate at 37˚C in a 5% CO2 atmosphere for 12 days. For the second stimulation, 1 x 106 instead of 2 x 106 responder cells were used in the culture described above.
IFNγ secretion assay
Responder CD4 or CD8 T cells (1 x 105) were stimulated with paraformaldehyde (PFA) (0.2%)-treated autologous EBV-B cells (1 x 105) pre-pulsed with the peptides for 30 min. The cells were then washed and suspended in 100 µl of RPMI medium, and treated with bi-specific CD45 and IFNγ antibody (IFNγ catch reagent) (2 µl) for 5 min on ice. The cells were then diluted in AIM-V medium (1 ml) and placed on a slow rotating device (Miltenyi Biotec) to allow IFNγ secretion at 37ºC in a 5% CO2 atmosphere. After incubation for 50 min, the cells were washed with cold buffer and treated with PE-conjugated anti-IFNγ (detection reagent), and FITC-conjugated anti-CD4 or anti-CD8 mAb. After incubation for 10 min at 4ºC, the cells were washed and analyzed with a FACS Calibur (Becton Dickinson).
Tetramer construction and staining
HLA-peptide tetramers were produced as described previously (29, 30). A*0206 (27), A24 (31), A31 (32) and B51 (33) tetramers were used. For staining, cells were incubated with tetramer at a concentration of 20 µg/ml for 15 min at room temperature, followed by incubation with FITC-conjugated anti-CD8 mAb (Miltenyi Biotec) on ice for 15 min and analyzed with a FACS Calibur (Becton Dickinson).
Intracellular Foxp3 staining
Triple staining was carried out using monoclonal antibodies (mAb). Staining with CD4 mAb (eBioscience, San Diego, CA) and CD25 mAb (Becton Dickinson) was performed according to the manufacturer's instructions. Intracellular Foxp3 staining using clone PC101 (eBioscience) was carried out using the Foxp3 Staining Buffer Set (eBioscience). The analysis was carried out on a FACS Calibur (Becton Dickinson).
Immunohistochemistry (IHC)
IHC was performed as described (4). E978 (4) and EMR8-5 (Funakoshi, Tokyo, Japan) (34) mAbs were used for the analysis of NY-ESO-1 and HLA class I expression, respectively.