Abstract
The cytotoxic T lymphocyte antigen-4 (CTLA-4) molecule on T cells acts to maintain homeostasis by regulating the proliferation of recently activated T cells. Blockade of CTLA-4 by anti-CTLA-4 antibody enhances T cell responses and has elicited significant tumor regression in some cancer patients. Clinical trials are ongoing to investigate the efficacy of anti-CTLA-4 antibody as a cancer therapeutic. Reports from several clinical trials have documented the occurrence of adverse events in patients treated with anti-CTLA-4 antibody which have some similarities with autoimmune conditions and have been termed immune-related adverse events (irAEs). Most irAEs are reversible with corticosteroid therapy. Some investigators suggest that irAEs occur in the same patients who have anti-tumor responses as a result of the anti-CTLA-4 antibody. Immunologic mechanisms to explain why irAEs occur in some patients have not been reported. Here we report that bladder cancer patients treated with anti-CTLA-4 antibody have increased levels of the Th1 cytokine IFN-γ detected in plasma samples. Although IFN-γ is a potent anti-tumor and inflammatory cytokine, increased levels of IFN-γ were not associated with irAEs in our patients. However, in one patient who experienced an irAE consisting of ischemic papillopathy and optic neuritis, we documented high pre-therapy levels of the Th2 cytokine IL-10 which decreased after treatment with anti-CTLA-4 antibody. The decrease in plasma IL-10 concentration coincided with the patient's irAE. We propose that decreased levels of IL-10 after treatment with anti-CTLA-4 therapy may be responsible for irAEs in some patients and needs to be further investigated in larger studies.
Keywords: clinical trial, bladder cancer, ipilimumab, IL-10, adverse event
Introduction
T cell responses are initiated by antigen receptor stimulation but are regulated by several intrinsic and extrinsic regulatory circuits to ensure an effective immune response to pathogens while minimizing damage from attack to self-antigens. Some of the pathways involved in the former can frustrate effective responses to cancer. Optimal T cell activation requires signals to be delivered through the T cell receptor (TCR) and costimulatory molecules, such as CD28 (1, 2). CD28 ligation on antigen-inexperienced T cells by its receptors B7-1 and B7-2 plays a crucial role in initial T cell priming (3-5). However, CD28-mediated T cell expansion is opposed by cytolytic T lymphocyte-associated antigen 4 (CTLA-4), which also binds B7-1 and B7-2 and functions to attenuate the T cell proliferation of recently activated T cells (6-8). Blockade of the inhibitory signals mediated by CTLA-4 has been shown to enhance T cell responses and induce tumor rejection in a number of animal models (9, 10). A monoclonal antibody to human CTLA-4 has been found to elicit objective responses in clinical trials (11-17) and is a promising new immunotherapeutic agent for the treatment of cancer patients.
Treatment with anti-CTLA-4 antibody has been associated with complete and partial tumor regression in some patients (11-17). Anti-CTLA-4 therapy has also been associated with toxicities referred to as immune-related adverse events (irAEs) (11-17). The reported irAEs encompass inflammatory conditions such as dermatitis, colitis, hepatitis, uveitis, and hypophysitis. Molecular mechanisms to explain the occurrence of anti-tumor responses or irAEs seen in some patients are currently under investigation.
We recently found that anti-CTLA-4 therapy led to increased expression of the T cell molecule known as inducible costimulator (ICOS) on CD4 T cells (18). CD4+ ICOShi T cells from treated patients had greater production of the Th1 cytokine interferon-gamma (IFN-γ) as opposed to the Th2 cytokine interleukin-10 (IL-10), which has previously been linked to ICOS-expressing T cells (19). A number of studies have shown that successful anti-tumor responses were associated with the production of IFN-γ (20-22) and tumor rejection was compromised in mice that lack the receptor for IFN-γ (23). In contrast, IL-10 has been associated with immunoregulatory mechanisms and can be produced by regulatory T cells, thus leading to suppression of effector T cell responses (24-26).
Here, we report that bladder cancer patients treated with anti-CTLA-4 therapy on a pre-surgical clinical trial had measurable increases in plasma concentrations of IFN-γ. We further demonstrate that one patient had decreased IL-10 concentration after treatment with anti-CTLA-4 therapy, which was associated with an irAE consisting of ischemic papillopathy with subsequent optic nerve atrophy. This is the first report of ischemic papillopathy likely due to anti-CTLA-4 therapy. This is also the first report of biological mechanisms and measurable markers from plasma samples that can potentially be used to monitor patients who may experience anti-tumor responses, possibly due to increased IFN-γ levels, and those who may experience irAEs, possibly due to decreased IL-10 levels.
Results
Increased IFN-γ levels in patients treated with anti-CTLA-4 antibody
We are currently accruing bladder cancer patients onto a pre-surgical clinical trial wherein 6 patients will receive 2 doses of anti-CTLA-4 antibody at 3 mg/kg per dose and then proceed to surgery as show in Figure 1. To date, 4 patients have completed all treatments and follow-up visits as per protocol.
Figure 1.
Clinical trial schema. Bladder cancer patients are treated with 2 doses of anti-CTLA-4 antibody at 3 mg/kg at study weeks 0 and 3 prior to undergoing surgery at study week 7 and post-operative follow-up visits at study weeks 11-15 and 23-24. Blood is collected as: pre-therapy samples prior to administration of the first dose of antibody at study week 0; after dose #1 at study week 3 prior to administration of dose #2; after dose #2 at study week 7 prior to surgery; and at each post-op visit.
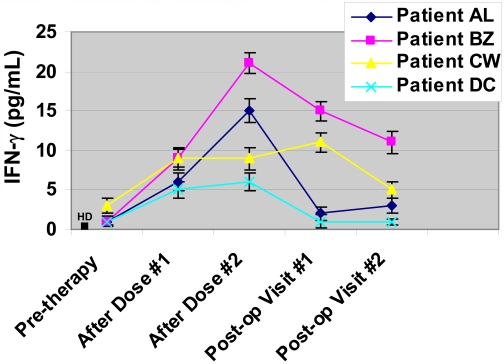
Plasma samples obtained throughout the protocol were assessed for two known Th1 cytokines, IL-2 and IFN-γ. IL-2 levels were low at approximately 1-2 pg/mL in healthy donors (n = 10) and pre-therapy plasma samples from treated patients, without any detectable difference noted after treatment with anti-CTLA-4 antibody (data not shown). However, IFN-γ was noted to increase in plasma samples from all 4 treated patients (Figure 2). All 4 patients had at least a 3-fold increase in IFN-γ concentrations after treatment with anti-CTLA-4 antibody as compared to pre-therapy levels. IFN-γ concentrations increased after the first and second doses of anti-CTLA-4 antibody. Healthy donors (n = 10) had very low levels of IFN-γ detected in their plasma samples, which were consistent with pre-therapy levels measured for the bladder cancer patients. IFN-γ concentrations tended to decrease in the absence of further anti-CTLA-4 therapy and each patient had lower levels of IFN-γ detected in plasma samples obtained post-operatively.
Figure 2.
Increased plasma concentrations of IFN-γ detected after administration of anti-CTLA-4 antibody. All 4 patients analyzed thus far had elevated levels of IFN-γ detected in plasma samples collected after the first and second doses of anti-CTLA-4 antibody as compared to pre-therapy levels. IFN-γ levels decreased in the post-operative setting consistent with no further administration of antibody. Healthy donors (HD, n = 10) were found to have low concentrations of plasma IFN-γ similar to the pre-therapy values seen in the bladder cancer patients.
Decreased IL-10 levels in one patient treated with anti-CTLA-4 antibody
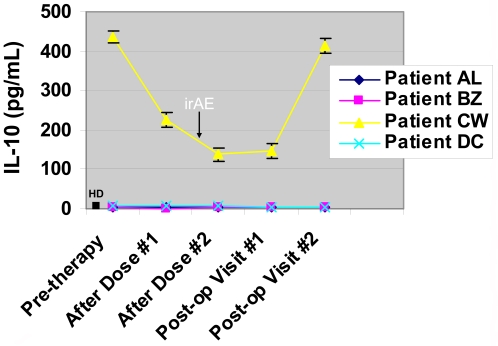
Plasma samples from all patients were also assessed for two known Th2 cytokines, IL-4 and IL-10. IL-4 levels were noted to be low, at approximately less than and up to 1 pg/mL in healthy donors and both pre- and post-therapy patient samples (data not shown). Similarly, IL-10 was found to be low at approximately 2-4 pg/mL in all healthy donors and both pre and post-therapy patient samples except for one patient (patient CW, Figure 3). Patient CW was found to have a high pre-therapy plasma concentration of IL-10 of approximately 436 pg/mL. IL-10 levels in this patient decreased to about 225 pg/mL after the first dose of anti-CTLA-4 antibody and then to approximately 139 pg/mL after the second dose of antibody. The patient continued to have decreased levels of IL-10 (148 pg/mL) in the post-operative setting even after discontinuation of anti-CTLA-4 antibody. Eventually the IL-10 concentration increased to 415 pg/mL but, during the time that patient CW experienced decreased levels of plasma IL-10 he developed a serious irAE consisting of ischemic papillopathy with subsequent optic nerve atrophy.
Figure 3.
Decreased plasma concentration of IL-10 in one patient after treatment with anti-CTLA-4 antibody. Patients treated with anti-CTLA-4 antibody were found to have low levels of plasma IL-10 concentrations, similar to values seen for healthy donors (HD, n = 10), except for one patient (patient CW). Patient CW had a high pre-therapy concentration of plasma IL-10 which decreased after treatment with anti-CTLA-4 antibody and then increased again in the post-operative setting which lacks anti-CTLA-4 antibody administration. Patient CW's immune-related adverse event (irAE) coincided with the decrease in IL-10 levels (black arrow).
Anterior uveitis, followed by ischemic papillopathy, in a patient treated with anti-CTLA-4 antibody
Patient CW was enrolled onto the anti-CTLA4 clinical trial after appropriate informed consent was obtained. The patient is a 72-year old male with type II diabetes that was previously controlled with oral medications. The patient's medical history is also significant for hypertension, hypercholesterolemia, and gastroesophageal disease reflux disease with associated Barrett's esophagus. The patient had recurrent localized bladder cancer despite prior treatment with intravesical bacille Calmette-Guérin (BCG) therapy and therefore was recommended to undergo radical cystoprostatectomy surgery as treatment for his disease. The patient's surgical history consists of prior knee replacement, laparoscopic cholecystectomy, hemorrhoid surgery, and prior transurethral resections of bladder tumors. The patient's family history is significant for a sister with a known autoimmune disease, systemic lupus erythematous (SLE). The patient was treated with anti-CTLA-4 antibody as per protocol on study weeks 0 and 3, prior to radical cystoprostatectomy surgery at study week 7 (Table 1).
Table 1.
Documented events for patient CW while enrolled on a clinical trial protocol consisting of treatment with anti-CTLA-4 antibody.
One day prior to surgery the patient noticed a 'conjunctivitis-like' reaction in both eyes. He had difficulty opening his eyes, photophobia and soreness. On examination best corrected visual acuity was 20/25 +2 in the right eye and 20/25 +3 in the left eye. Color vision was normal and slit lamp examination of the anterior chamber revealed a severe (4+) iritis. Posterior segment exam revealed few cells in the anterior vitreous and normal optic discs. Visual fields by automated perimetry were also normal. Baseline visual fields and nerve fiber analysis of the optic nerve head was within the normal range. The patient was diagnosed with bilateral uveitis and he was treated with prednisolone acetate 1% steroid eye drops hourly. The eye drops were gradually tapered to 3 times a day, approximately every 8 hours, with improvement in vision and successful treatment of the uveitis.
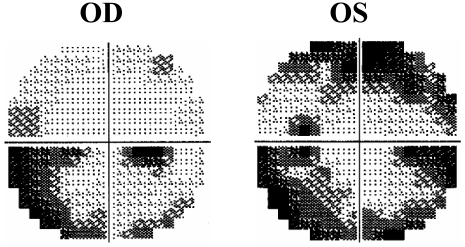
The patient then noticed a subacute worsening in vision for which he saw a retinal surgeon in his hometown and was diagnosed as having 'optic neuritis and retinal edema'. His local physician administered intravenous (i.v.) dexamethasone 20 mg immediately after speaking with physicians at our center. The patient was transferred to our center and continued to receive steroids i.v. consisting of methylprednisolone 250 mg every 6 hours. His vision at the time of hospital admission was 20/25 in the right eye and 20/200 in the left. There was a subtle left afferent pupillary defect. He now had significant central and peripheral visual field defects. There was no uveitis. The optic nerve heads were moderately swollen with multiple cotton wool spots on the nasal aspects of the disc bilaterally giving these regions a whitish appearance while the rest of the nerve was erythematous. An area of superficial retinal whitening suggesting ischemia was seen in the fovea, with the rest of the macula and periphery being normal. A fluorescein angiography was done which did not show any choroidal or macular ischemia. Optical coherence tomography (OCT) showed grossly increased nerve thickness due to disc edema. Additional testing showed bilateral nerve fiber loss in superonasal and inferotemporal quadrants of the nerve head when compared to baseline. Nerve fiber loss increased over the next 3 weeks. Humphrey visual field (HVF) was worse in the left eye and more so in the superior field. Both eyes demonstrated a cecocentral loss mapped on the 10-2 HVF, with peripheral superior and inferior defects in an arcuate manner on 30-2 HVF (Figure 4). An infectious cause was not suspected for the patient's visual impairment as the patient was afebrile and had no symptoms suggestive of infectious etiology for his visual impairment. Fluorescent treponemal antibody (FTA) was reactive despite no history or clinical features of syphilis and this reverted back to non-reactive several weeks later. Temporal arteritis was suspected although temporal arteries were pulsatile and non-tender. The patient denied headache, jaw claudication, polymyalgias and amaurotic events. The patient had a temporal artery biopsy several weeks after being on steroids and this was negative for inflammatory changes. Additional studies including magnetic resonance imaging (MRI) of the brain and orbits were normal.
Figure 4.
Visual examination of patient CW. Humphrey's visual field 30-2 (study week 13) of the right eye (OD) with an inferior arcuate scotoma and of the left eye (OS), with both superior and inferior arcuate scotomas.
The patient had a fluctuating course as outlined in Table 1. He is still being followed and his eye exam at study week 24 showed some improvement in his vision. His most recent exam documented continued improvement in visual fields and vision of 20/20 in the right and 20/50 in the left eye. The patient reported improved vision at his most recent clinic visit with improved ability to read and drive. Systemic steroid therapy consisting of prednisone is being tapered with addition of low-dose methotrexate.
Thus, coinciding with decreased plasma concentrations of IL-10 after administration of anti-CTLA-4 antibody, patient CW developed bilateral severe anterior uveitis which was treated successfully and soon after developed bilateral ischemic papillopathy. The patient's vision gradually improved but with some persistent visual field loss more in the left eye than the right eye.
The patient's laboratory value of elevated lipase, which suggested possible pancreatitis, as well as his initial complaint of blurry vision, which coincided with his diagnosis of uveitis and then ischemic papillopathy, correlated with decreased plasma levels of IL-10 as shown in Figure 3. The patient's plasma concentration of IL-10 was recently noted at week 24 to be increased again and similar to the pre-therapy value. The patient's visual findings also appear to be improving as of week 24.
Discussion
Anti-CTLA-4 antibody is a promising new cancer therapeutic that has been associated with tumor regression and improved clinical benefit in some patients with widespread metastatic disease (11-17). However, anti-CTLA-4 therapy has also been associated with irAEs including dermatitis, colitis, and as reported here for the first time, ischemic papillopathy, which consists of inflammation within the small blood vessels associated with the optic nerve thus leading to optic nerve atrophy. There have been reports of anterior uveitis associated with optic nerve swelling and visual loss without mention of ischemic manifestations (27). Many irAEs associated with anti-CTLA-4 antibody can be managed with corticosteroid therapy. In order to improve the clinical benefits of this novel agent and minimize its toxicities, detailed investigations are necessary to provide clues regarding the molecular mechanisms responsible for tumor regression and irAEs induced by anti-CTLA-4 antibody. Here, we present the first data to suggest that decreased IL-10 levels may be associated with irAEs. It is possible that patients with high pre-therapy levels of IL-10 may not be suitable candidates for anti-CTLA-4 therapy; however, in patients with metastatic cancer where there is disease progression despite multiple different therapies, the potential benefits versus risks of anti-CTLA-4 antibody must be weighed and treatment instituted after consideration of each individual case by the treating physician. Patients with high pre-therapy levels of plasma IL-10 may need to be closely monitored for irAEs while on anti-CTLA-4 therapy and systemic corticosteroids should be considered for treatment of any ensuing irAE.
IL-10 is reported to have immunoregulatory functions and it is possible that in patients with indolent autoimmune-like conditions, high levels of IL-10 exist to suppress autoimmunity. Regulatory T cells identified as CD4+ FOXP3+ T cells have been reported to produce IL-10 as a way to suppress effector T cells (24-26). Patient CW had approximately 4% CD4+ FOXP3+ T cells measured in his pre-therapy sample, which was lower than the frequency of CD4+ FOXP3+ cells found in pre-therapy samples from patients AL (approx. 17%) and BZ (approx. 30%); however, patient DC was also noted to have low frequency of CD4+ FOXP3+ cells in his pre-therapy sample (approx. 7%) (data not shown). Therefore, frequency of CD4+ FOXP3+ T cells may play a role in determining the occurrence of irAEs in patients treated with anti-CTLA-4 therapy; however, this will have to be investigated in a larger cohort of treated patients. Despite having a low frequency of CD4+ FOXP3+ T cells in his pre-therapy blood sample, patient CW had high levels of IL-10 present in his pre-therapy plasma sample which may reflect the functional status of CD4+ FOXP3+ T cells or other cells within the immune system. There are likely to be several mechanisms responsible for irAEs in patients and these will need to be identified as we continue to study treated patients.
Our data also demonstrated increased IFN-γ levels in 4 treated patients. Interestingly patient BZ, who received anti-CTLA-4 antibody at study weeks 0 and 3, had elevated levels of plasma IFN-γ detected in his blood sample collected at week 11 even though this was 8 weeks after the last dose of antibody had been administered. All other patients had their blood samples drawn at week 7 to assess cytokine profiles after the second dose of antibody but patient BZ's week 7 visits were delayed to week 11 due to a non-drug related pre-operative cardiac intervention. The elevated IFN-γ levels detected in patient BZ was attributed to anti-CTLA-4 therapy but it may also have been related to the cardiac procedure undergone by the patient. Elevated IFN-γ levels may possibly be associated with irAEs; however, although all 4 patients had increased concentrations of IFN-γ, the only patient on our study that developed an irAE was patient CW who had decreased IL-10 levels. It is likely that IFN-γ may be associated with anti-tumor responses. This concept will have to be investigated in a larger clinical trial with anti-CTLA-4 antibody in the metastatic disease setting where clinical correlation can be assessed.
Our trial and the data reported here provide biological markers that can be used to further understand the clinical outcomes associated with anti-CTLA-4 therapy, thus leading to improvement in the efficacy and safety profile of this novel cancer therapeutic agent.
Abbreviations
- CTLA-4
cytotoxic T lymphocyte antigen-4
- irAEs
immune-related adverse events
Acknowledgements
The authors wish to acknowledge the entire Genitourinary Medical Oncology and Urology Departments at M. D. Anderson Cancer Center for helpful suggestions in the implementation of the neoadjuvant anti-CTLA-4 antibody clinical trial and management of all patients on the protocol. We also thank research nurses Brenda Moomey, Peggy Robichaux, and Dallas Williams for their assistance in conducting the clinical trial; Marla Johnson, Jason Love, Cherie Perez and Alana Bethea for their assistance in data management and protocol amendments; and Erin Horne and Loretta Patterson for coordinating patient visits and blood draws. The authors are also grateful to Drs. Rachel Humphrey, Axel Hoos and Ramy Ibrahim at Bristol-Myers Squibb for their enthusiastic support and guidance in designing and conducting the Ipilimumab neoadjuvant clinical trial.
This work was supported in part by: Physician-Scientist Program Award and Institutional Research Grant from The University of Texas M. D. Anderson Cancer Center (UTMDACC), Career Development Award from the American Society of Clinical Oncology, a Gillson Longenbaugh Foundation Award, a Carl C. Anderson, Sr. & Marie Jo Anderson Charitable Foundation Award, and a Clinical Investigator Award from the Cancer Research Institute (all to P.S.). Bristol-Myers Squibb sponsored and funded the clinical trial of neoadjuvant Ipilimumab for bladder cancer patients.
References
- 1.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 2.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 3.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signaling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 5.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 6.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 7.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing affects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression up activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 10.van Elas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anticytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodi FS, Mihm MC, Soiffer RJ, Haluksa FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, Topalian SL, Haworth LR, Levy C, Rosenberg SA, Sherry RM. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korman A, Yellin M, Keler T. Tumor immunotherapy: preclinical and clinical activity of anti-CTLA4 antibodies. Curr Opin Investig Drugs. 2005;6:582–591. [PubMed] [Google Scholar]
- 15.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. http://www.cancerimmunity.org/v8p1/080102.htm [PMC free article] [PubMed] [Google Scholar]
- 17.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T cell inhibition and application to cancer therapy. Immunological Rev. 2008 doi: 10.1111/j.1600-065X.2008.00649.x. in press. [DOI] [PubMed] [Google Scholar]
- 18.Liakou CI, Kamat A, Ng Tang D, Chen H, Sun J, Troncoso P, Logotehtis C, Sharma P. CTLA-4 blockade increases IFNγ-producing CD4(+)ICOS(hi) cells to shift the ratio of effector to regulatory T cells in cancer patients. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- 19.Lohning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 21.Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15:148–154. doi: 10.1016/s0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 22.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi S. Regulatory T cells: key controllers immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MR, Chan CC, Yang JC, Rubin BI, Gracia GJ, Sen HN, Csaky KG, Rosenberg SA. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother. 2004;27:478–479. doi: 10.1097/00002371-200411000-00008. [DOI] [PubMed] [Google Scholar]
Materials and methods
Patients
Bladder cancer patients with diagnoses of urothelial carcinoma who are candidates for radical cystectomy were consented on an Institutional Review Board (IRB)-approved clinical trial. The trial allows 6 patients to receive 2 doses of Ipilimumab anti-CTLA-4 antibody at 3 mg/kg prior to surgery, which is performed 4 weeks after the last dose of antibody. Ipilimumab is a fully human monoclonal immunoglobulin (IgG1) specific for human CTLA-4 (CD152). The antibody is given at a dose of 3 mg/kg each time, with a 3-week interval between doses. Blood was collected pre-therapy before the first dose, at week 3 prior to the second dose (post-therapy week 3) and at week 7 prior to surgery (post-therapy week 7). All patients were monitored for safety. This is an ongoing clinical trial and to date 4 of 6 patients have completed all treatments, including surgical removal of their bladders and post-operative follow-up visits. The results reported here reflect data obtained from all 4 treated patients. One patient, patient BZ, who received both doses of anti-CTLA-4 antibody at weeks 0 and 3, had a delay in blood collection and surgery such that his week 7 visits occurred at week 11 due to a non-drug related preoperative cardiac intervention. Healthy donor blood was obtained from volunteers (n = 10).
Cytokine analysis
Plasma from the blood samples obtained was analyzed using a Th1/Th2 Ultra Sensitive kit (Meso Scale Diagnostics [MSD], Gaithersburg, MD) to determine concentrations of IFN-γ, IL-2, IL-4 and IL-10. Analysis was performed according to the manufacturer's instructions. For plasma data, averages with standard deviations were calculated from three separate experiments with individual samples tested in triplicate wells for each experiment. The limit of detection for the assay is 0.3 pg/mL for IFN-γ and 0.5 pg/mL for IL-10.