Abstract
In vitro measures of immune responsiveness toward tumors provide relevant information regarding the prevention and metastatic potential of cancer. In addition, the compartmentalization of immune responses is likely to be an important factor in dictating host antitumor immune responses. We have previously demonstrated that injection of antibody against B cells diminished pulmonary antitumor defenses. In the current study, we determined the effect of B cells on antitumor cellular responses against a lung metastatic tumor, MADB106. Lung B cells displayed sustained surface expression of CD80 and CD86, as compared to spleen B cells, in the presence of MADB106 tumor. Removal of B cells from lung lymphocyte cultures resulted in diminished IFN-γ secretion and tumor lysis, whereas removal of B cells from spleen lymphocytes exposed to tumor resulted in elevated IFN-γ and increased tumor lysis. Furthermore, a correlative increase in CD80 and CD86 co-stimulatory molecule expression by lung B cells was observed in mice subjected to MADB106 tumor. These findings provide additional evidence of the importance of pulmonary B cell responses in tumor defenses.
Keywords: rats, lung, spleen, B lymphocytes, MADB106, cancer, immunologic cytotoxicity
Introduction
Immune-associated defense against tumor development can be considered a double-edged sword by promoting tumor resistance, as well as facilitating tumor escape mechanisms. NK cell and cytotoxic T lymphocyte (CTL) activation has historically been shown to play an important role in controlling the growth and metastatic spread of numerous tumor malignancies (1). Conversely, B cells and humoral responses supported by CD4+ adaptive immune responses have been shown to foster tumor survival. For example, Qin et al. (2) documented the negative impact of B cells on tumor resistance. Recently, using two NK-sensitive in vivo experimental rat tumor models, we found B cells participate in the defense against tumor development in the lung by demonstrating a direct relationship between higher levels of circulating B cells and tumor resistance, and also demonstrating that depletion of B cells correlated with increased lung tumor metastasis in a similar manner to that of NK cells (3, 4). Thus, the known complexities of tumor immune responses lend credence for investigations that may explain these conflicts.
Assessment of immune function, particularly cytolytic responses against tumor, has traditionally been carried out using cells obtained from either spleen or blood; this apparently is done because cells from these compartments are readily available and/or can be obtained in large number. However, the mechanism by which NK cells or CTLs directly destroys tumor cells is by release of granzyme and perforin to lyse the tumor cell, so that this interaction requires close physical proximity of the immune cell and the tumor cell. The vast majority of lung tumor studies have produced metastases by injecting tumor cells intravenously, resulting in lung tumor development. Given the mechanism by which NK and cytolytic cells control tumor, assessment of their activity in the target organ, or by lung lymphocytes, would seem to be required. For this reason, we assessed effects using lung lymphocytes, as these were potentially most relevant to surveillance of lung tumors, and compared effects with those seen using splenic lymphocytes as typically performed by measurement of tumor lytic function. Interestingly, in a search of over 300 citations related to immune-mediated tumor defenses spanning more than 30 years, we found only two studies that had obtained NK cells from, or examined the action of NK and cytolytic cells within, the compartment wherein the tumor target resided.
The results from the current study demonstrate that lung B cells displayed sustained CD80 and CD86 co-stimulatory molecular surface expression as compared to spleen B cells. Removal of B cells from lung lymphocytes resulted in diminished IFN-γ secretion and tumor lysis, whereas removal of B cells from spleen lymphocytes exposed to tumor resulted in elevated IFN-γ and increased tumor lysis. These findings corresponded with the contribution of marginal zone spleen B cells, which upon depletion led to higher levels of tumor lysis. Furthermore, a correlative increase in CD80 and CD86 co-stimulatory molecule expression by lung B cells was observed in mice subjected to MADB106 tumor. In total, the studies presented here establish the importance of the assessment of antitumor activity in the context of the tumor's microenvironment and confirm a potential role for B cells in supportive lung antitumor responses.
Results
Expression of CD80 and CD86 by lung and spleen B cells in response to MADB106 tumor
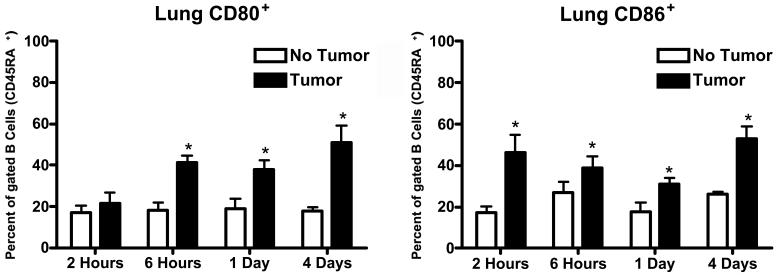
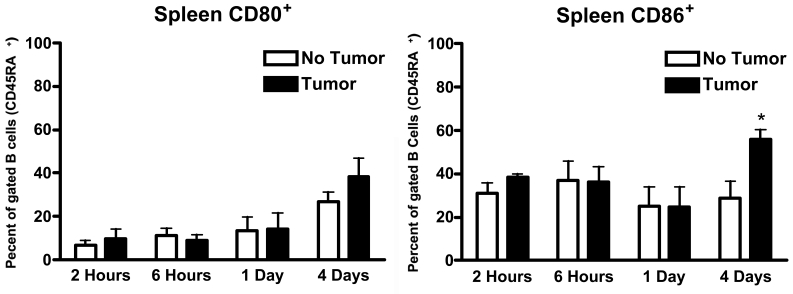
Figure 1 and Figure 2 show the expression of CD80 and CD86 molecules on the surface of B cells (CD45RA+) as the result of in vitro incubation with MADB106 tumor cells for varying periods of time. At the end of the incubation period, lymphocytes were collected and expression of CD80 and CD86 on the surface of B cells was determined by flow cytometry. As can be seen in Figure 1 and Figure 2, B cells taken from lung and from spleen displayed a different propensity to express CD80 and CD86 when incubated with MADB106 tumor cells. Lung B cells showed a statistically significant increase in the expression of CD80 following incubation with the tumor cells. This expression was seen fairly rapidly − it is evident after 6 hours of incubation - and was also present after incubation for 24 and 96 hours (Figure 1). Spleen B cells, in contrast, did not show increased expression of CD80 for all incubation periods tested up to 96 hours (Figure 2). Regarding expression of CD86, lung B cells showed a significant increase in expression of this cell surface molecule in response to MADB106 tumor cells for all incubation times assayed, starting with incubation for 2 hours. Interestingly, the expression of CD86 was clearly evident after 2 hours of incubation, then apparently declined somewhat, but was markedly seen again following 96 hours of incubation (Figure 1). Spleen B cells also showed tumor-induced expression of CD86, but this was seen only after 2 and 96 hours of incubation, and not with incubation for 6 and 24 hours (Figure 2). Thus, the expression of both CD80 and CD86 in response to tumor was more pronounced on B cells coming from lung than on B cells coming from spleen.
Figure 1.
Expression of CD80 and CD86 by lung B cells in response to MADB106 tumor. Total lung cells were incubated in the presence of MADB106 tumor cells. The expression of CD80 and CD86 on B cells (gated on CD45RA+ cells) was determined using flow cytometry. Vertical bars and error bars represent the mean ± SE (n = 6). Asterisks indicate statistical difference (P less than or equal to 0.05) in CD80 and CD86 molecule expression by lung B cells in the presence or absence of MADB106 tumor.
Figure 2.
Expression of CD80 and CD86 by spleen B cells in response to MADB106 tumor. Total spleen cells were incubated in the presence of MADB106 tumor cells. The expression of CD80 and CD86 on B cells (gated on CD45RA+ cells) was determined using flow cytometry. Vertical bars and error bars represent the mean ± SE (n = 6). Asterisks indicate statistical difference (P less than or equal to 0.05) in CD80 and CD86 molecule expression by spleen B cells in the presence or absence of MADB106 tumor.
Production of IFN-γ by lung and spleen lymphocytes in response to MADB106 tumor cells
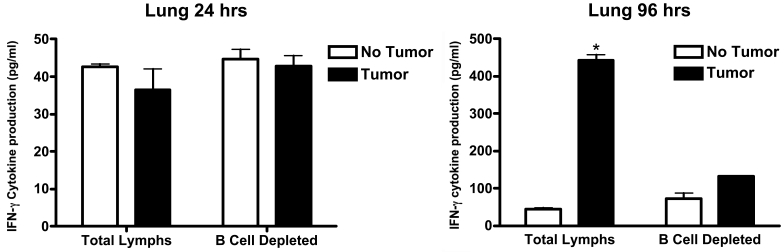
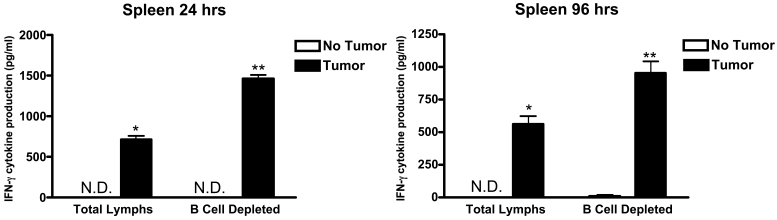
Figure 3 and Figure 4 show the production of IFN-γ that resulted when lymphocytes were incubated with MADB106 tumor cells for varying periods of time. The assay was carried out using undifferentiated (total) lung or spleen lymphocytes and also using lung or splenic lymphocytes from which B cells had been removed prior to incubation. At the end of the incubation period (2, 6, 24, or 96 hours), the amount of IFN-γ present in the supernatant was measured. After two or six hours of incubation, no IFN-γ was detected in the supernatant of either lung or spleen lymphocytes by ELISA, so no values for these two time periods are shown in Figure 3 and Figure 4. For lung lymphocytes, significant amounts of IFN-γ were detected in the supernatant after 24 hours of incubation, but (a) addition of tumor cells to the incubation was not observed to increase IFN-γ production, and (b) deletion of B cells from the total population of lymphocytes was also without effect. However, as shown in Figure 3, different effects were seen with lung lymphocytes at the end of 96 hours of incubation. At this time, first, IFN-γ was significantly increased by the presence of tumor cells and, second, removal of B cells from the lymphocyte population prevented this increase in IFN-γ from occurring. Turning to what occurred when spleen lymphocytes were used in the incubation, the findings were different. At the end of 24 hours of incubation of spleen lymphocytes, the presence of tumor cells was found to have markedly stimulated production of IFN-γ. Moreover, if spleen lymphocytes were removed from the total lymphocyte population, the amount of IFN-γ detected was significantly increased, thereby indicating that the presence of B cells in the spleen lymphocyte population acted to inhibit the production of IFN-γ stimulated by the presence of tumor cells (Figure 4). The same results were seen at the end of the 96-hour incubation − the presence of tumor cells significantly increased IFN-γ production and removal of B cells from the total lymphocyte population led to a larger increase in the amount of IFN-γ detected (Figure 4). In summary, MADB106 tumor cells increased production of IFN-γ when incubated with either lung or spleen lymphocytes in vitro, but the influence of B cells was opposite in lymphocytes from the two sources − for lung lymphocytes B cells were essential for tumor cell-induced stimulation of IFN-γ, while for spleen lymphocytes B cells inhibited tumor cell-induced IFN-γ production.
Figure 3.
Production of IFN-γ by lung lymphocytes in response to MADB106 tumor cells. Total lung lymphocytes were incubated in the presence of MADB106 tumor cells. At selected time intervals (2, 6, 24 and 96 h), the levels of IFN-γ cytokine were determined in culture supernatants. In addition, B cells were removed from total lung lymphocyte cultures. After 1 and 4 days of culture, supernatants were collected and the level of IFN-γ determined using ELISA techniques. Vertical bars and error bars represent the mean ± SE (n = 4). Asterisks indicate statistical differences (P less than or equal to 0.05) in the cytokine production by lung cells.
Figure 4.
Production of IFN-γ by spleen lymphocytes in response to MADB106 tumor cells. Total spleen lymphocytes were incubated in the presence of MADB106 tumor cells. At selected time intervals (2, 6, 24 and 96 h), the levels of IFN-γ cytokine were determined in culture supernatants. In addition, B cells were removed from total splenic lymphocyte cultures. After 1 and 4 days of culture, supernatants were collected and the level of IFN-γ determined using ELISA techniques. Vertical bars and error bars represent the mean ± SE (n = 4). N.D. indicates IFN-γ production was not detectable. An asterisk (*) indicates a statistical difference (P less than or equal to 0.05) in the cytokine production by spleen cells. Asterisks (**) indicate a statistical difference (P less than or equal to 0.05) in the cytokine production between total and B cell-depleted groups.
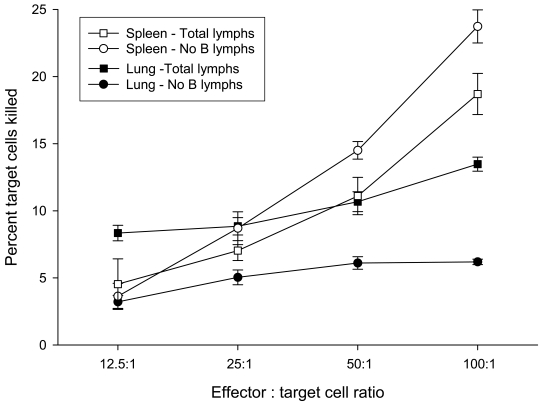
Lytic activity of lung and spleen lymphocytes against MADB106 tumor
Figure 5 shows the results from measuring in vitro tumoricidal activity of lung and spleen lymphocytes directed against MADB106 tumor cells. As with the previous measurement, this assay was carried out using undifferentiated (total) lung or spleen lymphocytes and also using lung or spleen lymphocytes from which B cells had been removed. Figure 5 shows the percentage of tumor cells killed as the ratio of effector to target cells was increased. As expected, in all cases the percentage of target cells killed increased as the number of effector cells employed in the assay increased (i.e., E:T ratio increased), thereby validating the assay procedure. The data shown in Figure 5 were analyzed by analysis of variance (ANOVA). To determine whether the presence of B cells affected lytic activity of lung or spleen lymphocytes, separate analyses were conducted for lung and for spleen lymphocytes with or without B cells present in the assay [each a two-way ANOVA − lymphocyte composition (total or B cells removed) x E:T ratios (12:5, 25, 50, 100:1)]. In both analyses, the main effect of lymphocyte composition was statistically significant (lung: F = 162.0, P < 0.001; spleen: F = 31.7, P < 0.02), which indicated that removal of B cells from the total lymphocyte population produced a significant change in the lytic activity of both lung and spleen lymphocytes. The main effect of E:T ratio was also significant in both analyses (lung: F = 18.6, P < 0.001; spleen: F = 328.6, P < 0.001), thus verifying that killing increased with increasing E:T ratio. But as can be seen clearly in Figure 5, the effect of removing B cells from lung and spleen lymphocytes was not the same − removing B cells from lung lymphocytes decreased killing while removing B cells from spleen lymphocytes increased killing. Whether this difference was statistically significant was tested by carrying out a three-way ANOVA, in which the lymphocyte source (lung or spleen) was added as a factor to the analysis described above (i.e., lymphocyte composition and E:T ratio being the other two factors). In this analysis, the interaction of lymphocyte source (lung and spleen) and lymphocyte composition (total and B cells removed) was highly significant (F = 168.6, P < 0.001), thereby confirming that the effect on target cell killing produced by the removal of B cells from lung lymphocytes and from spleen lymphocytes was significantly different. In summary, in vitro measurement of lytic activity directed against MADB106 target cells by lung and spleen lymphocytes revealed that the lytic activity of lymphocytes taken from lung was increased by the presence of B cells, whereas the lytic activity of lymphocytes taken from spleen was reduced by the presence of B cells.
Figure 5.
Lytic activity of lung and spleen lymphocytes against MADB106 tumor. The influence of B cells on tumor lysis was determined by comparing the percentage lysis of MADB106 tumor cells in the presence or absence of B cells. Total lymphocytes or lymphocytes depleted of B cells from lung and spleen tissues were incubated with viable fluorescently-labeled MADB106 tumor cells for 90 min. Tumor lysis was measured by propidium iodine (PI) staining of labeled MADB106 tumor cells. Percentage lysis was expressed as the percentage of fluorescent positive tumor cells staining positive for PI in the presence of lymphocyte cultures minus the percentage of PI-labeled tumor cells in the absence of lymphocyte cultures. The data shown were analyzed by analysis of variance (ANOVA). The main effect of E:T ratio was significant in both analyses (lung: F = 18.6, P < 0.001; spleen: F = 328.6, P < 0.001), thus verifying that killing increased as the E:T ratio increased. Separate analyses were conducted for lung and for spleen lymphocytes, with or without B cells, present in the assay [each a two-way ANOVA − lymphocyte composition (total or B cells removed) x E:T ratios (12:5, 25, 50, 100:1)]. In both analyses, the main effect of lymphocyte composition was statistically significant (lung: F = 162.0, P < 0.001; spleen: F = 31.7, P < 0.02). Data is expressed as the mean ± S.E. (n = 3) at a given effector:target (E:T) ratio.
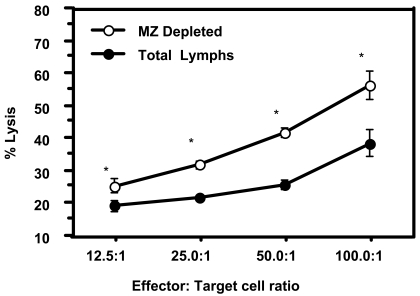
Marginal zone B cells influence spleen cell tumor lytic capacity
Previous studies have documented the presence of marginal (MZ) and follicular zone (FZ) B cell subpopulations within the spleen lymphoid compartment to have distinct functional properties in mediating immune responses (5). We therefore determined the tumoricidal effect of spleen lymphocytes when MZ B cells were removed as compared to the total spleen cell population. Figure 6 demonstrates that removal of MZ B cells from the spleen population results in a significant increase in the killing of MADB106 tumor cells. MZ B cells were not present in resident lung tissues (data not shown). These findings suggest a potential mechanism supporting the spleen's B cells' suppressive effect on antitumor cell responses.
Figure 6.
Marginal zone B cells influence spleen cell tumor lytic capacity. The influence of marginal zone (MZ) B cells on tumor lysis was determined by comparing the percentage lysis of MADB106 tumor cells in the presence or absence of MZ B cells. Total lymphocytes or lymphocytes depleted of MZ B cells from spleen were incubated with viable fluorescently-labeled MADB106 tumor cells for 90 min. Tumor lysis was measured by propidium iodine (PI) staining of labeled MADB106 tumor cells. Percentage lysis was expressed as the percentage of fluorescent positive tumor cells staining positive for PI in the presence of lymphocyte cultures minus the percentage of PI-labeled tumor cells without lymphocyte cultures. The data shown were analyzed by analysis of variance (ANOVA). Data is expressed as the mean ± S.E. (n = 3) at a given effector:target (E:T) ratio. Asterisks indicate statistical differences (P less than or equal to 0.05) compared between total and MZ B cell-depleted groups at each E:T ratio.
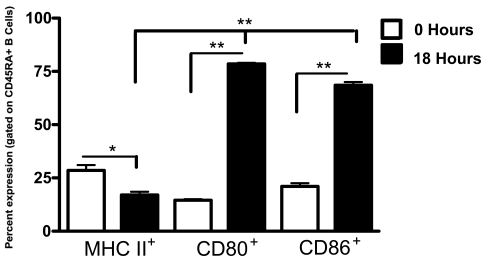
In vivo induction of CD80+ and CD86+ lung B cells in response to MADB106 tumor
To begin to confirm our in vitro findings, rats were administered 1 x 106 MADB106 tumor cells by intravenous injection. We determined the extent of MHC II, CD80 and CD86 expression by B cells isolated from the lungs 18 h following tumor injection. Significant (P less than or equal to 0.001) increases in both CD80 and CD86 co-stimulatory surface molecule expression were found on lung B cells of rats receiving MADB106 tumor cells (Figure 7). No significant differences in MHC II expression were observed.
Figure 7.
In vivo induction of CD80+ and CD86+ lung B cells in response to MADB106 tumor. B cells were purified from the lungs 18 h following tumor injection. We determined the percentage of B cells that expressed MHC II, CD80 and CD86 co-stimulatory surface molecules. The data shown were analyzed by analysis of variance (ANOVA). Data is expressed as the mean ± S.E. (n = 3). Asterisks indicate statistical differences (P less than or equal to 0.001) between rats receiving tumor or PBS sham injection.
Discussion
We have previously demonstrated that intravenous administration of antibody against NK cells had a significant effect on lung tumor clearance within hours after introduction of tumor. Interestingly, a similar effect was also found upon administration of antibody against B cells, suggesting that B cells may be involved in an antimetastatic effector function in the lung. At first glance, these findings seemed in stark contrast to earlier studies suggesting that B cells and other immune responses portray a counterprotective role against tumor development and metastasis (2, 6, 7).
In vitro experimentation, such as cytolytic assays and cytokine production, has been widely accepted as measures of immune competency. Surprisingly, few studies have addressed whether these measures can differ given the source of the cells and lead to misinterpretation of tumor defense properties related to a given in vivo experimental tumor model. Therefore, in light of our in vivo findings, the aim of this study was to determine the influence of B cells on cellular immune tumor lytic properties in the context of different microenvironments, i.e. spleen versus lung. Here we provide experimental evidence that lung B cells, but not spleen B cells, support antitumor effects.
In our initial study, co-culture of tumor cells with lung lymphocytes resulted in a selective rapid up-regulation of the co-stimulatory molecules CD80 and CD86 on lung B cells. In contrast, spleen B cells in the presence of spleen lymphocytes demonstrated an inconsistent induced expression of CD86 and no induction of CD80. The co-stimulatory cell surface signaling molecules expressed by immune cells are critical for the initiation and transduction of cellular immune responses. Specifically, CD80/CD86 molecules, which are usually expressed on B lymphocytes and other APCs (8-10), interact with their co-receptor CD28/CTLA-4 expressed primarily on the surface of T cells and serve as reliable measures of immune cellular activation (11-13). In fact, studies have shown that alterations in the expression of co-stimulatory molecules on lymphocytes play a relevant role in the early cellular responses involved in tumor surveillance (14, 15). Although the functional significance of the observed differences was not determined in the present study, the rapid increase in CD80 and CD86 expression by lung B cells as opposed to spleen B cells is consistent with a higher responsive state toward MADB106 tumor cells. These results correspond with prior studies reporting that differential induction of co-stimulatory signaling molecules, such as CD80 and CD86, result in specific functional responses by B cells (11, 12). Thus, it is plausible that the rapid responsiveness of lung B cells via co-stimulatory molecule up-regulation could account for the activation of NK cells and other mediators influencing tumor lytic activity.
The major action of NK cells and CTLs in the defense against tumor development is through the release of granzyme and perforin to lyse tumor cells. Preferences in cytokine secretion in response to tumor cells have been shown to play an important role in protective versus pathological responses associated with tumor development (16-18). IFN-γ secretion plays an proactive role in tumor defenses, having a strong impact on the cytolytic effects of NK cells and CTLs (19, 20). Thus, studies were performed to determine the impact that lung and spleen B cells had on IFN-γ production by lymphocytes in response to tumor, with the expectation that influences on IFN-γ secretion would predict tumor lytic activity. Consistent with this idea, the removal of lung B cells from the total lung lymphocyte culture reduced IFN-γ production in the presence of tumor and correlated directly with the up-regulation of CD80 and CD86. Conversely, variable expression of CD86 and the lack of CD80 expression by spleen B cells were correlated with a significant suppression of tumor-induced IFN-γ production.
Lastly, when measuring the effect of B cells on tumor lysis we were able to measure, within a 90 min time interval, the extent of such an effect. We showed that when B cells were removed from the total lung lymphocyte cultures, the degree of tumor killing was markedly reduced after 90 min of culture. In contrast, the absence of B cells among total spleen cultures resulted in elevated tumor killing. Indeed, inherent differences between lung and spleen environments exist. For example, the spleen, which serves as a major lymphoid tissue, is known to comprise two major subpopulations of B cells, the marginal (MZ) and follicular (FZ) zonal populations. Importantly, studies have demonstrated that these subsets have divergent functions in their ability to prime naïve T cells (5, 21). Our finding that MZ B cells were absent in the lung (data not shown) and that spleen MZ B cells were found to suppress tumor lysis suggest a potential mechanism supportive of the different antitumor actions between lung and spleen B cell compartments. In support, studies have shown B cells to be immunosuppressive during later stages of tumor development. One possibility may be an effect of the involvement of MZ B cells infiltrating local sites of tumor development. Thus, how B cells respond to tumor or other antigens is likely to be influenced by the context of its environment, as well as the stage of tumor development, whereby at least in the lung early B cell responses promote tumor defense and perhaps take on an immunosuppressive role during the progression of disease.
In an attempt to begin to define the activation status by B cells in vivo, we determined whether B cells responding in the lungs following tumor injection displayed higher levels of CD80 and CD86 co-stimulatory molecules as was demonstrated in our in vitro studies. Consistent with our in vitro findings, we demonstrated an increase in CD80 and CD86 co-stimulatory molecule expression by lung B cells within 18 h following tumor injection. These findings suggest that an elevation in the activation status of B cells does occur in response to in vivo MADB106 tumor responses and provide the impetus for further investigation into their functional role in tumor defense.
In conclusion, the protective role of B cells against lung tumor development can be surmised from B cell's support of IFN-γ production and their corresponding positive influence on tumor killing. It has been previously shown that B cells can induce IFN-γ cytokine production by NK cells (22). How lung B cells may be regulating IFN-γ production in response to MADB106 tumor cells remains to be determined. IL-12 is known to exert potent antitumor effects through the increase in IFN-γ produced by NK, NKT and Th1 cells (19, 23-25). Moreover, the induction of IL-12 or other cytokine mediators may be a result of cellular interactions involving ligand-receptor pair recognition, possibly through CD80/CD86 cell receptor expression. Our data, demonstrating in vivo up-regulation in CD80 and CD86 co-stimulatory molecule expression by B cells in the lung after tumor injection, provide evidence of a functional response to tumor. In fact, Luque et al. (26) described that the expression of CD80 and CD86 molecules by EBV-transformed human B cell lines could enhance cytotoxicity by human NK cells. Other studies have shown that the expression of CD80 and CD86 molecules by immune cells influence cell-mediated cytotoxicity (27-29). Further studies are needed to test whether cell-cell interactions and other mechanisms are involved in the lung B cells' antitumor effect.
In the early 1980s, the studies of Wiltrout and colleagues (30) were the first to directly measure lung antitumor NK cell responses using effector cells of the target organ, while others interpreted NK cell activity by measuring lung clearance of radio-labeled tumor cells or used effector cells from sites readily accessible for experimental evaluation (e.g. spleen and blood). These studies showed that the extent to which the levels of NK activity measured using effector cells from other sites were different and may not reflect the comprehensive antitumor effects within tumor target organs. The studies presented here reinforce this idea by determining that B cells present in lung may be an important contributor to the antimetastatic effects associated with NK and CTLs. Thus, we believe that our findings will have broad implications in the interpretation of potential outcomes in studies investigating tumor/immune interactions, shedding light on the divergent mechanisms involved in local and peripheral tumor surveillance.
Abbreviations
- MZ
marginal zone
Acknowledgements
The authors would like to extend thanks to Kathy Boss-Williams, Ph.D., Jeff Moore and Sandra Parks for their assistance in completion of this manuscript. We thank the laboratory members of Robert Donahoe, Ph.D., as well as Pamela Lankford-Turner for the use of the FACS apparatus. This work was supported by the National Institutes of Health Grant 5 RO1 CA87923-03.
References
- 1.Foss FM. Immunologic mechanisms of antitumor activity. Semin Oncol. 2002;29(3 Suppl 7):5–11. doi: 10.1053/sonc.2002.33076. [DOI] [PubMed] [Google Scholar]
- 2.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 3.Quan N, Zhang Z, Demetrikopoulos MK, Kitson RP, Chambers WH, Goldfarb RH, Weiss JM. Evidence for involvement of B lymphocytes in the surveillance of lung metastasis in the rat. Cancer Res. 1999;59:1080–1089. [PubMed] [Google Scholar]
- 4.Demetrikopoulos MK, Goldfarb RH, Zhang ZB, Weiss JM. Blood level of B and CD4+ lymphocytes measured before induction of an experimental tumor in rats predicts tumor progression and survival. Cancer Epidemiol Biomarkers Prev. 2000;9:609–617. [PubMed] [Google Scholar]
- 5.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 6.Schneider-Brachert W, Tchikov V, Merkel O, Jakob M, Hallas C, Kruse ML, Groitl P, Lehn A, Hildt E, Held-Feindt J, Dobner T, Kabelitz D, Kronke M, Schutze S. Inhibition of TNF receptor 1 internalization by adenovirus 14.7K as a novel immune escape mechanism. J Clin Invest. 2006;116:2901–2913. doi: 10.1172/JCI23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majima T, Ichikura T, Chochi K, Kawabata T, Tsujimoto H, Sugasawa H, Kuranaga N, Takayama E, Kinoshita M, Hiraide H, Seki S, Mochizuki H. Exploitation of interleukin-18 by gastric cancers for their growth and evasion of host immunity. Int J Cancer. 2006;118:388–395. doi: 10.1002/ijc.21334. [DOI] [PubMed] [Google Scholar]
- 8.Boussiotis VA, Freeman GJ, Gribben JG, Nadler LM. The role of B7-1/B7-2:CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol Rev. 1996;153:5–26. doi: 10.1111/j.1600-065x.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 9.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 10.Tamada K, Harada M, Okamoto T, Takenoyama M, Ito O, Matsuzaki G, Nomoto K. Specific antitumor activity of tumor-infiltrating lymphocytes expanded first in a culture with both anti-CD3 monoclonal antibody and activated B cells and then in a culture with interleukin-2. Cancer Immunol Immunother. 1995;41:339–347. doi: 10.1007/BF01526553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002;277:7766–7775. doi: 10.1074/jbc.M105902200. [DOI] [PubMed] [Google Scholar]
- 12.Terrazzano G, Zanzi D, Palomba C, Carbone E, Grimaldi S, Pisanti S, Fontana S, Zappacosta S, Ruggiero G. Differential involvement of CD40, CD80, and major histocompatibility complex class I molecules in cytotoxicity induction and interferon-gamma production by human natural killer effectors. J Leukoc Biol. 2002;72:305–311. [PubMed] [Google Scholar]
- 13.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 14.Rao KL, Varalakshmi C, Kumari AL, Khar A. Interaction between B.7 and CD28 costimulatory molecules is essential for the activation of effector function mediating spontaneous tumour regression. Scand J Immunol. 1999;49:633–640. doi: 10.1046/j.1365-3083.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim CW, Choi SH, Chung EJ, Lee MJ, Byun EK, Ryu MH, Bang YJ. Alteration of signal-transducing molecules and phenotypical characteristics in peripheral blood lymphocytes from gastric carcinoma patients. Pathobiology. 1999;67:123–128. doi: 10.1159/000028061. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Agnola C, Biragyn A. Clinical utilization of chemokines to combat cancer: the double-edged sword. Expert Rev Vaccines. 2007;6:267–283. doi: 10.1586/14760584.6.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar-Onfray F, Lopez MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18:171–182. doi: 10.1016/j.cytogfr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Z, Xu X, Zhang Y, Xing J, Long J, Gu L, Wang X, Sun D, Ka W, Yao W, Wen Z, Chien S. Tumor-derived factors impaired motility and immune functions of dendritic cells through derangement of biophysical characteristics and reorganization of cytoskeleton. Cell Motil Cytoskeleton. 2007;64:186–198. doi: 10.1002/cm.20175. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara K, Yoshinaga SK, Lanier LL. Inducible costimulator costimulates cytotoxic activity and IFN-gamma production in activated murine NK cells. J Immunol. 2002;169:3676–3685. doi: 10.4049/jimmunol.169.7.3676. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, Saiki I, Okumura K. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001;31:1720–1727. [PubMed] [Google Scholar]
- 21.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 22.Michael A, Hackett JJ, Bennett M, Kumar V, Yuan D. Regulation of B lymphocytes by natural killer cells. Role of IFN-gamma. J Immunol. 1989;142:1095–1101. [PubMed] [Google Scholar]
- 23.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–1733. [PubMed] [Google Scholar]
- 24.Kelly JM, Takeda K, Darcy PK, Yagita H, Smyth MJ. A role for IFN-gamma in primary and secondary immunity generated by NK cell-sensitive tumor-expressing CD80 in vivo. J Immunol. 2002;168:4472–4479. doi: 10.4049/jimmunol.168.9.4472. [DOI] [PubMed] [Google Scholar]
- 25.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 26.Luque I, Reyburn H, Strominger JL. Expression of the CD80 and CD86 molecules enhances cytotoxicity by human natural killer cells. Hum Immunol. 2000;61:721–728. doi: 10.1016/s0198-8859(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 27.Chambers BJ, Salcedo M, Ljunggren HG. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 28.Geldhof AB, Raes G, Bakkus M, Devos S, Thielemans K, De Baetselier P. Expression of B7-1 by highly metastatic mouse T lymphomas induces optimal natural killer cell-mediated cytotoxicity. Cancer Res. 1995;55:2730–2733. [PubMed] [Google Scholar]
- 29.Yeh KY, Pulaski BA, Woods ML, McAdam AJ, Gaspari AA, Frelinger JG, Lord EM. B7-1 enhances natural killer cell-mediated cytotoxicity and inhibits tumor growth of a poorly immunogenic murine carcinoma. Cell Immunol. 1995;165:217–224. doi: 10.1006/cimm.1995.1208. [DOI] [PubMed] [Google Scholar]
- 30.Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, Reynolds CW. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985;134:2783–2789. [PubMed] [Google Scholar]
- 31.Simecka JW, Patel P, Davis JK, Ross SE, Otwell P, Cassell GH. Specific and nonspecific antibody responses in different segments of the respiratory tract in rats infected with Mycoplasma pulmonis. Infect Immun. 1991;59:3715–3721. doi: 10.1128/iai.59.10.3715-3721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones HP, Hodge LM, Fujihashi K, Kiyono H, McGhee JR, Simecka JW. The pulmonary environment promotes Th2 cell responses after nasal-pulmonary immunization with antigen alone, but Th1 responses are induced during instances of intense immune stimulation. J Immunol. 2001;167:4518–4526. doi: 10.4049/jimmunol.167.8.4518. [DOI] [PubMed] [Google Scholar]
- 33.Kruisbeek AM. Introduction: regulation of T cell development by the thymic microenvironment. Semin Immunol. 1999;11:1–2. doi: 10.1006/smim.1998.0161. [DOI] [PubMed] [Google Scholar]
Materials and methods
Animals
Male and female Fischer 344 rats (Charles River) at least six months of age were used in this study. Rats were housed in microisolator cages under positive-pressure ventilation. The animals were given food and water ad libitum. All experiments were performed in compliance with the University's institutional committee for the use of animals (IACUC).
Lymphocyte isolation
Cells were isolated from the lungs as described previously (31, 32). Briefly, lungs were perfused with sterile PBS without magnesium or calcium to minimize contaminating blood cells. The lungs were separated into individual lobes and finely minced. Minced tissue was suspended in RPMI 1640 (Gibco, Invitrogen, Corp., Carlsbad, CA) medium containing 300 U/ml Clostridium histolyticum type I collagenase (Worthington Biochemical, Freehold, NJ), 50 U/ml DNase (Sigma, St. Louis, MO), 10% FBS (Gibco), HEPES, and antibiotic/antimytotic solution (Sigma) and incubated at 37˚C for 90-120 min. After incubation, the digestion mixture was passed through a 250 µm nylon mesh filter to remove undigested tissue. Cell types were purified from cell suspensions by density gradient centrifugation using Lympholyte Rat (Accurate Chemicals, Westbury, NY). A single cell suspension of spleen cells was prepared by mashing spleens through a nylon mesh filter. Red blood cells were removed using ACK lysis buffer (33). The spleen cells were collected and washed twice in RPMI wash media (Gibco) containing antibiotic/antimytotic cocktail.
Tumor cell line
The MADB106 tumor, which is a chemically-induced adenocarcinoma derived from the pulmonary metastasis of a Fischer (344) rat, was used in all studies (30). The MADB106 cell line was maintained in complete culture media (RPMI 1640; Invitrogen, Corp., Grand Island, NY) supplemented with antibiotic/antimytotic cocktail (Gibco) and 10% FBS and expanded in sterile T75 tissue culture flasks at 37˚C and 5% CO2 culture conditions. To control for variability due to possible mutations, cells were limited to ten passages in culture prior to experimentation. Cells were harvested using 2% Na2EDTA solution by mechanical disruption using a cell scraper to remove adherent cells from the surface of the culture flask. The tumor cells were suspended in sterile PBS and washed twice prior to use. Lymphocyte viability was determined by Trypan blue staining.
Culture conditions
Lymphoid cells were cultured in 96-well round-bottom microtiter plates in RPMI 1640 (Gibco) supplemented with 10% FBS (Gibco), HEPES, 10 U/ml rIL-2 (eBioscience, San Diego, CA), antibiotic/antimytotic solution (Gibco) and 50 µM 2-β-ME (Sigma). Lymphocytes were incubated at 37˚C and 5% CO2. Lymphocytes were co-cultured at a density of 1 x 106 cells/well, in the absence or presence of viable tumor cells at a ratio of 100: 1 (lymphocyte to tumor), in a volume of 200 µl/well of culture media. Supernatants were collected at selected time intervals and stored at -80˚C until assayed for cytokine levels.
Preparation of B lymphocytes
For in vitro and in vivo experiments, total lymphocyte cultures were depleted of B lymphocytes using magnetic bead separation techniques (Dynal, Brown Deer, WI). Total lung and spleen cells were incubated with an optimal concentration of biotinylated anti-CD45RA magnetic beads (Biosource, Camarillo, CA) for 30 min at 4˚C. For depletion of marginal zone (MZ) B cells, spleens were incubated with biotinylated anti-MZB (Biosource). After the incubation period, anti-CD45RA+ labeled cells were deleted from the total cell population by passing cells through a magnetic apparatus (Dynal). Lung and spleen cells were subjected to two successive rounds of B lymphocyte depletion. Cell fractions depleted of 95% or more of B lymphocytes, as determined using flow cytometry, were used for subsequent experimentation.
For in vivo experimentation, lung B cells were purified using magnetic bead separation techniques (Dynal, Brown Deer, WI). Total lung cells were incubated with an optimal concentration of biotinylated anti-CD45RA magnetic beads (Biosource, Camarillo, CA) for 30 min at 4˚C. Subsequently, positively selected B cells were analyzed by flow cytometry.
In vitro NK cell tumor lysis assay
To evaluate NK-mediated tumor lysis, PKH-26 dye (Sigma) was used to label NK target MADB106 tumor cells. Briefly, 3 x 106 MADB106 tumor cells per ml were incubated at 28˚C for 3 min with an optimal concentration of PKH-26. After incubation, an equal volume of 1% BSA in RPMI 1640 medium was added to the cell suspension and incubated for an additional 1 min. Following the staining procedure, tumor cells were washed twice by centrifugation with RPMI 1640 medium supplemented with 5% FBS. The labeled MADB106 cells were tested for the intensity of fluorescence. The ability of NK cells to lyse MADB106 cells was determined using the protocol that follows. Briefly, lymphocytes obtained from lung or spleen, preparations of total lymphocytes or total lymphocytes depleted of B lymphocytes, were incubated with MADB106 tumor target cells in 75x12 mm polypropylene tubes at selected effector: target cell ratios (12.5:1, 25.0:1, 50.0:1 and 100.0:1) in triplicate. After gentle centrifugation, cells were incubated for 90 minutes at 37˚C and 5% CO2. After the incubation period, 0.5 pM final concentration of propidium iodide (PI) was added to each tube and placed on ice until analyzed using flow cytometry methods. NK tumor lysis was expressed as the percentage of PKH-26+ PI+ staining of the gated tumor cell population. Percentage lysis was expressed as the percentage of fluorescent positive tumor cells staining positive for PI in the presence of lymphocyte cultures minus the percentage of PI-labeled tumor cells without lymphocyte cultures.
Immunofluorescent staining
Immunophenotyping of cells was performed using immunofluorescent staining techniques. Two-color immunofluorescent staining was performed using a combination of phycoerythrin (PE), peridinin chlorophyll-a protein (PerCP), or fluorescein isothyocyanate (FITC)-labeled antibodies specific for rat CD45RA (OX-33), CD80 (3H5), CD86 (24F), MHC II (OX-6), and Pan T (OX52). All fluorescent antibodies were purchased from BD PharMingen, San Diego, CA. Unstained cells were used as negative controls. Cells bearing different markers were identified using a FACScan cytometer (Becton Dickenson, Mountain View, CA) and analysis was performed using CellQuest software (Becton Dickenson).
Cytokine ELISA assays
The amount of cytokine in culture supernatants was determined by capture ELISA. Rat IFN-γ protein levels were measured using rat IFN-γ ELISA sets (BD PharMingen). IFN-γ cytokine production was determined using rat IFN-γ ELISA set (eBiosourse, San Diego, CA). Briefly, Easy-wash flat-bottom 96-well microtiter plates (Nalge, Nunc, Rochester, NY) were coated overnight with 100 µl mAb specific for each cytokine. Plates were then washed 5 times using wash buffer (PBS/0.05% Tween) followed by the addition of 200 µl of wash buffer supplemented with 10% FBS to prevent non-specific binding and incubated for 2 h. After blocking plates, 100 µl of supernatant and IFN-γ recombinant cytokine was placed in wells. Following overnight incubation at 4˚C, plates were washed 5 times with washing buffer. 100 µl of biotinylated mouse anti-rat IFN-γ cytokine was added to the appropriate wells and incubated for 1 h. To detect cytokine levels, avidin-HRP and 3, 3', 5, 5'-tetramethylbenzidine substrate (BD PharMingen) were allowed to react on the plate for 30 minutes. 100 µl of 0.1 M NaHPO4 stopping solution (BD PharMingen) was added and plates were read at an absorbance of 450 nm using MicroQuant Spectrophotometer (Bio-Tek Instruments, INC., Winooski, Vermont). Cytokine concentrations were determined by comparing standard curves generated from rat recombinant IFN-γ protein standard provided with ELISA kits (BD PharMingen) using log/log quadratic linear regression analysis provided by Juniper software (KC Junior, Bio-Tek Instruments, Inc.).
Tumor cell injection
To assess in vivo responses to MADB106 tumor, rats were administered 1 x 106 MAB106 tumor cells intravenously via tail vein injection.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 4.0 for MacIntosh (GraphPad Software, San Diego, USA). For multi-experimental group analysis, data were subjected to analysis of variance (univariate ANOVA), followed by Post-hoc tests (Tukey) for group differences. For analysis of two-group differences, Student's t test was employed, followed by Post-hoc analysis. All data are expressed as the mean ± SEMs. The two-tailed level of significance was set at P less than or equal to 0.05.