Abstract
Tumor cells and the immune system play a lethal "pas de deux" during tumor development. However, it is not clear which role the innate immune system plays in these interactions. We studied the interaction of normal spleen cells (NSCs) with tumor cells expressing low levels of MHCI on the cell surface. This interaction induces increased MHCI expression on the MHCIlow tumor cells by a cell-cell contact-dependent, IFN-γ-mediated mechanism. The effector cells responsible for the increased IFN-γ production were identified as CD4+ CD1d-independent NKT cells, NK1.1+ NK cells and CD4+ CD11c+ DCs. The possible three cell collaboration is not activated by MHCIhigh tumor cells or normal fibroblasts. Kinetic experiments showed that the increase in IFN-γ production induced by MHCIlow tumor cells happens in two consecutive waves, an early peak around 12 hours, followed by a second more important peak around day 2-3. Thus, we propose that CD4+ CD1d-independent NKT cells are activated by the MHCIlow tumor cells, they release IFN-γ stimulating DCs to produce IL-12, which in turn activates NK cells to produce large amounts of IFN-γ. The recognition mechanism used by the CD4+ CD1d-independent non-classical NKT cells is unknown. Monoclonal antibody (mAb) blocking experiments using antibodies against either activating or inhibitory receptors or co-receptors on NKT/NK cells gave no conclusive results. Moreover, NSCs from either normal or MHCII-/- mice augmented MHCI expression on MHCIlow tumors, excluding a significant role of CD4-MHCII interactions in the system. Hence the initial recognition mechanism in this system still awaits further experimentation.
Keywords: in vitro, spleen cells, cultured tumor cells, MHCI, innate immunity
Introduction
Expression of major histocompatibility complex class I (MHCI) molecules on the cell surface serves as an identity flag used in cell-cell interactions and transmembrane signaling (1, 2). MHCI antigens are expressed at various levels from about day 12 of embryonic life by all cells in the body with exceptions such as brain, renal tubular or pancreatic acinar cells (1, 3). Thus, MHCI expression is regulated and may change in response to the environment. It is not known whether regulation of MHCI expression is autonomous or induced by external factors such as type I (α and β) or type II (γ) interferons, TNF-α or other molecules (1). IFN-γ is one of the major external regulators of MHCI expression; it is produced mainly by T, NKT and NK cells but also, to a lesser extent, by macrophages and dendritic cells (DCs) (4-6). IFN-γ exerts its function upon interaction with the IFN-γ receptor (IFNγR) that is ubiquitously expressed on all cells (4).
Tumor cells are self cells with abnormalities caused by structural changes in, or altered levels of, expression of household proteins. These abnormalities may be due to irreversible genomic instability in the tumor cells, or to reversible selection exerted on regulatory elements for genes encoding cell growth factors (7, 8). As a selective response to immune destruction, tumor cells use several escape strategies, many of which involve down-regulation of MHCI molecules or other molecules of the antigen-presenting machinery (7-12). Consequently, tumor cells do not express tumor-specific peptides on the outer membrane and thus cannot be recognized by cytotoxic T lymphocytes (CTLs). However cells other than CTLs attack tumor target cells directly: natural killer (NK) cells, polymorphonuclear (PMN) leukocytes and macrophages/DCs do not recognize tumor cells via peptide/MHCI ligands. The latter seem involved in the recently described natural immunity against tumor cells (13). Another phenomenon known since pioneering studies in Stockholm is that tumor cells cultured in vitro may down-regulate their MHCI molecules, whereas these cells injected in vivo regain normal levels of MHCI expression (14). However, the effector cells responsible for this phenomenon have not been identified. Moreover, mice deficient in the innate immune system show a higher incidence of tumor cell induction and out-growth compared to wild-type mice (7, 8). Thus, cells from the innate immune system may play a role in the regulation of MHCI expression on cells with which they interact.
Several tumor models use peptides, from proteins against which specific T cell receptor (TCR) αβ transgenic mice have been made, as tumor antigens (9, 10, 15, 16). These model systems have demonstrated that tumor cells may be killed directly by specific T cells or indirectly by released IFN-γ. However, most systems showed that despite an overwhelming excess of tumor-specific T cells in the mice, the tumor cells will kill the hosts (9, 15). The tumor cells used in these studies expressed rather low levels of MHCI at the cell surface. One possibility is that the tumor-specific CTLs had difficulties in recognizing and killing the tumor cells due to their low expression of MHCI, i.e. the induction of higher levels of MHCI on the tumor cells should render them sensitive to the tumor-specific CTLs in vivo (17). In order to verify this notion and to determine whether effector cells in normal spleen (NSCs) could regulate MHCI expression on MHCIlow tumor cells, we cultured the tumor cells with NSCs in vitro. In such co-cultures, splenic NK cells could eliminate the MHCIlow expressing cells and (i) leave the MHCIhigh cells intact, and/or (ii) induce high MHCI expression on the residual tumor cells. The present paper represents our efforts to understand how the interaction between the innate immune components of NSCs and MHCIlow tumor cells leads to the induction of high MHCI expression and enhanced sensitivity of the tumor cells to CTLs.
Results
Normal spleen cells induce augmented MHCI expression on MHCIlow tumor cells
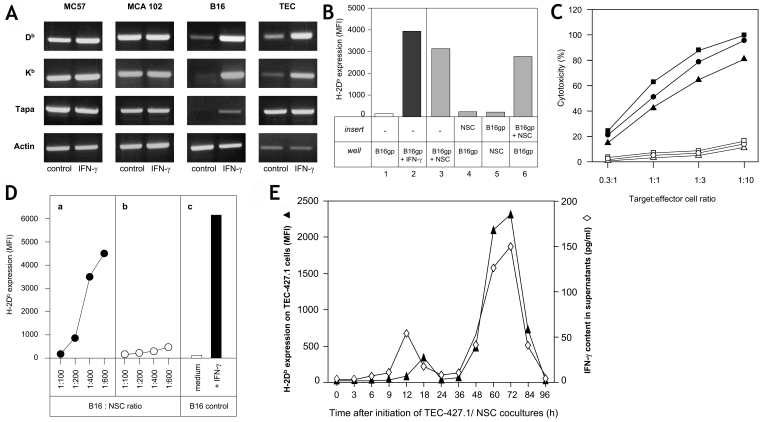
In the present study, we used three MHCIlow tumor cell lines of different origin: B16 melanoma cells, MCA102 fibrosarcoma cells and TEC-427.1 thymus epithelial cells. These cells are MHCIlow due to different defects: B16 cells are deficient in Db, Kb and tapasin expression, TEC-427.1 cells are deficient Db and Kb expression, and MCA102 cells display no defects related to Kb, Db or tapasin (Figure 1A). However, despite their different inherent defects, co-culture of NSCs with MHCIlow tumor cells causes increased cell-surface expression of MHCI on the tumor cells (Figure 1B). Thus, the MHCIlow phenotype on the three tumor cell lines is reversible. However, the increased expression of MHCI molecules was not stable over time, and cloning of the MHCIhigh tumor cells did not yield stable MHCIhigh clones (not shown). These results indicated that the MHCIhigh membrane expression was induced, rather than due to selection of MHCIhigh variant cells after the elimination of MHCIlow tumor cells by NK cells in the NSC population. Co-culture of NSCs with MHCIhigh cells like L12R4 lymphoma or MC57 fibrosarcoma cells did not influence MHC expression on the tumor cells (not shown). Moreover, MHCIlow tumor cells that had been induced to express increased MHCI surface levels and then down-regulated their MHCI expression, were sensitive to a new induction of increased MHCI expression (not shown). Thus, the data suggest that recognition of MHCIlow tumor cells by NSCs induces the increase in MHCI expression.
Figure 1.
The increase in MHCI expression on tumor cells is dependent on cell-cell contact between NSCs and tumor cells. (A) MC57, MCA102, B16 and TEC tumor cells were cultured in the absence (control) or presence of IFN-γ for two days. Then, mRNA was isolated, cDNA synthesized using primers specific for Db, Kb, tapasin (tapa) or actin, and the PCR products were separated in agarose gels. DNA fragments were revealed by BET. MCA102 and B16 cells were MCA102gp and B16gp cells, respectively. (B) B16gp cells (2.5 x 104) were cultured for 3 days alone or with 300 pg/ml IFN-γ in 3 ml wells as negative (column 1) or positive controls (column 2). Alternatively, B16gp cells were cultured with NSCs (10 x 106) in 3 ml wells (column 3), or B16gp cells were cultured in the wells and NSCs in the insert (column 4) and vice versa (column 5). B16gp cells were also cultured in the well with a mixture of B16gp and NSCs in the insert (column 6). The data in columns 1-6 are referred to as groups 1-6 in the text. H-2Db expression of the tumor cells in the wells was measured by flow cytometry using FITC-labeled anti-Db mAb (28.14.4S). (C) B16gp (squares), MCA102gp (circles) and TEC-427.1 cells (triangles) were cultured alone (open symbols) or with IFN-γ (100 pg/ml) (closed symbols) for 3 days. The tumor cells were then harvested, washed and assayed for sensibility to the cytotoxic activity of alloreactive T cells (BALB/c anti-B6 T cell line) at different target:effector cell ratios. The data are means of quadruplicates, and variations did not exceed 10% (not shown). (D) NSCs from wild-type (a) or IFN-γ-/- deficient mice (b) were co-cultured for 3 days with B16gp cells at ratios of 600:1, 400:1, 200:1 or 100:1. The B16gp cells were then harvested and H-2Db expression was measured by flow cytometry. Tumor cells were either cultured in medium alone (negative control) or stimulated with 100 pg/ml of IFN-γ (positive control) (c). (E) NSCs (3 x 106/ml) were cultured in quadruplicates with MHCIlow TEC-427.1 (5 x 104/ml) tumor cells for four days. After 3, 6, 9, 12, 18, 24, 36, 48, 60, 72, 84, and 96 hours 1 ml of supernatant was isolated. These supernatants were analyzed for IFN-γ content by ELISA (open diamonds) and for their capacity to induce an increase in MHCI expression by flow cytometry on TEC-427.1 cells with FITC anti-Db mAb (MFI on quadruplicates were less than 6%) on MHCIlow tumor cells (filled diamonds). Standard deviations of quadruplicates of IFN-γ determinations did not exceed 10%. Four experiments were performed with identical results.
In order to find out whether the increased MHCI expression on the tumor cells was induced by cell-cell contact or by release of soluble factors, we performed a series of experiments such as those depicted in Figure 1B. The negative control group is MHCIlow tumor cells cultured in medium (group 1) and the positive control group 2 represents B16 cells cultured with suboptimal concentrations of IFN-γ. The results from groups 3-6 showed that NSCs and B16gp cells have to be in contact in the same compartment in order to induce the phenomenon (compare the results from group 3 with those from groups 4 and 5). However, when NSCs and B16 cells were cultured together in one of the compartments (group 6), one or more soluble factors were released that could induce an increase in MHCI expression in tumor cells cultured alone in the opposite compartment. Three more experiments with variations in tumor cells, NSCs or tumor cell-NSC mixtures in different compartments confirmed the conclusion that the recognition of MHCIlow tumor cells by NSCs causes release of soluble factors that can augment MHCI expression in tumor cells. The consequence of the increase in MHCI expression is that the tumor cells become more sensitive to specific cytolytic T cells (Figure 1C).
As culture of MHCIlow tumor cells in IFN-γ-containing supernatant from L12R4 lymphoma cells (18) increased the MHCI expression (Figure 1, panels A and B), IFN-γ was a possible candidate as the soluble effector factor released by the NSCs. We therefore compared the levels of MHCI expression on MHCIlow tumor cells co-cultured with spleen cells from normal or IFN-γ-/- mice. As can be seen in Figure 1D, spleen cells from IFN-γ-/- mice do not induce increased MHCI expression on MHCIlow tumor cells. Thus, our data presented so far clearly demonstrate that IFN-γ is directly involved in the described phenomenon (see also Discussion). Moreover, kinetic experiments showed that release of IFN-γ by NSCs recognizing MHCIlow tumor cells happens in two waves, an early peak around 12 hours and a second, more important peak, around day 2-3. These peaks are completely superimposable with the two peaks of increased MHCI expression caused by the same supernatants (Figure 1E).
We conclude that NSCs recognize MHCIlow tumor cells and then release IFN-γ, which induces augmented MHCI expression via the IFNγR on these cells (4).
Identification of the NSC-inducer cells responsible for increased MHCI expression in MHCIlow tumor cells
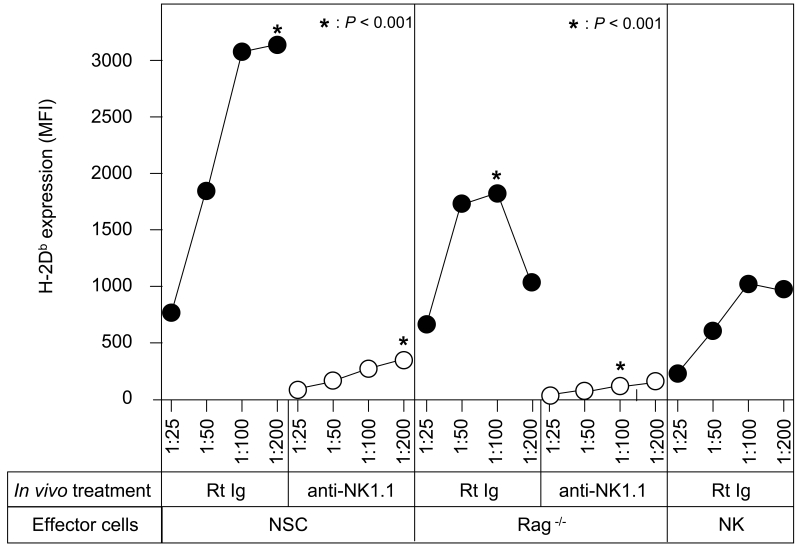
One of the hypotheses behind the present experiments is that the innate immune system participates in the regulation of MHCI expression on tumor cells. Consequently, "NSCs" from Rag-/- mice that lack immune cells with rearranged receptors (TCR or Ig) should induce augmented MHCI expression on MHCIlow tumor cells. The data in Figure 2 (a summary of three independent experiments) show that MHCI expression on MHCIlow tumor cells is increased by Rag-/- NSCs, albeit less well than NSCs from wild-type mice. Thus, something is missing in NSCs from Rag-/- mice. Two T cell types, NKT and γδ T cells, are considered part of the innate immune system (19, 20) and these cells are absent in Rag-/- NSCs. Therefore, we tried to demonstrate whether these T cells had a function in our system. Both NKT and NK cells from B6 mice express the NK1.1 marker. In vivo depletion of NK1.1+ cells by PK136 anti-NK1.1 mAb (21) reduced the capacity of normal, as well as Rag-/-, spleen cells to induce MHCI expression in MHCIlow tumor cells (Figure 2; P values are very significant). Purified NK cells (using a NK cell separation kit removing CD3+ CD4+ CD8+ T cells, CD11c+ DCs and CD19+ B cells) augment MHCI expression on MHCIlow tumor cells, but less efficiently than Rag-/- spleen cells (Figure 2). These data indicate that both NKT and NK cells are important effector cells in the observed phenomenon. The residual cells in the Rag-/- spleen cell population, DCs, macrophages and eosinophils have no activity by themselves.
Figure 2.
Innate immune cells induce an increase in MHCI expression on MHCIlow tumor cells. B16 tumor cells were cultured for three days with different amounts of NSCs from either wild-type B6 or Rag-/- B6 mice , or with different amounts of purified NK cells (Miltonyi NK-cell Kit), and then harvested, washed and analyzed for H-2d membrane expression by flow cytometry. Normal or Rag-/- mice were injected with normal rat Ig (RtIg) or PK136 anti-NK1.1 mAb on days -3 and -2. P values (*) are given for some groups.
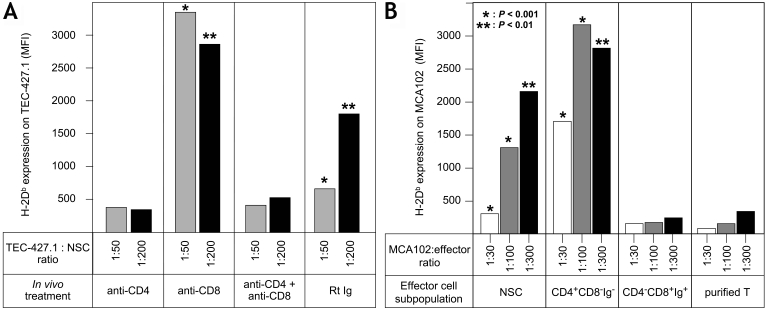
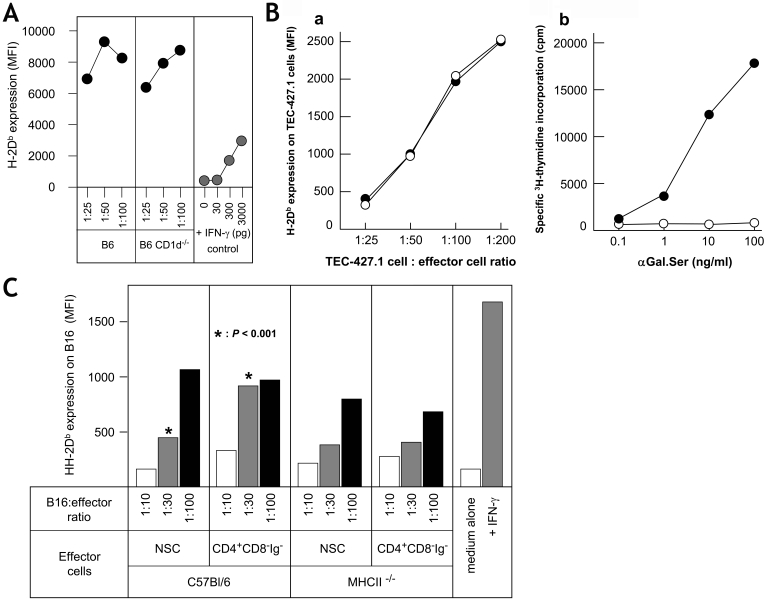
In order to investigate whether αβ or γδ T cells were possible effector cells, we performed in vivo and in vitro cell fractionation experiments. B6 mice were injected with 500 µg of anti-CD4 mAb, anti-CD8 mAb or a mixture of both mAb on days -5 and -2 before harvest of the spleen cells. NSCs from anti-CD4 mAb treated mice had lost the ability to induce MHCI expression on the tumor cells, whereas NSCs from anti-CD8 mAb treated mice had a significantly enhanced effect on MHCI expression in MHCIlow tumor cells (Figure 3A). Thus, important effector cells in the system are CD4+ cells. CD8+ T cells may have a regulatory activity, as removal of CD8+ cells in in vitro fractionation experiments also causes a significant functional increase in the residual CD4+ cell population (Figure 3B). Removal of γδ T cells (or CD4+ CD25+ Treg cells) from the CD4+ population had no impact (not shown). Conventional αβ T cells (CD4+ CD8+ NK1.1-) purified using the Miltenyi Isolation Kit (removing B cells, DCs, macrophages, NK and NKT cells) showed no MHCI enhancing effect (Figure 3B). This experiment was repeated two more times with the same results. These data strongly indicate that the missing effector cells in Rag-/- NSCs are CD4+ NK1.1+ NKT cells. In order to distinguish between CD1-dependent and CD1-independent NKT cells, we investigated NSCs from CD1-/- B6 mice. The data in Figure 4A demonstrate that NSCs from wild-type, as well as from CD1-/- mice were equally efficient in augmenting MHCI expression on MHCIlow tumor cells. Additional experiments demonstrated that although spleen cells from B6 Jα18-/- mice show no response to αGal.Ser, i.e. lack of conventional CD1-restricted NKT cells (22), they increased MHCI expression on MHCIlow tumor cells as well as on those of control B6 mice (Figure 4B). Vα14-Jα18 negative NKT cells express a less restricted TCRαβ repertoire (22). It was therefore of interest to analyze whether the CD4+ NKT cells in our system were dependent on MHCII expression for their function. B6 NSCs and spleen cells from B6 MHCII-/- mice were cultured with B16 melanoma cells for three days. The tumor cells were then tested for increased MHCI expression. The data in Figure 4C show that NSCs from MHCII-/- mice do increase MHCI expression on MHCIlow tumor cells but less well than NSCs from normal mice (n = 3). However, the difference between the effect seen with NSCs versus that seen with MHCII-/- knockout (KO) cells was not significant, although always in the same direction. These results indicate that an interaction between CD4 on NKT cells and MHCII on DCs may play a partial role in the initial events in our system.
Figure 3.
NSC effector cells in the system are CD4+ CD8- non-conventional T cells. (A) B6 mice were injected on days -5 and -2 with 500 µg anti-CD4 or anti-CD8 mAb [control mice received 500 µg normal rat Ig (RtIg)]. Spleen cells were harvested on day 0 and cultured for three days with TEC or B16 cells (at two different rations, 50:1 and 200:1). Increased expression of Db was measured by flow cytometry. Variations in MFI were below 10% and are not given. P values are indicated in order to show the significant difference in MHCI augmenting capacity between NSCs from normal and from anti-CD8 mAb treated mice: *, P < 0.001; **, P < 0.01. Results with TEC cells are shown. (B) B6 NSCs were separated into CD4+ CD8- Ig- cells (>95% CD3+) and CD4- CD8+ Ig+ cells (approx. 10% CD8+ and >70% CD19+), or purified T cells (>97% CD3+) using the T-cell Kit from Miltonyi (this kit remove granulocytes, macrophages, DCs, NK, NKT and B cells). They were then cultured with MCA102 cells for three days, and increased MHCI expression on the tumor cells was analyzed using flow cytometry. Results are expressed as means of MFI measurements. Variations were <10% and thus standard deviations are not indicated. In contrast, the indicated P values show that removal of CD8+ and Ig+ cells increases the MHCI-augmenting capacity of the residual effector cells in the NSC population.
Figure 4.
Effector NKT cells are CD1d- Jα18- and MHCII-independent. (A) NSCs from B6 mice or B6 CD1d-/- deficient mice were harvested and cultured for 3 days with B16gp cells at different ratios (NSC:B16gp ratios were 100:1, 50:1 or 25:1) and H-2Db expression on tumor cells were analyzed. B16gp cells cultured either alone or with medium of different concentrations of IFN-γ served as negative and positive controls. (B) Spleen cells from normal B6 mice (closed circles) or from B6 Jα18-/- mice (open circles) were cultured with TEC-427.1 tumor cells for 3 days. Tumor cells were then isolated and analyzed for increased H-2Db expression. Alternatively, the two different spleen cell populations were stimulated with different concentrations of αGal.Ser for 4 days (quadruplicates), the last 24 hours in the presence of 1 µCi 3H-thymidine. Standard deviations for MFI and 3H-thymidine incorporation (cpm) were <10% and <5%, respectively, and are thus not indicated. (C) B16 cells were cultured for 3 days with either medium (negative control), 100 pg/ml IFN-γ (positive control), or with four different types of effector cells (white bars, 5 x 106 cells; gray bars, 10 x 106 cells; black bars, 20 x 106 cells): 1) NSCs; 2) NSCs where Ig+ and CD8+ cells were removed by incubation with anti-mouse Ig beads and with anti-CD8 mAb beads and fractionation on magnetic columns; 3) NSCs from MHCII-/- KO mice; and 4) NSCs from KO mice where Ig+ and CD8+ cells were removed. Comparisons between wild-type and MHCII-/- deficient cell populations revealed no significant differences. Only purified CD4+ cells from NSCs (at 10 x 106 cells/well) were significantly more effective compared to non-fractionated cells (see also Figure 3). In three different experiments, NSCs from MHCII-/- deficient mice were always less efficient compared to NSCs from wild-type B6 mice; however, the difference was not statistically significant.
Thus, all data presented show that CD1d-independent CD4+ NKT cells (23), which do not have an absolute MHCII requirement, are important effector cells in our system. Furthermore, preliminary experiments demonstrated that significant numbers of DX5+ TCRβ+ NKT cells, as well as DX5- TCRβ- NK cells, contained intracellular IFN-γ (unpublished data).
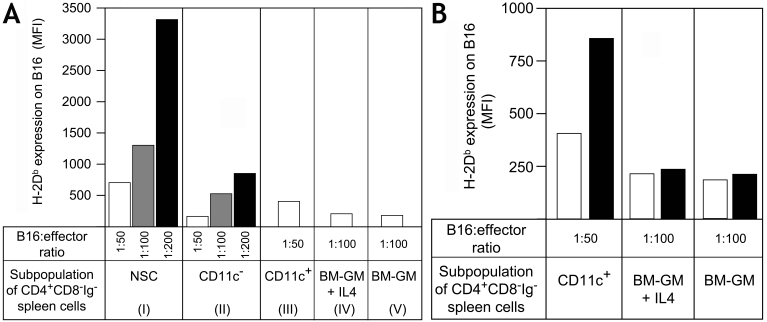
In vitro depletion of CD11c+ cells from NSCs, Rag-/- spleen cells or anti-NK1.1 mAb treated Rag-/- spleen cells showed decreased activity of the residual cells (not shown). Separation of the CD4+ CD8- Ig- spleen cells into CD11c- and CD11c+ cells showed that the CD11c- cells had about 4-fold lower activity than non-separated cells (Figure 5A, groups I and II). The CD11c+ fraction exhibited a 2-fold decrease in MHCI augmenting activity (group III). Bone marrow (BM)-derived DCs expanded in culture with GM-CSF (with or without IL-4) do not induce enhanced MHCI expression on MHCIlow tumor cells alone (Figure 5A, groups IV and V). We then tried to reconstitute the reduced response of CD11c- cells with the BM-derived DCs (100:1 ratio with tumor cells) or with the CD11c+ cells (50:1 ratio with tumor cells). As shown in Figure 5B, the CD11c+ cells from spleen synergize very significantly with the splenic CD11c- cells. In contrast the BM-derived DCs do not cooperate with the CD11c- cells. This finding fits with the observation that BM-derived DCs (± IL-4) contains very few CD4+ CD11c+ cells (<0.1%) in comparison with NSCs (approx. 2%, data not shown). Thus, CD4+ CD11c+ DCs (24, 25) play an important role as effector cells in the described experimental system. Their major function may be the production of IL-12 (see below).
Figure 5.
Spleen DCs, but not BM-derived DCs, collaborate in the MHCI augmenting function of NSCs. B6 spleen cells were depleted of CD11c+ cells by two passages on anti-CD11c magnetic bead columns (CD11c- cells, <0.1% positive cells). Eluted CD11c+ cells were purified one additional time on magnetic columns (>80% positive). (A) The three different subsets were cultured at different ratios (50:1, 100:1, 200:1) with B16 cells for 3 days. For CD11c+ cells only 50:1 ratios are shown (100:1, MFI approx. 974 ± 87; 200:1, MFI approx. 1.469 ± 141). BM cells were cultured with 20 ng/ml GM-CSF ± IL-4 (10 ng/ml) for 7 days (approx. 45% and 59% CD11c+ cells, respectively) and cultured with B16 cells at ratio of 100:1. (B) CD11c+ cells, BM-GM and BM-GM + IL-4 cells were also cultured without (open bars) or with CD11c- cells (black bars) in order to measure cooperative effects between these cells. Increased MHCI expression was measured by flow cytometry (MFI values are given). Standard deviations were <10%.
Collectively, our experiments show that recognition of MHCIlow tumor cells and subsequent production of IFN-γ is performed by either CD4+ CD1d-independent NKT cells, NK1.1+ NK cells or CD4+ CD11c+ DCs. These three cell types seem to work most efficiently in synergy (see further below).
Recognition of MHCIlow tumor cells by NSCs
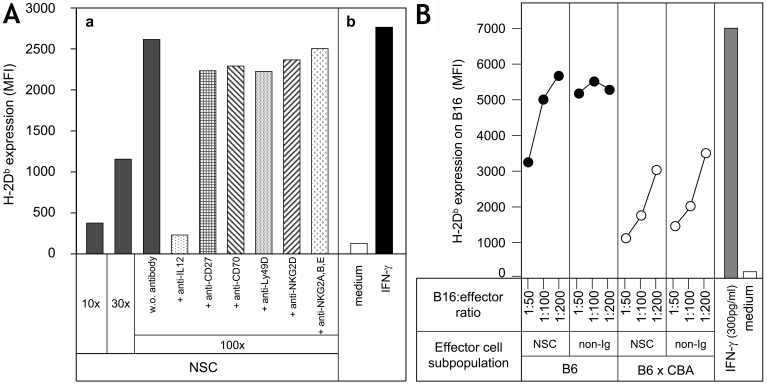
The data presented so far suggest that activation of NKT or NK cells by MHCIlow tumor cells may take place via activating receptors like NKG2D, CD27 or Ly49D/H (20, 26-28). To elucidate how the effector cells in our system may recognize the MHCIlow tumor cells, we tried to block the interaction between NSC effectors and tumor cells using mAb. CD4+ CD8- Ig- NSCs were used in order to avoid complications arising from the presence of irrelevant cells in mAb inhibition experiments (i.e. Fc receptor positive cells). The effector cells were incubated for three hours at 4˚C with the mAb to optimize their interaction, and the mAb was not washed away. Monoclonal antibodies against the activation receptors or co-receptors CD27, NKG2D, and Ly49D on NKT or NK cells were analyzed for receptor-blocking activity in our system. As can be seen in Figure 6A, mAb against these receptors as well as against their ligands did not influence the capacity of CD4+ NSCs to augment MHCI expression on the three different MHCIlow tumor cells (only data from B16 cell experiments are shown). Blocking of the inhibitory receptors Ly49A, Ly49C or NKG2A/CD94 had no effect in our system (not shown). Only mAb against the IL12p70 molecules completely blocked the induction of augmented MHCI expression on MHCIlow tumor cells (Figure 6A). Thus, DC-derived biologically active IL-12 plays an important role in the effector cell cooperation observed.
Figure 6.
Possible mechanisms of recognition of MHCIlow tumor cells by NSCs. (A) NSCs were incubated with the indicated mAb (or purified MIg from normal mice = w.o.) for three hours at 4˚C, and then added directly to B16gp tumor cells. Control NSC-B16 cell cultures at 1:10, 1:30 and 1:100 ratios were made, whereas mAb-treated cultures were analyzed at a 1:100 ratio. In vitro culture was for 3 days; tumor cells were then harvested and analyzed for H-2Db expression by flow cytometry. (B) Spleen cells or non-Ig+ cells (approx. T cells, >90% CD3+) from B6 (closed circles) or (B6 x CBA) F1 (open circles) mice were cultured with B16gp cells for 3 days. The tumor cells were then isolated and analyzed for expression of H-2Db by flow cytometry. The capacity to induce an increase in MHCI expression on MHCIlow tumor cells by B6 effector cells is significantly higher (P < 0.001) than by (B6 x CBA) F1 cells.
A comparative study of NSCs and T, NKT, NK (non-Ig) cells from B6 or (B6 x CBA) F1 mice showed that the F1 cells had 4-fold less activity compared to B6 cells. This could be a gene dose-effect by differential expression of activating and inhibitory receptors on H-2b homozygous versus H-2bxk heterozygous effector cells (28). Thus, whatever the nature of the receptor(s) on NKT or NK cells recognizing MHCIlow tumor cells, the activation of augmented MHCI expression, i.e. production of IFN-γ, is optimal for syngeneic H-2b homozygous effector cells.
Discussion
The present experiments were performed with three tumor cell lines of different origin and of MHCIlow phenotype due to various reversible mechanisms (Figure 1A). Our data showed that co-culture of MHCIlow tumor cells with NSCs induced increased MHCI expression by a cell-cell contact-dependent, IFN-γ-mediated mechanism (Figure 1B). In addition, the effector cells were found among the innate immune cells of the spleen (Figure 2, Figure 3, Figure 4, Figure 5). The frequency and/or activity of the effector cells responsible for the observed phenomenon are different in various lymphoid organs, being highest in spleen and lymph nodes, and lowest in bone marrow, thymus and peritoneum (not shown). IFN-γ was the only direct effector molecule detected in the supernatant from NSC-tumor cell cultures: (i) Purified IFN-γ increased MHCI expression on MHCIlow tumor cells (see Materials and Methods); (ii) Depletion of IFN-γ from different T or NKT/NK cell supernatants with high concentrations of IFN-α, IFN-β, TNF-α, IL-4, IL-13 etc. removed the capacity to augment MHCI expression on MHCIlow tumor cells (see Materials and Methods); (iii) Spleen cells from IFN-γ-/- mice did not augment MHCI expression on MHCIlow tumor cells (Figure 1D); activation of NSCs from IFN-γ-/- mice by concanavalin A did not produce cytokines which could augment MHCI expression on MHCIlow tumor cells (not shown). Thus, IFN-γ is the direct effector molecule in the present system and does not act as a "helper" molecule in the induction of other molecules with the capacity to augment MHCI expression. We conclude that IFN-γ is the sole effector molecule responsible for the increased MHCI expression on MHCIlow tumor cells at both the induction and effector level.
Cell fractionation experiments have demonstrated that the major cell subsets involved in the described phenomenon are CD4+ CD1d-independent Jα18-/- NKT cells, CD4+ CD11c+ DCs, and NK1.1+ NK cells. CD4+ or CD8+ TCRαβ+ T cells, TCRγδ+ T cells, CD4+ CD25+ regulatory T cells, B cells or macrophages are not implicated (Figure 2, Figure 3, Figure 4, Figure 5). It seems that DCs, NKT and NK cells function most efficiently together: (i) NSCs from Rag-/- mice function less well than NSCs from wild-type mice (Figure 2), (ii) purified NK cells function less well than NSCs from normal or Rag-/- mice (Figure 2) and (iii) both CD11c+ and CD11c- sub-populations of CD4+ CD8- Ig- cells work suboptimally, and they collaborate when remixed before culture with tumor cells (Figure 5). Moreover, kinetic experiments have shown that the induction of augmented MHCI expression on MHCIlow tumor cells happens in two waves, an early peak around day 1 and a second more important peak around day 2-3, with decreased synthesis of IFN-γ from day 4-5 (Figure 1E). Addition of mAb against IL-12 to cultures of NSCs and MHCIlow tumor cells abolishes the MHCI-inducing capacity of the effector cells. Furthermore, the finding that the presence of MHCII molecules influences only slightly the increase of MHCI expression on MHCIlow tumor cells indicates a possible (but not decisive) role for CD4-MHCII interactions in the initial recognition events between CD4+ NKT cells and MHCII+ DCs. Therefore, it appears that the most probable scenario for our collaborative system is that NKT cells recognize the "missing self" (14, 29) on MHCIlow tumor cells, release stored IFN-γ which induces DC to produce IL-12 that in turn activates NK cells (20, 26-31). This phenomenon may in turn be regulated by CD8+ T cells (Figure 3).
Thus, a key question is how the NKT cells recognize MHCIlow tumor cells and initiate the possible three-cell collaboration with DCs and NK cells. NKT cells may be divided into three categories based on their reactivity to α-galactosylceramide (αGal.Cer), their TCRα-chain diversity and their CD1d dependency: Type I classical "invariant" NKT cells have invariant Vα14-Jα18 TCRα chains and react to αGal.Cer in a CD1d-dependent manner. Type II nonclassical NKT cells do not react to αGal.Cer and have diverse TCR chains but are CD1d dependent. Type III NKT cells are CD1d independent, do not respond to αGal.Cer, and possess diverse TCR chains (22, 32). The finding that the NKT cells involved in the present system were independent of CD1d ligand and of Jα18 expressing T cell receptors defines them as type III NKT cells and appears to exclude the recognition of tumor cell lipids, a process performed mostly by the invariant Vα14+ CD1d-restricted iNKT (type I) cells (33, 34). It follows that probable receptors were the activating receptors NKG2D or Ly49D/H (KAR; killer activation receptor), inhibitory receptors like NKG2A/CD94, Ly49A and Ly49C (KIR; killer inhibitory receptor), or co-receptors like CD2, CD27 or CD28 (35) on NKT or NK cells. However, none of the anti-KAR/KIR/co-receptor mAbs influenced the capacity of NSC effector cells to induce MHCI expression on MHCIlow tumor cells, despite the fact that these mAbs actively block NKT or NK cell cytotoxicity against YAC (MHCIlow) target cells (Figure 6A). Therefore, we suggest that the recognition event under study may be carried out by either (i) an unknown receptor, (ii) ligand-receptor pairs not analyzed in our experiments, e.g. CD48-2B4 (36, 37) or (iii) a combination of activating and inhibitory receptors or their level of expression (Figure 6B) (28, 37, 38). The latter possibility is supported by the finding that B6 NSCs are far more effective compared to (B6 x CBA) F1 NSCs (Figure 6B), probably due to differences in expression of KAR and KIR among B6 and CBA strains (28). The NKT cells active in the present system may resemble the recently described IL-17 producing NKT cells, which are CD4+ and express a diverse TCR repertoire (32).
Previous experiments have demonstrated that MHCI expression in tumor cells is influenced by many factors (7, 8, 10, 16, 17). Injection of tumor cells with down-regulated MHCI expression into syngeneic mice causes up-regulation of MHCI expression on MHCIlow tumor cells (14). Thus, the in vivo environment up-regulates MHCI expression as was observed in the in vitro experiments presented. These in vivo data have been repeated with the B16 cell line used in this study (unpublished data). So, what level of MHCI expression is advantageous for tumor cells in their interactions with the immune system? High levels of MHCI expression may suppress CTL responses due to the action of inhibitory receptors (38), intermediate levels of MHCI expression would favor CTL killing, and low levels of MHCI expression favors NK cell cytotoxicity (26, 39). At the start of neoblastic cell development, it would be an advantage for the tumor cells to maintain high levels of MHCI expression, as the frequency of tumor-specific CTLs is very low and the number of NKT and NK cells is high. Thus, MHCIlow tumor cells produced many fewer tumor colonies compared to MHCIhigh tumor cells when injected into naive B6 mice (14, 40, 41). The present data indicate that innate NKT, DC and NK effector cells are involved in this phenomenon.
It is a mystery how tumor cells can resist the presence of high numbers of very active tumor-specific CTLs in "in vivo" tumor models (9, 10, 15, 16). IFN-γ, produced early during recognition of MHCIlow tumor cells by the innate immune system, has (i) an up-regulatory function on MHCI expression, rendering the MHCIlow tumor cells vulnerable to CTLs (Figure 1C), (ii) a tumorocidal effect on the tumor cells (10, 42) and (iii) an anti-angiogenetic effect on the tumor stroma cells (9, 43, 44). Thus, it is surprising that the innate and adaptive immune systems cannot cope with the development of tumor cell out-growth in such tumor model systems (see 9, 15, 16, 45). It is interesting to note that if the P14 TCRαβ transgenic mice are first immunized with live lymphocytic choriomeningitis virus (LCMV), they then become resistant to tumor growth (15, 45). This indicates that components of the innate immune system may have to be activated (probably via Toll-like receptors interacting with DNA or RNA from dying tumor cells) in order to function optimally. This idea is supported by the observed IL-12 dependency of the MHCI regulation (Figure 6A), and by our findings that tumor-specific responses are more efficient in conventional mice compared to specific-pathogen-free (SPF) mice (46). The reason for this is an inefficiently activated innate immune system in SPF mice. Yet another possibility is that important components of the innate immune system do not come into contact with the tumor cells (blocked by stromal cells), and thus cannot optimally regulate the level of MHCI on the tumor cells (9). As shown here, cell-cell contact is necessary for the induction of IFN-γ production and subsequent increase in MHCI expression. Along the line of the ideas developed in this paper, yeast vaccines encoding tumor antigens that activate dendritic cells and elicit protective cell-mediated immunity are now being used in clinical cancer immunotherapy (47, 48).
In conclusion, our data have shown that NKT, DC and NK cells from the innate immune system regulate MHCI expression on MHCIlow tumor cells by a cell-cell contact-dependent, IFN-γ-mediated mechanism. The innate effector cells recognize the tumor cells by an unknown mechanism, and the recognition event does not induce memory. We propose that the innate immune system takes part in the environmental factors that regulate MHCI expression in normal and neoblastic cells.
Abbreviations
- BM
bone marrow
- MFI
mean fluorescence intensity
- MHCI
MHC class I
- NSC
normal spleen cell
Acknowledgements
The present work was supported by institutional grants from the CNRS, Laboratoires Pierre Fabre, and from the European Union. The authors wish to thank Professor B. Arnold (Heidelberg, Germany) for critically reviewing the manuscript. We gratefully acknowledge the gifts of cells from Doctors F. Melchers (Berlin, Germany), P. Ricciadi-Castagnoli (Milan, Italy) and A. M. Schmitt-Verhulst (Marseille, France) and of CD1d knock-out mice from Dr. K. Benlagha (Paris, France). We very much appreciate the generous gift of αGal.Cer/CD1d tetramers and the collaboration with Dr. Dalam Ly, London, Ontario, Canada.
References
- 1.Tanaka K, Yoshioka T, Bieberich C, Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- 2.Dissing S, Geisler C, Rubin B, Plesner T, Claesson M. T cell activation. II. Activation of human T-lymphoma cells by crosslinking of their MHC class I antigens. Cell Immunol. 1990;126:196–210. doi: 10.1016/0008-8749(90)90312-f. [DOI] [PubMed] [Google Scholar]
- 3.Ozato K, Wan YJ, Orrison BM. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985;82:2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 5.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 6.Taieb J, Chaput, Ménard C, Apetot L, Ullrich E, Bonmort M, Péquignot M, Casares N, Terme M, Flament C, Opolon P, Lecluse Y, Métivier D, Tomasello E, Vivier E, Ghiringhelli F, Martin F, Klatzman D, Poynard T, Tursz T, Raposo G, Yagita H, Ryffel B, Kroemer G, Zitvogel L. A novel dendritic cell subset involved in tumor immunosurveillance. Nature Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 7.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 9.Ganss R, Arnold B, Hämmerling GJ. Mini-review: Overcoming tumor-intrinsic resistance to immune effector function. Eur J Immunol. 2004;34:2635–2641. doi: 10.1002/eji.200425474. [DOI] [PubMed] [Google Scholar]
- 10.Schüler T, Blankenstein T. Cutting edge: CD8+ effector T cells reject tumors by direct antigen recognition but indirect action on host cells. J Immunol. 2003;170:4427–4431. doi: 10.4049/jimmunol.170.9.4427. [DOI] [PubMed] [Google Scholar]
- 11.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res. 2001;61:1095–1099. [PubMed] [Google Scholar]
- 12.Yee C, Greenberg P. Modulating T-cell immunity to tumors: new strategies for monitoring T-cell responses. Nat Rev Cancer. 2002;2:409–419. doi: 10.1038/nrc820. [DOI] [PubMed] [Google Scholar]
- 13.Cui Z, Willingham MC, Hicks AM, Alexander-Miller MA, Howard TD, Hawkins GA, Miller MS, Weir HM, Du W, DeLong CJ. Spontaneous regression of advanced cancer: Identification of a unique genetically determined, age-dependent trait in mice. Proc Natl Acad Sci U S A. 2003;100:6682–6687. doi: 10.1073/pnas.1031601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prévost-Blondel A, Zimmermann C, Stemmer C, Kulmburg P, Rosenthal FM, Pircher H. Tumor-infiltrating lymphocytes exhibiting high ex vivo cytolytic activity fail to prevent murine melanoma tumor growth in vivo. J Immunol. 1998;161:2187–2194. [PubMed] [Google Scholar]
- 16.Mocikat R, Braumüller H, Gumy A, Egeter O, Ziegler H, Reusch U, Bubeck A, Louis J, Mailhammer R, Riethmuller G, Koszinowski U, Röcken M. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 17.Böhm W, Thoma S, Leithäuser F, Möller P, Schirrmbeck R, Reimann J. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161:897–908. [PubMed] [Google Scholar]
- 18.Gonthier M, Llobera R, Arnaud J, Rubin B. Self-reactive T cell receptor-reactive CD8+ T cells inhibit T cell lymphoma growth in vivo. J Immunol. 2004;173:7062–7069. doi: 10.4049/jimmunol.173.11.7062. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanigushi M, Seino KI, Nakayama T. The NKT cell system: Bridging innate and acquired immunity. Nat Immunol. 2003;4:1164–1165. doi: 10.1038/ni1203-1164. [DOI] [PubMed] [Google Scholar]
- 21.Seaman WE, Sleisenger M, Ericksson E, Koo GC. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol. 1987;138:4539–4544. [PubMed] [Google Scholar]
- 22.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 23.Eberl G, Lees R, Smiley ST, Tanigushi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 24.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 25.Martin P, del Hoyo GM, Anjuère F, Ruiz SR, Arias CF, Marín AF, Ardavín C. Concept of lymphoid versus myeloid dendritic cell lineages revisited: both CD8alpha(-) and CD8alpha(+) dendritic cells are generated from CD4(low) lymphoid-committed precursors. Blood. 2000;96:2511–2519. [PubMed] [Google Scholar]
- 26.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 27.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 28.Kane KP, Silver ET, Hazes B. Specificity and function of activating Ly-49 receptors. Immunol Rev. 2001;181:104–114. doi: 10.1034/j.1600-065x.2001.1810108.x. [DOI] [PubMed] [Google Scholar]
- 29.Kärre K, Ljunggren HG, Piontel G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 30.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelak A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 31.Crowe NY, Coquet JM, Berzins SP, Kyparissousdis K, Keating R, Pellicci SG, Hayakawa Y, Godfrey DL, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gumperz JE, Roy C, Makowska A, Lum D, Sigita M, Podrebarac T, Koezuka Y, Porcelli SA, Caedell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Oshima H, Hayakawa Y, Akiba H, Atsuka M, Kobata T, Kobayashi K, Ito M, Yagita H, Okumura K. CD27-mediated activation of murine NK cells. J Immunol. 2000;164:1741–1745. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 36.Velikovsky CA, Deng L, Chlewiski LK, Fernandez MM, Kumar V, Mariuzza RA. Structure of natural killer receptor 2B4 bound to CD48 reveals basis for heterophilic recognition in signalling lymphocyte activation molecule family. Immunity. 2007;27:572–584. doi: 10.1016/j.immuni.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chlewicki LK, Velikovsky A, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244). J Immunol. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 38.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol. 2004;4:190–198. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 39.Krogsgaard M, Davis MM. How T cells 'see' antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 40.Gorelik E, Gunji Y, Herberman RB. H-2 antigen expression and sensitivity of BL6 melanoma cells to natural killer cell cytotoxicity. J Immunol. 1988;140:2096–2102. [PubMed] [Google Scholar]
- 41.Kawano YI, Tanigushi K, Toshitani A, Nomoto K. Synergistic defense system by cooperative natural effectors against metastasis of B16 melanoma cells in H-2 associated control: Different behavior of H-2+ and H-2- cells in metastatic processes. J Immunol. 1986;136:4729–4734. [PubMed] [Google Scholar]
- 42.Kakuta S, Tagawa Y, Shibata S, Nanno M, Iwakura Y. Inhibition of B16 melanoma experimental metastasis by interferon-gamma through direct inhibition of cell proliferation and activation of anti-tumour host mechanisms. Immunology. 2002;105:92–100. doi: 10.1046/j.0019-2805.2001.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of IFN gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–4100. [PubMed] [Google Scholar]
- 44.Strasly M, Cavallo F, Geuna M, Mitola S, Colombo MP, Forni G, Bussolino F. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J Immunol. 2001;166:3890–3899. doi: 10.4049/jimmunol.166.6.3890. [DOI] [PubMed] [Google Scholar]
- 45.Blohm U, Roth E, Brommer K, Dumrese T, Rosenthal FM, Pircher H. Lack of effector cell function and altered tetramer binding of tumor-infiltrating lymphocytes. J Immunol. 2002;169:5522–5530. doi: 10.4049/jimmunol.169.10.5522. [DOI] [PubMed] [Google Scholar]
- 46.Rubin B. Natural immunity influences the level of autoimmune responses against cancer. Scand J Immunol. 2008 doi: 10.1111/j.1365-3083.2008.02220.x. in press. [DOI] [PubMed] [Google Scholar]
- 47.Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7:625–629. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 48.Franzusoff A, Duke RC, King TH, Lu Y, Rodell TC. Yeasts encoding tumour antigens in cancer immunotherapy. Expert Opin Biol Ther. 2005;5:565–575. doi: 10.1517/14712598.5.4.565. [DOI] [PubMed] [Google Scholar]
- 49.Faas SJ, Rothstein JL, Kreider BL, Rovera G, Knowles BN. Phenotypically diverse mouse thymic stromal cell lines which induce proliferation and differentiation of hematopoietic cells. Eur J Immunol. 1993;23:1201–1214. doi: 10.1002/eji.1830230602. [DOI] [PubMed] [Google Scholar]
- 50.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukins 2, 3, 4 or 5 using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 51.Schneider C, Newman RA, Sutherland R, Asser U, Greaves MF. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
Materials and methods
Mice
B6 Rag-/- and B6 MHCII-/- mice were obtained from CDTA, Orléans, France. B6, (B6 x CBA) F1, CBA/J and BALB/c mice were purchased from JANVIER (Le Genest-St-Isle, France). B6 IFN-γ-/- and B6 Jα18-/- mice were provided by Animalerie d'IFR30 Hôpital Purpan, Toulouse. B6 CD1d-/- mice were kindly given to us by Dr. Kamel Benlagha, Hôpital St Vincent de Paul, Paris. Cell depletion in vivo was performed by i.p. injection of 500 µg purified monoclonal antibody GK1.5 anti-CD4, 2.43 anti-CD8, PC61 anti-CD25, or PK136 anti-NK1.1 on days -3 and -2 before harvest of spleens and thymuses (see below).
Cells
The following H-2b tumor cell lines were maintained in 5-10% FCS/RPMI 1640 (FCS/R) or DMEM medium: Fibrosarcoma MC57, melanoma B16.F10, fibrosarcoma MCA102, and thymus epithelial TEC-427.1 cells (49). Minigene transfected B16 or MCA102, termed B16gp or MCA102pg, were described previously (15, 45). NK cell-sensitive YAC cells were obtained from Dr. E. Vivier, CIML, Marseille. L12R4 cells are H-2b T-lymphoma cells that produce IFN-γ upon stimulation with phorbol ester and ionomycin (18).
BALB/c anti-B6 CTL lines were produced by stimulation of purified BALB/c T cells with mitomycin C-treated C57Bl/6 spleen cells (50 µg/ml), followed by stimulation every 10 days with mitocymin C-treated B6 spleen cells and 2-10% X6310 cell supernatant (IL-2) (18, 50).
Spleen cells were treated by a short (1 min) contact with NH4Cl, medium was added and the cells washed twice. Dendritic cells (DCs) were produced by culturing bone marrow cells in GM-CSF ± IL-4 containing medium; they were used after 6-8 days of culture (6). GM-CSF and IL-4 were from supernatants of B16.GM-CSF cells (Dr. P. Ricciardi-Castagnoli, Milan, Italy) and X63IL4 cells (50), respectively.
Antibody-coated magnetic beads from Miltenyi Biotec (Auburn, CA) were used to separate the different subpopulations in the spleen. The following beads were employed: Anti-Thy1, anti-CD4, anti-CD8, anti-T cell kit, anti-NK cell kit, anti-CD11c and anti-mouse immunoglobulin (MIg) or anti-rat Ig (RIg), using procedures described by the manufacturer. Separation of CD25+/-, TCRβ+/- or TCRγδ+/- cells was performed with purified splenic T cells incubated with rat anti-mouse CD25, TCRβ or TCRγδ mAb, followed by washing and further incubation with anti-RIg beads. The purity of the fractionated cells was verified by flow cytometry (>90%).
Monoclonal antibodies
Most mAbs used in this study were obtained from BD-Pharmingen or eBioscience (San Diego, USA). Large amounts of mAbs were produced in our laboratory by culture of hybridoma cells and passage of the supernatants through protein G-Sepharose columns. Pure mAbs were eluted with 0.2 M citric acid, pH 2.8, in 1 M Tris, pH 8.0. Eluted antibodies were concentrated by ammonium sulphate precipitation and dialysis, and were adjusted to 1 mg/ml. AN18 anti-IFN-γ mAb (from Dr. Anne-Marie Schmitt-Verhulst, CIML, Marseille) was purified and coupled covalently to protein G-Sepharose using dimethyl pimelimidate as described (51). Control mouse Ig (MIg) (for in vitro and in vivo antibody treatments) was produced as described above by passing normal mouse serum through a protein G-Sepharose column. Antibodies used in functional blocking assays were: anti-IL12p70 (clone 9A5), anti-CD2 (clone RM2-5), anti-CD27 (clone LG.3A10), anti-CD28 (clone 37.51), anti-CD70 (clone FR70), anti-Ly49A (clone A1), anti-Ly49D (clone 4E5), anti-I-Ab (clones 25-9-3 and 25-9-17) from BD-Pharmingen and anti-Ly49C,I,F,H (clone 14B11), anti-NKG2D (clones C7 and CX5), anti-NKGAA,C,E (clone 20d5), and anti-Rae-1 (clone CX1) from eBioscience.
Purification of IFN-γ
IFN-γ was purified from day 3 supernatants of (i) B16 cells co-cultured with NSCs, (ii) NSCs stimulated with high amounts of IL-2, (iii) NSCs stimulated with concanavalin A, (iv) BALB/c anti-B6 T cells stimulated with B6 NSCs or (v) phorbol 12-myristate 13-acetate (PMA)/ionomycin-stimulated L12R4 cells (18) using affinity chromatography. All supernatants were passed through anti-IFN-γ (AN18)-Sepharose columns (1 ml/min); the beads were then washed and absorbed IFN-γ was eluted with 0.2 M citric acid into tubes with 1 M Tris, pH 8.5. IFN-γ containing fractions (ELISA, see below) were pooled, dialyzed against Dulbecco's phosphate buffered saline (DPBS) and stored at -80˚C. In most experiments, a large pool of IFN-γ (100 ng/ml) from L12R4 cells was used. It was stabilized with 0.2 mg/ml of bovine serum albumin (Fraction V, >0.5% pure, Sigma).All five supernatants contained IFN-α, IFN-β or TNF-α; however, supernatants depleted of IFN-γ did not augment MHCI expression on MHCIlow tumor cells (not shown).
Flow cytometric analysis
Surface marker analysis
Cells were washed twice in 0.8% BSA/DPBS with 0.02% azide and then incubated with 0.1 µg of mouse Fc-blocking reagent (BD-Pharmingen) and with mAb conjugated with different fluorochromes for 20 min at room temperature. After two washes, the cells were analyzed in a LSRII (Becton-Dickinson) using Diva software.
MHC expression analysis
Tumor cells were collected, washed and incubated with anti-Kb, anti-Db or anti-I-Ab mAb. Cell-absorbed mAb was revealed by FITC-labeled goat anti-mouse IgG antibody (Sigma-Aldrich). Results are expressed as mean fluorescence intensity (MFI). A standard curve was established in each experiment using a L12R4 supernatant with known IFN-γ content.
Experimental design and statistics
Normal spleen cells (NSCs) or fractionated NSCs were cultured with the tumor cells (50,000 cells/well) at different ratios in 3 ml/well (12-well plates) using FCS/R medium. After different incubation times (most often 3 days), non-adherent cells were removed by vigorous pipetting and the adherent tumor cells were detached by trypsin treatment. Tumor cells and NSCs were separated by inserts with a 0.4 µm membrane using either 12-well (3 ml) or 6-well plates (8 ml) (Greiner, Bioscience, Germany).
In each of 67 experiments, controls included: (i) tumor cells cultured alone (MFI = 119 ± 34); (ii) tumor cells cultured with 100 pg/ml of IFN-γ (purified from L12R4 supernatant; MFI = 2,187 ± 263), most often the tumor cells were cultured with 30, 100, 300, and 1,000 pg/ml in order to obtain a standard curve; (iii) tumor cells cultured with 2.5 x 106 NSCs (1:50 ratio) (MFI = 2,742 ± 487); and (iv) L12R4 cells (MFI = 1,936 ± 236) as flow cytometry control. Variations in specific MFI (MFI of IFN-γ stimulated group - MFI of control group) of each experimental point did not exceed 10% and thus standard errors are not given for clarity. Significance was determined by the Student's t-test. P values are provided in Figures where judged necessary. The number of experiments performed to answer a given question is given as n; if not mentioned, n is always three or higher.
Growth inhibition (cytotoxicity) assay
The cytotoxic activity of effector cells (treated for 20 min with 20 µg mitomycin C/ml) against tumor cells was measured by a growth inhibition assay as described (18). Tumor cells (104) were added to flat-bottom, 96-well microtiter plates, and different amounts of effector cells were co-cultured for two days. Then, 30 µl of MTT (1 mg/ml) was added for 4 h at 37˚C, followed by centrifugation, treatment with 150 µl DMSO, and measurement of OD540. The percentage cytotoxicity was calculated as previously described (18).
Cytokine ELISA
IFN-γ, IL-2, IL-4, IL-13, and TNF-α cytokine contents in culture supernatants were determined using BD Pharmingen capture ELISA kits (San Diego, USA) as recommended by the manufacturer. The results are expressed in pg/ml, calculated according to a standard curve obtained with recombinant cytokine.
PCR
Tumor cells were grown either in the absence or presence of optimal concentrations of IFN-γ for two days. RNA was then extracted and cDNA was synthesized using standard conditions (18). The forward and reverse primers used were: 5'-GCGACGCGGAGAATCCGAGAT-3' and 5'-GCTGATCTGCGCCGCCATGT-3' for Db, 5'-CGCCTACGACGGCTGCGATTA-3' and 5'-CCCAAGAGGCACCACCACAGAT-3' for Kb, and 5'-GAGCCTGTCGTCATCACCAT-3' and 5'-AGCACCTTGAGGAGTCCGAG-3' for tapasin. The PCR products were separated in agarose gels and revealed with BET.