Abstract
Cancer vaccines have been demonstrated to be a promising strategy for treating human neoplastic disease, but one of the limitations of these vaccines remains the paucity of target antigens to which to direct an effective immune response. We hypothesize that sperm fibrous sheath proteins may be a new class of useful antigens for developing successful cancer vaccines. This hypothesis is supported by the expression of two sperm fibrous sheath proteins, called sperm protein 17 and calcium-binding tyrosine-phosphorylation regulated protein, in tumors of unrelated histological origin and their capability to induce T cell-based immune responses.
Keywords: human, sperm, Sp17, CABYR, tumor antigens
Background
Cancer vaccines are an attractive therapeutic option for patients with primary and secondary malignancies; they rely on the manipulation of the immune system and its effector functions to eradicate tumor cells (1, 2). However, progress in the development of cancer vaccines for human malignancies has been slow, in part due to the paucity of suitable target antigens. Therefore in order to develop successful T cell-based therapies for the treatment of cancer, it is important to identify additional tumor antigens. Antigens expressed by neoplasms have principally been classified as: (a) shared tumor antigens; (b) differentiation antigens; (c) products of mutated genes, viral genes, over-expressed and amplified genes; (d) products of spliced variant genes; (e) common cancer-associated auto-antigens; (f) cancer-independent auto-antigens; and (g) products of under-expressed genes (3, 4). In addition, the existence of antigens expressed by the tumor endothelium has recently been described (5).
The hypothesis
The expression of sperm protein 17 (Sp17) and calcium-binding tyrosine-phosphorylation regulated protein (CABYR) in tumors of unrelated histological origin, and their natural localization in the human sperm fibrous sheath (FS), has led us to hypothesize that FS proteins might identify a new potential class of target antigens useful for developing cancer vaccines.
The human sperm fibrous sheath
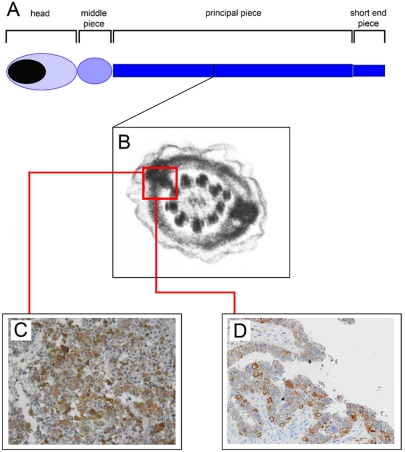
It is known that the flagellum of human spermatozoa (Figure 1A) consists of four distinct segments: (a) the connecting piece adjacent to the head; (b) the middle piece defined by a tightly packed helical array of mitochondria surrounding the cytoskeletal structures of the flagellum; (c) the principal piece, and (d) the short end piece (6).
Figure 1.
Proteins located in the sperm fibrous sheath may be useful target to develop effective immunotherapeutic strategies. (A) Schematic diagram showing the structure of the human spermatozoon. (B) Electron micrograph showing a cross-section of the sperm fibrous sheath. The FS is a unique cytoskeletal structure of the spermatozoon. The cancer-testis antigen Sp17 belongs to the fibrous sheath proteins family and has been found in several tumors of unrelated histological origin, including benign pituitary adenomas (C) and primary ovarian carcinomas (D).
The major cytoskeletal structures are the axoneme, also present in cilia, and the outer dense fibers and FS, which are unique to spermatozoa. The FS is a unique cytoskeletal entity which underlies the plasma membrane, surrounds the axoneme and outer dense fibers, and defines the extent of the principal piece of the sperm flagellum (7). It consists of two longitudinal columns connected by closely arranged semicircular ribs (Figure 1B) that assemble from the distal to the proximal end throughout spermatogenesis (7, 8). Although the function of the FS is unclear, it seems to serve as a scaffold for both glycolytic enzymes and constituents of signaling cascades, and it is well positioned to play a role in the regulation of sperm motility (7).
Several proteins localized in the FS have been identified, including Sp17, CABYR, AKAP3, AKAP4, TAKAP-80, Rhopilin, Ropporin, GSTM5 and fibrousheathin (7-12), although there are undoubtedly more to be reported. Of these proteins, Sp17 and CABYR have been analyzed in more detail.
Sperm protein 17: wider expressed than originally thought
A family of tumor-associated antigens, called cancer-testis (CT) antigens, has been found to be expressed in a limited number of normal human tissues and various human tumors of unrelated histological origin (13). One of these, Sp17, has been identified as a CT antigen in multiple myeloma, other blood malignancies, and ovarian cancer. Messenger RNA encoding Sp17 was detected in 17% of patients with multiple myeloma and in the primary tumor cells from 70% of patients with primary ovarian carcinoma (14, 15). At the protein level, Sp17 has been found in human germinal cells of the testis (except in the case of spermatogonia) (16), the ciliated epithelia of the respiratory airways, and both the male and female reproductive systems (17). It has also recently been found in the synoviocytes of females affected by rheumatoid arthritis (18) and the melanophages of cutaneous melanocytic lesions (19), as well as in a proportion of primary nervous system tumors (20) and a subset of esthesioneuroblastomas (21). As it is expressed in germinal cells and also various neoplastic tissues (Figure 1, C and D), Sp17 is more widely distributed in humans than originally thought. Although the function of Sp17 is still unknown, the high degree of sequence conservation throughout its N-terminal half and the presence of an A-kinase anchoring protein (AKAP)-binding motif within this region suggests that it might play a regulatory role in a protein kinase A (PKA)-independent AKAP complex in both germinal and neoplastic cells. Wang et al. (22) have recently suggested that the regulation of Sp17 gene expression in multiple myeloma cells occurs by promoter methylation. Although DNA methylation is not the primary control mechanism regulating the expression of most tissue-specific genes, their results indicate that promoter methylation can serve as the main regulatory mechanism for the expression of Sp17 in ARK-B, ARP-1, RPMI-8226 and KMS-11 multiple myeloma tumor cell lines (22).
Interestingly, human leukocyte antigen (HLA) class I-restricted Sp17-specific cytotoxic T lymphocytes (CTLs) were generated successfully from the peripheral blood of three patients with ovarian carcinoma at the time of disease presentation (15). These CTLs were able to lyse autologous Epstein-Barr virus-transformed lymphoblastoid cells in a Sp17-dependent manner. The CTLs also lysed Sp17-positive autologous tumor cells, suggesting that Sp17 is processed and presented in association with the HLA class I molecules in Sp17-positive tumor cells in a concentration and configuration that could be recognized by recombinant protein-primed CTLs. Tumor cell killing by the CTLs appeared to be mediated through the perforin pathway. Flow cytometric analysis of the CTLs indicated that they were predominantly CD8 in phenotype and produced interferon-γ and scant amounts of interleukin-4 (15).
CABYR: a novel cancer-testis antigen
CABYR is a calcium-binding tyrosine phosphorylation-regulated protein originally isolated from human spermatozoa (10). It is encoded by the gene CABYR on chromosome 18 at 18q11.2; the protein localizes to the principal piece of the sperm flagellum in association with the FS and exhibits calcium binding when phosphorylated during capacitation (10). Although CABYR was initially reported to be testis specific, recently it has been observed in lung (23) and brain tumors (24), suggesting that CABYR may be expressed in tumors of unrelated histological origin. Using a combination of conventional RT-PCR and real-time PCR to determine the expression levels of CABYR-a, CABYR-b, and CABYR-c in 16 normal tissues, 15 cell lines, and 36 lung cancer tissues, Luo et al. (23) have also concluded that CABYR is immunogenic in some cancer-bearing patients and that CABYR might be a useful candidate antigen for lung cancer immunotherapy. In addition to the analysis of mRNA expression, they used immunohistochemistry to analyze CABYR protein expression in lung cancer. The frequent expression of CABYR protein in lung cancer tissues and the absence of CABYR protein in non-tumoral lung tissues confirmed the result from analyzing CABYR at the mRNA level (23). Thus, the immunohistochemical analysis of CABYR provided more evidence supporting CABYR as a CT antigen in lung cancer.
Conclusions
Since the first cloning of a human tumor antigen (24), the identification of tumor antigens capable of eliciting an immune response in cancer patients and the development of immunogenic cancer vaccines targeting these antigens has represented formidable tasks for tumor immunologists (1, 2, 25).
Recently, cancer vaccines have been demonstrated to be a valid strategy to approach the treatment of human cancer. One of the limitations that remain is the scarcity of valid target antigens for effective cancer vaccines.
Interestingly, a post-meiotic expression pattern of Sp17 and CABYR was observed which increases the possibility for these two antigens to be used for effective cancer vaccines. It is well known that testis is an immune privileged site due to the presence of the blood-testis barrier. However, many of the identified CT antigens, including BAGE (26), PLU-1 (27), MORC (28), TEX-15 and TDRD1 (29), are expressed in spermatogonia located in the basal compartment of the seminiferous epithelium, which represents the non-protected side of the blood-testis barrier. Thus, cancer vaccines targeting these CT antigens may result in reduction or elimination of spermatogenesis and permanent injury to the testes of the patients. In contrast, vaccines targeting CT antigens, including Sp17 (16), CABYR (23), OY-TES1 (30), TPTE (31) and ADAM2 (32), which lie on the protected side of the blood-testis barrier (i.e. the adluminal compartment of the seminiferous epithelium) may reduce this risk (33, 34).
We have hypothesized here that sperm FS proteins, like Sp17 and CABYR, might constitute a new class of target antigens useful for developing cancer vaccines. This hypothesis can be tested and needs to be systematically validated as we have described before (35). If proven, the present hypothesis would not only increase the number of available target antigens in cancer vaccines, but would also add new knowledge on the relationship between human germ cell development and carcinogenesis (13, 36-38).
Abbreviations
- CT
cancer-testis
- FS
fibrous sheath
Acknowledgements
The authors are grateful to Dr. Elena Donetti for providing the electron microscopy image of sperm fibrous sheath, Drs. Fabio Grizzi and Barbara Franceschini for their immunohistochemistry contribution and Teri Fields for her assistance in editing this manuscript. W. Martin Kast holds the Walter A. Richter Cancer Research Chair.
References
- 1.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 2.Brinkman JA, Hughes SH, Stone P, Caffrey AS, Muderspach LI, Roman LD, Weber JS, Kast WM. Therapeutic vaccination for HPV induced cervical cancers. Dis Markers. 2007;23:337–352. doi: 10.1155/2007/245146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preuss KD, Zwick C, Bormann C, Neumann F, Pfreundschuh M. Analysis of the B-cell repertoire against antigens expressed by human neoplasms. Immunol Rev. 2002;188:43–50. doi: 10.1034/j.1600-065x.2002.18805.x. [DOI] [PubMed] [Google Scholar]
- 4.Dermime S, Armstrong A, Hawkins RE, Stern PL. Cancer vaccines and immunotherapy. Br Med Bull. 2002;62:149–162. doi: 10.1093/bmb/62.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, Grone HJ, Hallahan DE, Reisfeld RA, Debus J, Niethammer AG, Huber PE. Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11:6270–6279. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- 6.Sadler TW. Langman's medical embryology. Baltimore (MD): Williams & Wilkins; 1985. [Google Scholar]
- 7.Eddy EM, Toshimori K, O'Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–115. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 8.Fujita A, Nakamura K, Kato T, Watanabe N, Ishizaki T, Kimura K, Mizoguchi A, Narumiya S. Ropporin, a sperm-specific binding protein of rhophilin, that is localized in the fibrous sheath of sperm flagella. J Cell Sci. 2000;113:103–112. doi: 10.1242/jcs.113.1.103. [DOI] [PubMed] [Google Scholar]
- 9.Verdier Y, Farre G, Rouet N, Kele Z, Janaky T, Boue F. Identification of a new, testis-specific sperm antigen localized on the principal piece of the spermatozoa tail in the fox (Vulpes vulpes). Biol Reprod. 2005;72:502–508. doi: 10.1095/biolreprod.104.032623. [DOI] [PubMed] [Google Scholar]
- 10.Naaby-Hansen S, Mandal A, Wolkowicz MJ, Sen B, Westbrook VA, Shetty J, Coonrod SA, Klotz KL, Kim YH, Bush LA, Flickinger CJ, Herr JC. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev Biol. 2002;242:236–254. doi: 10.1006/dbio.2001.0527. [DOI] [PubMed] [Google Scholar]
- 11.Mei X, Singh IS, Erlichman J, Orr GA. Cloning and characterization of a testis-specific, developmentally regulated A-kinase-anchoring protein (TAKAP-80) present on the fibrous sheath of rat sperm. Eur J Biochem. 1997;246:425–432. doi: 10.1111/j.1432-1033.1997.t01-1-00425.x. [DOI] [PubMed] [Google Scholar]
- 12.Turner RM, Musse MP, Mandal A, Klotz K, Jayes FC, Herr JC, Gerton GL, Moss SB, Chemes HE. Molecular genetic analysis of two human sperm fibrous sheath proteins, AKAP4 and AKAP3, in men with dysplasia of the fibrous sheath. J Androl. 2001;22:302–315. [PubMed] [Google Scholar]
- 13.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 14.Lim SH, Wang Z, Chiriva-Internati M, Xue Y. Sperm protein 17 is a novel cancer-testis antigen in multiple myeloma. Blood. 2001;97:1508–1510. doi: 10.1182/blood.v97.5.1508. [DOI] [PubMed] [Google Scholar]
- 15.Chiriva-Internati M, Wang Z, Salati E, Timmins P, Lim SH. Tumor vaccine for ovarian carcinoma targeting sperm protein 17. Cancer. 2002;94:2447–2453. doi: 10.1002/cncr.10506. [DOI] [PubMed] [Google Scholar]
- 16.Grizzi F, Chiriva-Internati M, Franceschini B, Hermonat PL, Soda G, Lim SH, Dioguardi N. Immunolocalization of sperm protein 17 in human testis and ejaculated spermatozoa. J Histochem Cytochem. 2003;51:1245–1248. doi: 10.1177/002215540305100916. [DOI] [PubMed] [Google Scholar]
- 17.Grizzi F, Chiriva-Internati M, Franceschini B, Bumm K, Colombo P, Ciccarelli M, Donetti E, Gagliano N, Hermonat PL, Bright RK, Gioia M, Dioguardi N, Kast WM. Sperm protein 17 is expressed in human somatic ciliated epithelia. J Histochem Cytochem. 2004;52:549–554. doi: 10.1177/002215540405200414. [DOI] [PubMed] [Google Scholar]
- 18.Takeoka Y, Kenny TP, Yago H, Naiki M, Gershwin ME, Robbins DL. Developmental considerations of sperm protein 17 gene expression in rheumatoid arthritis synoviocytes. Dev Immunol. 2002;9:97–102. doi: 10.1080/1044667021000095186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschini B, Grizzi F, Colombo P, Soda G, Bumm K, Hermonat PL, Monti M, Dioguardi N, Chiriva-Internati M. Expression of human sperm protein 17 in melanophages of cutaneous melanocytic lesions. Br J Dermatol. 2004;150:780–782. doi: 10.1111/j.0007-0963.2004.05887.x. [DOI] [PubMed] [Google Scholar]
- 20.Grizzi F, Gaetani P, Franceschini B, Di Ieva A, Colombo P, Ceva-Grimaldi G, Bollati A, Frezza EE, Cobos E, Rodriguez y Baena R, Dioguardi N, Chiriva-Internati M. Sperm protein 17 is expressed in human nervous system tumors. BMC Cancer. 2006;6:23. doi: 10.1186/1471-2407-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bumm K, Grizzi F, Franceschini B, Koch M, Iro H, Wurm J, Ceva-Grimaldi G, Dimmler A, Cobos E, Dioguardi N, Sinha UK, Kast WM, Chiriva-Internati M. Sperm protein 17 expression defines 2 subsets of primary esthesioneuroblastoma. Hum Pathol. 2005;36:1289–1293. doi: 10.1016/j.humpath.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Zhang Y, Ramsahoye B, Bowen D, Lim SH. Sp17 gene expression in myeloma cells is regulated by promoter methylation. Br J Cancer. 2004;91:1597–1603. doi: 10.1038/sj.bjc.6602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo C, Xiao X, Liu D, Chen S, Li M, Xu A, Liu J, Gao S, Wu S, He D. CABYR is a novel cancer-testis antigen in lung cancer. Clin Cancer Res. 2007;13:1288–1297. doi: 10.1158/1078-0432.CCR-06-1742. [DOI] [PubMed] [Google Scholar]
- 24.Hsu HC, Lee YL, Cheng TS, Howng SL, Chang LK, Lu PJ, Hong YR. Characterization of two non-testis-specific CABYR variants that bind to GSK3beta with a proline-rich extensin-like domain. Biochem Biophys Res Commun. 2005;329:1108–1117. doi: 10.1016/j.bbrc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 25.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 26.Boel P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, Coulie P, Boon T, van der Bruggen P. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 27.Madsen B, Tarsounas M, Burchell JM, Hall D, Poulsom R, Taylor-Papadimitriou J. PLU-1, a transcriptional repressor and putative testis-cancer antigen, has a specific expression and localisation pattern during meiosis. Chromosoma. 2003;112:124–132. doi: 10.1007/s00412-003-0252-6. [DOI] [PubMed] [Google Scholar]
- 28.Inoue N, Hess KD, Moreadith RW, Richardson LL, Handel MA, Watson ML, Zinn AR. New gene family defined by MORC, a nuclear protein required for mouse spermatogenesis. Hum Mol Genet. 1999;8:1201–1207. doi: 10.1093/hmg/8.7.1201. [DOI] [PubMed] [Google Scholar]
- 29.Loriot A, Boon T, De Smet C. Five new human cancer-germline genes identified among 12 genes expressed in spermatogonia. Int J Cancer. 2003;105:371–376. doi: 10.1002/ijc.11104. [DOI] [PubMed] [Google Scholar]
- 30.Ono T, Kurashige T, Harada N, Noguchi Y, Saika T, Niikawa N, Aoe M, Nakamura S, Higashi T, Hiraki A, Wada H, Kumon H, Old LJ, Nakayama E. Identification of proacrosin binding protein sp32 precursor as a human cancer/testis antigen. Proc Natl Acad Sci U S A. 2001;98:3282–3287. doi: 10.1073/pnas.041625098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong XY, Su YR, Qian XP, Yang XA, Pang XW, Wu HY, Chen WF. Identification of two novel CT antigens and their capacity to elicit antibody response in hepatocellular carcinoma patients. Br J Cancer. 2003;89:291–297. doi: 10.1038/sj.bjc.6601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scanlan MJ, Gordon CM, Williamson B, Lee SY, Chen YT, Stockert E, Jungbluth A, Ritter G, Jager D, Jager E, Knuth A, Old LJ. Identification of cancer/testis genes by database mining and mRNA expression analysis. Int J Cancer. 2002;98:485–492. doi: 10.1002/ijc.10276. [DOI] [PubMed] [Google Scholar]
- 33.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filippini A, Riccioli A, Padula F, Lauretti P, D'Alessio A, De Cesaris P, Gandini L, Lenzi A, Ziparo E. Control and impairment of immune privilege in the testis and in semen. Hum Reprod Update. 2001;7:444–449. doi: 10.1093/humupd/7.5.444. [DOI] [PubMed] [Google Scholar]
- 35.Chiriva-Internati M, Grizzi F, Bright RK, Kast WM. Cancer immunotherapy: avoiding the road to perdition. J Transl Med. 2004;2:26. doi: 10.1186/1479-5876-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa FF, Le Blanc K, Brodin B. Concise review: cancer/testis antigens, stem cells, and cancer. Stem Cells. 2007;25:707–711. doi: 10.1634/stemcells.2006-0469. [DOI] [PubMed] [Google Scholar]
- 37.Kalejs M, Erenpreisa J. Cancer/testis antigens and gametogenesis: a review and "brain-storming" session. Cancer Cell Int. 2005;5:4. doi: 10.1186/1475-2867-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zendman AJ, Ruiter DJ, Van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194:272–288. doi: 10.1002/jcp.10215. [DOI] [PubMed] [Google Scholar]