Abstract
This study evaluates HLA gene expression and tumor infiltration by B-cells, macrophages, dendritic cells, T-helper and cytotoxic T-lymphocytes in response to short-term preoperative treatment with cyclooxygenase inhibitors. Patients with colorectal carcinoma were randomized to receive oral NSAID (indomethacin or celebrex) for three days preoperatively; controls received esomeprazol. Peroperative tumor biopsies and normal colon tissue were analyzed by microarray, quantitative PCR and immunohistochemistry. Efficacy of short-term systemic NSAID treatment was confirmed by measurement of PGE2 production in blood monocytes from healthy volunteers. NSAID treatment upregulated genes at the MHC locus on chromosome 6p21 in tumor tissue, but not in normal colon tissue, from the same patient. 23 of the 100 most upregulated genes belonged to MHC class II. HLA-DM, -DO (peptide loading), HLA-DP, -DQ, -DR (antigen presentation), granzyme B, H, perforin and FCGR3A (CD16) (cytotoxicity) displayed increased expression, as did CD8, a marker of cytotoxic T-lymphocytes, while HLA-A and -C expression were not increased by NSAID treatment. MHC II protein (HLA-DP, -DQ, -DR) levels and infiltration by CD4+ T-helper cells of tumor stroma increased upon NSAID treatment, while CD8+ cytotoxic T-lymphocytes increased in both tumor stroma and epithelium. Molecules associated with immunosuppressive T regulatory cells (FOXP3, IL-10) were significantly decreased in indomethacin-exposed tumors. Standard oral administration of NSAID three days preoperatively was enough to increase tumor infiltration by seemingly activated immune cells. These findings agree with previous information that high prostanoid activities in colorectal cancer increase the risk for reduced disease-specific survival following tumor resection.
Keywords: clinical trial, colorectal cancer, NSAID, immunohistochemistry, cellular immunity
Introduction
A growing amount of information demonstrates that eicosanoids are involved in complex processes related to cancer progression, such as tumorigenesis, angiogenesis, metastasis and immune response (1-5). Accordingly, several clinical studies have implied benefit of non-steroidal anti-inflammatory drugs (NSAIDs), particularly in patients with colorectal tumors (2-4, 6, 7). Our own research has provided evidence that tumor progression is attenuated by nonselective NSAIDs which decrease systemic inflammation, prolong survival and improve quality of life (8-11). In attempts to screen for genome-wide effects of indomethacin treatment in colorectal tumor cells, we performed expression profiling on microarrays focusing on findings in the MHC (major histocompatibility complex) locus on chromosome 6p21, a region associated with more than 100 disease conditions (12). This 3.6 Mb genomic sequence encodes the transplantation HLA- genes and others of importance for defense reactions and fundamental cellular processes (12). Interestingly, human tumors frequently lose expression of HLA molecules, and reduction or total loss has been confirmed in colorectal carcinoma (13-15). Cells participating in the immune response may thus fail to exert function without adequate MHC signaling in tumor cells, with the exception of natural killer (NK) cells which may recognize MHC class I-negative tumor cells. In the present study we describe the expression levels observed for genes representing HLA class I and II, markers for antigen presenting cells and for immune cells such as T-helper and cytotoxic T-lymphocytes (CTLs), pro-apoptotic cytotoxins, and NK cells in tumor tissue following preoperative oral NSAID treatment. These markers suggest that NSAIDs interact with colorectal cancer progression by alterations affecting immune surveillance.
Results
Microarray profiling was used to profile expression of the immune response, whereas quantitative PCR and immunohistochemistry were used to follow up on suggestive observations of RNA and protein levels.
Microarrays
Pooled tumor cDNA from six indomethacin-treated patients versus pooled tumor cDNA from six placebo-treated control patients were hybridized on whole human oligo microarrays. Results from three technical replicates were merged and 41059 features out of 44290 were informative. The expression levels of 2278 genes were found to have changed when filtering on two-fold up- or down-regulation (643 were upregulated and 1635 were downregulated). One major finding was a cluster of upregulated genes from the MHC locus on chromosome 6p21, comprising a 3.6 Mb sequence. In the list of the top 100 upregulated genes, 23 belonged to MHC class II genes and three belonged to the MHC region. Results from a "yellow control experiment" with self-self competition showed a mean ratio of 1.02 ± 0.123 (SD). Evaluation of Treg-associated molecules (FOXP3, IL-10, CTLA4, CCL22) showed significantly decreased levels of FOXP3 and IL-10 in tumor tissue from indomethacin-treated patients (P < 0.05), while the levels of CTLA4 and CCL22 were not altered. Hybridization analyses on pooled RNA indicated that MHC class II genes were not upregulated in normal mucosa from indomethacin-treated patients as was observed in tumor tissue (not shown).
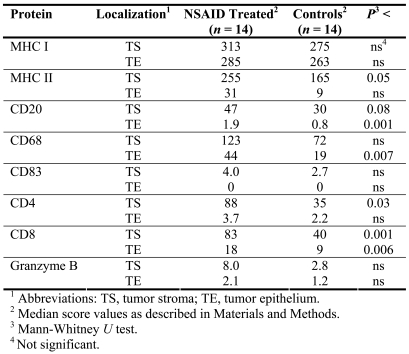
Real-time PCR
In our analyses of samples from individual patients, we focused on genes representing HLA class I and II, markers for antigen presenting cells, T-helper and effector cells (such as cytotoxic T-lymphocytes), pro-apoptotic cytotoxins, and membrane receptors triggering lysis by NK cells, granulocytes and macrophages. PCR expression values for alpha- and beta-chains of HLA molecules were summarized and presented for whole molecules, as well as for functional groups - i.e. HLA-DM, -DO for peptide loading, HLA-DP, -DQ, -DR for antigen presentation, and granzyme B, H and perforin representing cytotoxicity. The results of the microarray analysis for the six patients analyzed individually agreed with the results from the corresponding pooled RNA. Ten out of seventeen genes/functional groups showed significantly increased expression (P < 0.05-0.008) and three showed a trend to increased expression in tumors in NSAID-treated patients (Table 1). Increased expression of HLA-A or HLA-C was not observed in NSAID-treated patients based on individual quantitative PCR analyses (Table 1).
Table 1.
Transcript analysis by quantitative PCR of tumor RNA from individual patients.
Immunohistochemistry
Low or undetectable levels of MHC II protein in tumor epithelial cells were most frequent in the control group compared to tumors from NSAID-treated patients: 8/14 (57%) in the control group versus 4/14 (29%) in the NSAID-treated group, with a cutoff score of 10 or less (the maximum score measured being 400). The remaining patients (score > 10) had higher score values for MHC II protein in tumor epithelial cells (Figure 1). All patients stained positive for MHC II protein in tumor stroma, and MHC I protein in both tumor stroma and tumor epithelial cells. In NSAID-treated patients, both MHC class I and II protein had high scores in stroma and epithelial cells, with a significant increase in HLA-DP, -DQ, and -DR (Table 2). Antigen presenting cells, CD20+ B cells and CD68+ macrophages were significantly increased in the tumor epithelial area in NSAID-treated patients. There was also a trend to significance (P < 0.1) for CD20+ cells in tumor stroma. CD83+ protein, a marker for dendritic cells, did not show a significant change. The number of CD4+ T-helper cells seen in tumor stroma following NSAID treatment was significantly higher. Infiltration of CD8+ cytotoxic T-lymphocytes was significantly increased in tumor stroma and in tumor epithelium from NSAID-treated patients. In stromal parts, CD8+ and CD68+ cells were often seen as a barrier at the invading border of tumor cells and/or close to escaping single tumor cells or invasive islets of tumor tissue (Figure 2). Double staining of samples taken at random showed co-localization of the pro-apoptotic protein perforin and CD8 protein, which confirms the capability of CD8+ T-lymphocytes to kill target cells (Figure 3). Immunohistochemical double staining of CD16 and CD56, as markers for NK cells, did not show co-localization of proteins in samples taken at random. Instead CD16 protein seemed to be present in macrophages and granulocytes, as judged from their nuclear morphology. CD56 protein was sparsely detected in some patients.
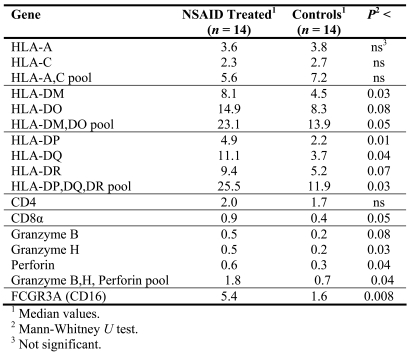
Figure 1.
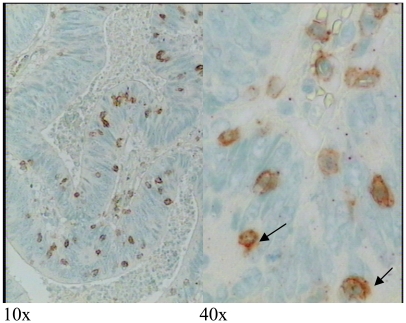
Immunohistochemistry of colon tumor tissue sections with antibody recognizing HLA-DP, -DQ, and -DR proteins. (A) Tumor epithelial cells and stroma. (B) Only stroma. (C) Negative control where the specific antibody has been substituted by normal mouse IgG. Protein stains brown. Background nuclei stain blue. Original magnification 40x.
Table 2.
Immunohistochemical evaluation of immune-associated molecules involved in peptide-signaling, antigen presentation, helper and effector cells, and pro-apoptotic cytotoxicity.
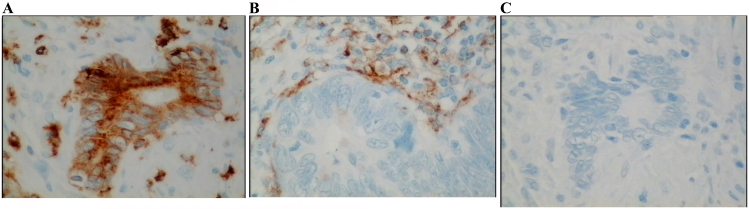
Figure 2.
Immunohistochemistry of colon tumor tissue section with antibody recognizing CD68 protein. Cells expressing CD68, a marker for macrophages, form a front along the invasive margin of tumor epithelial cells. Protein stains brown, background nuclei stain blue.
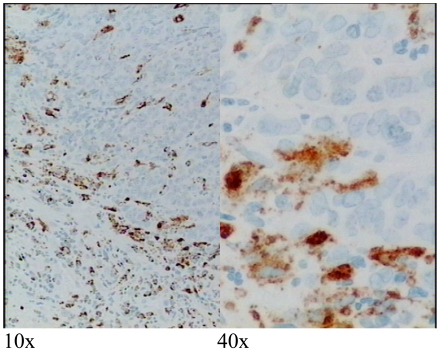
Figure 3.
Immunohistochemistry showing CD8+ cytotoxic T cells (brown staining) in tumor epithelium and dual-labeled pore-forming protein perforin in red (arrows). Background nuclei stain blue. Tumor cells which have been killed have left cleared spaces in the tumor epithelium.
Discussion
We have earlier reported that indomethacin treatment of patients with different types of solid cancer may prolong survival and improve physical functioning and quality of life when provided as palliative support (8). The explanation behind such improvements may be manifold, but local effects on tumor growth are involved. Evidence for this is an attenuation of angiogenesis, decreased tumor cell proliferation and increased tumor apoptosis (9-11, 16). The basis for this may be that COX-2 and 15-hydroxy-prostaglandin dehydrogenase expression in colorectal cancer predicts tumor tissue variation in PGE2 signaling on prostaglandin subtype receptor E1-4, where tumor tissue EP2 expression is a predictor of mortality (17, 18). However, it is also well known that prostanoids play a major role in the communication of immune responses, which represent complex interactions that may determine disease progression and metastasis. Therefore, it was considered important to evaluate local immune factors in the presence of preoperative treatment with NSAID, since it is recognized that NSAID can convert states of anergy into immune competence in malnourished and stressed patients (19, 20).
A major observation in the present study is that many genes belonging to the MHC locus on chromosome 6p21 were upregulated in human colon cancer during short preoperative treatment with conventional NSAIDs (indomethacin, celebrex). MHC genes control the synthesis of molecules that are essential for immune functions mediated by T-lymphocytes and NK cells (21). Antigen recognition by T-cells is dependent on the expression of HLA molecules by target cells. HLA molecules bind small antigenic peptides arising from enzymatically degraded proteins which are then presented on the cell surface, subsequently screened and recognized by the T-cell receptor. Normally, HLA class I molecules are expressed on all cells except red blood cells and cells of the testis, presenting intracellularly-derived peptide fragments to CD8+ cytotoxic T-lymphocytes, while HLA class II molecules, usually expressed on professional antigen presenting cells (APCs), present extracellularly-derived peptide fragments to CD4+ T-helper lymphocytes (22-27).
Intestinal epithelial cells may express low levels of HLA class II, although such expression is normally restricted to APCs, such as B-lymphocytes, macrophages and dendritic cells. Up-regulation of these molecules is associated with inflammation (13). Attempts have been made to turn tumor cells into antigen presenting cells by inducing HLA signaling, since human tumors often lose expression of HLA molecules, which may leave the immune system inactivated towards tumor cells (14, 28-30). Increased levels of PGE2 in colon cancer may negatively influence immunity. Arvind et al. (28) reported that SW1116 colon cancer cells expressed HLA class II antigens constitutively, especially HLA-DR. PGE2 reduced the expression of HLA-DR and removal of PGE2 restored the levels of HLA-DR, while PGF2α and LTB4 did not affect the expression of HLA-DR. Also, a colon cancer cell line (HT 29), which did not express HLA-DR constitutively, initiated HLA-DR expression when cells were treated with prostaglandin inhibitors like aspirin, indomethacin and sulindac. In contrast, HLA class I expression was not influenced by PGE2. Such effects agree with our present results that NSAIDs (indomethacin, celebrex) upregulated HLA class II expression in colon cancer tissue and MHC II protein in tumor epithelial cells following short-term preoperative treatment. All tumors displayed HLA class I protein in the tumor tissue without an upregulation of expression being observed, which thus seems to be enough for peptide presentation and CTL activation. PGE2 has been reported to suppress immune response by EP receptor signaling, where production of downstream targets such as chemokines and their receptors, associated with dendritic cells, macrophages and lymphocyte function, were inhibited (1, 31-35). PGE2 also downregulates cytokines (TNF-α, IFN-γ and IL-2) with T-helper cell-stimulatory function (Th1) and upregulates T-helper cells (Th2), as characterized by immunosuppressive cytokines such as IL-4, IL-6 and IL-10 (1, 36-41). This is conceptually in agreement with the results presented here showing that NSAID treatment increased the infiltration of B cells, macrophages, CD4+ T-helper cells, as well as CD8+ cytotoxic T-cells, in tumor tissue. We also observed increased RNA expression of granzyme H and perforin and a trend to increased granzyme B levels in such tumors capable of activating intracellular caspases that initiate apoptosis in target cells. Granzymes are released together with perforin, which is a pore-forming protein from the cytoplasmic granules of CTLs and NK cells (42-49). We conclude that such CTLs are ready to kill target cells based on the perforin protein seen in CD8+ cytotoxic T-lymphocytes, as evidenced by the "halos" surrounding condensed apoptotic tumor cells or the disruption of tumor cell patterns.
Several reports support the importance of activated tumor-specific CD8+ cytotoxic T-lymphocytes (50-53). Accordingly, Pagés et al. (54) reported that patients who suffered from colorectal cancer with no signs of metastatic spread (vascular emboli, lymphatic invasion or perineural invasion) had increased infiltration by immune cells (CTLs) with increased content of cytotoxins. Another study reported infiltration by CTLs in primary malignant melanoma and corresponding metastases, where CTLs were armed with cytotoxins only in primary tumors (55). Also, mobilization of granulocytes, lymphocytes and macrophages at the invasive border of gastrointestinal cancer were recently reported to be associated with improved survival (56, 57). Earlier studies have suggested that monocytes and macrophages are responsible for T-lymphocyte impairment by increased PGE2 production (58-61) There is a growing consensus based on vaccine trials that co-operation between CD4+ Th1 cells and activated CD8+ cytotoxic T-lymphocytes is necessary for adequate anti-tumor immune responses. The appearance of CD4+ CD25+/CD8+ CD25+ T regulatory cells (Tregs) was not functionally evaluated in the present study, but the expression of Treg-associated molecules (immunosuppressive FOXP3 and IL-10) decreased in indomethacin-exposed tumors in studies by others (62, 63).
It may well be that unspecific and specific COX inhibition exerts different effects in complex immune reactions that involve eicosanoids, although we believe that such differences are rather quantitative based on a systematic analysis of several unspecific, intermediately specific and specific cyclooxygenase inhibitors in our experimental models (64). Besides, indomethacin is the only NSAID resulting in survival differences in patients with malignancies so far (8). Thus, it was important for us to include indomethacin, but it was also tempting to include preliminary effects by more modern therapeutics, such as COX-2 inhibitors. Therefore, patients were randomized to receive either indomethacin or celebrex to form a preliminary group of NSAID-treated patients. Thus, the results presented here represent net effects of both unspecific and more specific COX-intervention, with patients on indomethacin or celebrex showing the same overall changes. Future research will eventually reveal fundamental differences between specific and unspecific COX-inhibition. Analysis of normal mucosa from NSAID-treated and control patients was performed to account for host reactions by NSAIDs. Comparison of expression ratios from array analyses of indomethacin-treated tumor tissue and normal mucosa confirmed that MHC class II genes were not upregulated in normal mucosa.
In conclusion, the results presented demonstrate that administration of NSAID for three days preoperatively was enough to turn the tumor microenvironment into what is, at least conceptually, a more favorable condition for patient outcome, with the appearance of tumor infiltration by immune cells with the potential to kill tumor cells. This agrees with observations in our previous investigations showing that COX activities, high tumor content of PGE2 and tumor expression of EP2 increased the risk for reduced survival (17, 65). Future studies should reveal detailed molecular events to explain the mechanism behind our potentially important observations with relevance for immune therapy of colon carcinoma, as well as surgical procedures.
Abbreviations
- COX
cyclooxygenase
- NSAID
non-steroidal anti-inflammatory drug
- PGE2
prostaglandin E2
Acknowledgements
This work was supported in part by grants from the Swedish Cancer Society (2014), the Swedish Research Council (08712), Tore Nilson Foundation, Assar Gabrielsson Foundation (AB Volvo), Jubileumskliniken Foundation, Inga-Britt & Arne Lundberg Research Foundation, Swedish and Göteborg Medical Societies and the Medical Faculty, Göteborg University, VGR 19/00, 1019/00, and the Wilhelm and Martina Lundgren Foundation.
References
- 1.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trifan OC, Hla T. Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J Cell Mol Med. 2003;7:207–222. doi: 10.1111/j.1582-4934.2003.tb00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann JR, DuBois RN. Cyclooxygenase-2 and gastrointestinal cancer. Cancer J. 2004;10:145–152. doi: 10.1097/00130404-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chell S, Kadi A, Williams AC, Paraskeva C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim Biophys Acta. 2006;1766:104–119. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, Richmond A, Strieter R, Dey SK, DuBois RN. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grau MV, Rees JR, Baron JA. Chemoprevention in gastrointestinal cancers: current status. Basic Clin Pharmacol Toxicol. 2006;98:281–287. doi: 10.1111/j.1742-7843.2006.pto_294.x. [DOI] [PubMed] [Google Scholar]
- 7.Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 8.Lundholm K, Gelin J, Hyltander A, Lonnroth C, Sandstrom R, Svaninger G, Korner U, Gulich M, Karrefors I, Norli B, Hafström L, Kewenter J, Olbe L, Lundell L. Anti-inflammatory treatment may prolong survival in undernourished patients with metastatic solid tumors. Cancer Res. 1994;54:5602–5606. [PubMed] [Google Scholar]
- 9.Gelin J, Andersson C, Lundholm K. Effects of indomethacin, cytokines, and cyclosporin A on tumor growth and the subsequent development of cancer cachexia. Cancer Res. 1991;51:880–885. [PubMed] [Google Scholar]
- 10.Lönnroth C, Svaninger G, Gelin J, Cahlin C, Iresjö BM, Cvetkovska E, Edström S, Andersson M, Svanberg E, Lundholm K. Effects related to indomethacin prolonged survival and decreased tumor growth in a mouse tumor model with cytokine dependent cancer cachexia. Int J Oncol. 1995;7:1405–1413. doi: 10.3892/ijo.7.6.1405. [DOI] [PubMed] [Google Scholar]
- 11.Lönnroth C, Andersson M, Lundholm K. Indomethacin and telomerase activity in tumor growth retardation. Int J Oncol. 2001;18:929–937. [PubMed] [Google Scholar]
- 12.Shiina T, Inoko H, Kulski JK. An update of the HLA genomic region, locus information and disease associations: 2004. Tissue Antigens. 2004;64:631–649. doi: 10.1111/j.1399-0039.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 13.Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol. 2003;46:33–57. doi: 10.1016/s1040-8428(02)00159-2. [DOI] [PubMed] [Google Scholar]
- 14.Hillman GG, Kallinteris NL, Lu X, Wang Y, Wright JL, Li Y, Wu S, Forman JD, Gulfo JV, Humphreys RE, Xu M. Turning tumor cells in situ into T-helper cell-stimulating, MHC class II tumor epitope-presenters: immuno-curing and immuno-consolidation. Cancer Treat Rev. 2004;30:281–290. doi: 10.1016/j.ctrv.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, Durrant LG. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118:6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson H, Lönnroth C, Wang W, Svanberg E, Lundholm K. Cyclooxygenase inhibition in early onset of tumor growth and related angiogenesis evaluated in EP1 and EP3 knockout tumor-bearing mice. Angiogenesis. 2005;8:339–348. doi: 10.1007/s10456-005-9023-8. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson A, Hansson E, Kressner U, Nordgren S, Andersson M, Wang W, Lonnroth C, Lundholm K. EP(1-4) subtype, COX and PPARgamma receptor expression in colorectal cancer in prediction of disease-specific mortality. Int J Cancer. 2007;121:232–240. doi: 10.1002/ijc.22582. [DOI] [PubMed] [Google Scholar]
- 18.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, Pretlow TP, Newman RA, Willis J, Dawson D, Markowitz SD. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markewitz A, Faist E, Lang S, Endres S, Fuchs D, Reichart B. Successful restoration of cell-mediated immune response after cardiopulmonary bypass by immunomodulation. J Thorac Cardiovasc Surg. 1993;105:15–24. [PubMed] [Google Scholar]
- 20.Gogos CA, Maroulis J, Zoumbos NC, Salsa B, Kalfarentzos F. The effect of parenteral indomethacin on T-lymphocyte subpopulations and cytokine production in patients under major surgical operations. Res Exp Med. 1995;195:85–92. doi: 10.1007/BF02576778. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Lora A, Algarra I, Collado A, Garrido F. Tumour immunology, vaccination and escape strategies. Eur J Immunogenet. 2003;30:177–183. doi: 10.1046/j.1365-2370.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–276. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 23.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 24.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpe AH, Abbas AK. T-cell costimulation--biology, therapeutic potential, and challenges. N Engl J Med. 2006;355:973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 26.Gerloni M, Zanetti M. CD4 T cells in tumor immunity. Springer Semin Immunopathol. 2005;27:37–48. doi: 10.1007/s00281-004-0193-z. [DOI] [PubMed] [Google Scholar]
- 27.Darrow TL, Abdel-Wahab Z, Seigler HF. Immunotherapy of Human Melanoma With Gene-Modified Tumor Cell Vaccines. Cancer Control. 1995;2:415–423. doi: 10.1177/107327489500200505. [DOI] [PubMed] [Google Scholar]
- 28.Arvind P, Papavassiliou ED, Tsioulias GJ, Qiao L, Lovelace CI, Duceman B, Rigas B. Prostaglandin E2 down-regulates the expression of HLA-DR antigen in human colon adenocarcinoma cell lines. Biochemistry. 1995;34:5604–5609. doi: 10.1021/bi00016a035. [DOI] [PubMed] [Google Scholar]
- 29.Wang RF. Enhancing antitumor immune responses: intracellular peptide delivery and identification of MHC class II-restricted tumor antigens. Immunol Rev. 2002;188:65–80. doi: 10.1034/j.1600-065x.2002.18807.x. [DOI] [PubMed] [Google Scholar]
- 30.Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C, Keiser N, Santaniello F, Tomasi TB. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 31.Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol. 2003;74:868–879. doi: 10.1189/jlb.0303116. [DOI] [PubMed] [Google Scholar]
- 32.Jing H, Yen JH, Ganea D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J Biol Chem. 2004;279:55176–55186. doi: 10.1074/jbc.M409816200. [DOI] [PubMed] [Google Scholar]
- 33.Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 34.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 35.Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 36.Salcedo R, Zhang X, Young HA, Michael N, Wasserman K, Ma WH, Martins-Green M, Murphy WJ, Oppenheim JJ. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood. 2003;102:1966–1977. doi: 10.1182/blood-2002-11-3400. [DOI] [PubMed] [Google Scholar]
- 37.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 38.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–1216. [PubMed] [Google Scholar]
- 40.Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holla VR, Wang D, Brown JR, Mann JR, Katkuri S, DuBois RN. Prostaglandin E2 regulates the complement inhibitor CD55/decay-accelerating factor in colorectal cancer. J Biol Chem. 2005;280:476–483. doi: 10.1074/jbc.M407403200. [DOI] [PubMed] [Google Scholar]
- 42.Trapani JA. Granzymes: a family of lymphocyte granule serine proteases. Genome Biol. 2001;2:REVIEWS3014. doi: 10.1186/gb-2001-2-12-reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 44.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 45.Waterhouse NJ, Trapani JA. CTL: Caspases Terminate Life, but that's not the whole story. Tissue Antigens. 2002;59:175–183. doi: 10.1034/j.1399-0039.2002.590301.x. [DOI] [PubMed] [Google Scholar]
- 46.Raja SM, Metkar SS, Froelich CJ. Cytotoxic granule-mediated apoptosis: unraveling the complex mechanism. Curr Opin Immunol. 2003;15:528–532. doi: 10.1016/s0952-7915(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 47.Clark R, Griffiths GM. Lytic granules, secretory lysosomes and disease. Curr Opin Immunol. 2003;15:516–521. doi: 10.1016/s0952-7915(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 48.Sedelies KA, Sayers TJ, Edwards KM, Chen W, Pellicci DG, Godfrey DI, Trapani JA. Discordant regulation of granzyme H and granzyme B expression in human lymphocytes. J Biol Chem. 2004;279:26581–26587. doi: 10.1074/jbc.M312481200. [DOI] [PubMed] [Google Scholar]
- 49.Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci U S A. 1999;96:5640–5644. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. http://www.cancerimmunity.org/v7p4/061216.htm [PMC free article] [PubMed] [Google Scholar]
- 51.Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10:309–313. [PubMed] [Google Scholar]
- 52.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res. 2006;66:7301–7309. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- 54.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 55.Parmiani G. Tumor-infiltrating T cells--friend or foe of neoplastic cells? N Engl J Med. 2005;353:2640–2641. doi: 10.1056/NEJMp058236. [DOI] [PubMed] [Google Scholar]
- 56.Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, Tuppurainen K, Makela J, Karttunen TJ, Makinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–2654. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Cuschieri A, Talbot IC, Weeden S. Influence of pathological tumour variables on long-term survival in resectable gastric cancer. Br J Cancer. 2002;86:674–679. doi: 10.1038/sj.bjc.6600161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wanebo HJ, Riley T, Katz D, Pace RC, Johns ME, Cantrell RW. Indomethacin sensitive suppressor-cell activity in head and neck cancer patients. The role of the adherent mononuclear cell. Cancer. 1988;61:462–474. doi: 10.1002/1097-0142(19880201)61:3<462::aid-cncr2820610310>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 59.Han T, Nemoto T, Ledesma EJ, Bruno S. Enhancement of T lymphocyte proliferative response to mitogens by indomethacin in breast and colorectal cancer patients. Int J Immunopharmacol. 1983;5:11–15. doi: 10.1016/0192-0561(83)90066-8. [DOI] [PubMed] [Google Scholar]
- 60.Balch CM, Dougherty PA, Cloud GA, Tilden AB. Prostaglandin E2-mediated suppression of cellular immunity in colon cancer patients. Surgery. 1984;95:71–77. [PubMed] [Google Scholar]
- 61.Soppi E, Eskola J, Ruuskanen O. Effects of indomethacin on lymphocyte proliferation, suppressor cell function, and leukocyte migration inhibitory factor (lmif) production. Immunopharmacology. 1982;4:235–242. doi: 10.1016/0162-3109(82)90005-4. [DOI] [PubMed] [Google Scholar]
- 62.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 63.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 64.Cahlin C, Gelin J, Andersson M, Lönnroth C, Lundholm K. The effects of non-selective, preferential-selective and selective COX-inhibitors on the growth of experimental and human tumors in mice related to prostanoid receptors. Int J Oncol. 2005;27:913–923. [PubMed] [Google Scholar]
- 65.Cahlin C, Lönnroth C, Arvidsson A, Nordgren S, Lundholm K. Growth associated proteins in tumor cells and stroma related to disease progression of colon cancer accounting for tumor tissue PGE2 content. Int J Oncol. 2008 in press. [PubMed] [Google Scholar]
- 66.Pachot A, Monneret G, Brion A, Venet F, Bohe J, Bienvenu J, Mougin B, Lepape A. Messenger RNA expression of major histocompatibility complex class II genes in whole blood from septic shock patients. Crit Care Med. 2005;33:31–38. doi: 10.1097/01.ccm.0000150958.20209.a3. Discussion 236-7. [DOI] [PubMed] [Google Scholar]
- 67.Nagarajan UM, Lochamy J, Chen X, Beresford GW, Nilsen R, Jensen PE, Boss JM. Class II transactivator is required for maximal expression of HLA-DOB in B cells. J Immunol. 2002;168:1780–1786. doi: 10.4049/jimmunol.168.4.1780. [DOI] [PubMed] [Google Scholar]
- 68.Luder CG, Lang C, Giraldo-Velasquez M, Algner M, Gerdes J, Gross U. Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J Neuroimmunol. 2003;134:12–24. doi: 10.1016/s0165-5728(02)00320-x. [DOI] [PubMed] [Google Scholar]
- 69.de la Calle-Martin O, Hernandez M, Ordi J, Casamitjana N, Arostegui JI, Caragol I, Ferrando M, Labrador M, Rodriguez-Sanchez JL, Espanol T. Familial CD8 deficiency due to a mutation in the CD8 alpha gene. J Clin Invest. 2001;108:117–123. doi: 10.1172/JCI10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Silva FS, Venturini DS, Wagner E, Shank PR, Sharma S. CD4-independent infection of human B cells with HIV type 1: detection of unintegrated viral DNA. AIDS Res Hum Retroviruses. 2001;17:1585–1598. doi: 10.1089/088922201753341997. [DOI] [PubMed] [Google Scholar]
- 71.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
Materials and methods
Patients
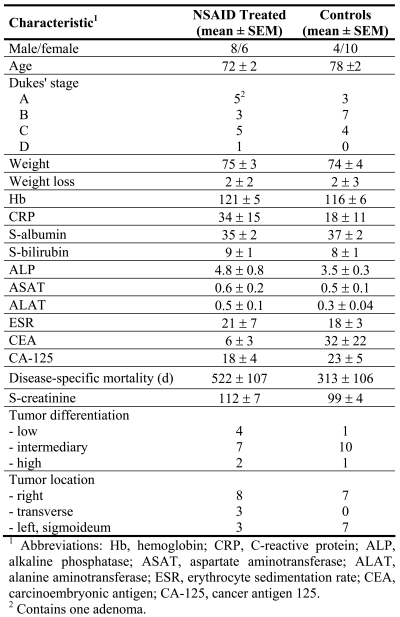
Patients who were to undergo primary and curative resection of colorectal cancer were randomized to receive NSAID or sham treatment during three days before surgery, between 1998 and 2004 at Sahlgrenska University Hospital, Gothenburg, Sweden. The patient group consisted of 16 females and 12 males with a median age of 74 ± 9 (SD) years (range 55-85 years). Tumors were histologically classified by certified pathologist as Dukes A (5), B (10), C (11) and D (1) corresponding to stages I-IV. One patient had villous adenoma but remained in the study. Tumor stages within treatment groups were as follows: in the controls (n = 14), Dukes A (2), B (7), C (5), and D (0); in the NSAID-treated patients (n = 14), Dukes A (3), B (3), C (6), D (1), and villous adenoma (1) (Table 3). NSAID treatment was indomethacin (Confortid, 50 mg x 2, Alpharma, n = 10) or Celebrex (100 mg x 2, Pfizer, n = 4) during 3 preoperative days, together with gastric prophylaxis (Nexium 40 mg x 1, Astra Zeneca, n = 14) which was also provided as a sham-treatment to all control patients (Table 3). None of our patients received perioperative radiochemotherapy, according to standard treatment criteria at our institution and individual judgment at the time of inclusion. None of the patients experienced objective or subjective side effects associated with NSAID or esomeprazol. There was no use of NSAID or NSAID-like drugs off the trial. The study (NCT00473980) was approved by the Committee for Ethics at the Medical Faculty, University of Gothenburg.
Table 3.
Patient characteristics before operation.
Assessment of medication efficacy
We used the capacity of white peripheral blood cells to produce PGE2 after endotoxin (LPS) challenge in vitro as a marker of NSAID treatment efficacy. Peripheral venous blood was drawn from healthy volunteers before and after consumption of indomethacin for three days as described above. EDTA mixed blood was diluted in RPMI 1640 (PAA Laboratories), layered on Lymphoprep (Nycomed) and centrifuged. White blood cells in the buffy coat were washed in medium and diluted in complete medium (RPMI 1640, 10% heat-inactivated FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 292 µg/ml L-glutamine) for a final concentration of 5 x 106 cells/ml. One milliliter per well was seeded in duplicate in a 24-well culture plate (Falcon). Lipopolysaccharide (LPS) (10 µg/ml cell suspension of Salmonella enteritides) was added to one well and saline to the duplicate well. Plates were kept at 37˚C in a humidified 5% CO2, 95% air cell incubator for three hours. Indomethacin (10 µg/ml cell suspension) was then added to inhibit further breakdown of arachidonic acid. The cell suspension was centrifuged for ten minutes at 300 x g at room temperature. The cell-free conditioned medium was kept at -20˚C until analysis of PGE2. Unconditioned culture medium was treated in the same way and saved for background PGE2 subtraction. PGE2 was extracted on Amprep C18 minicolumns (Amersham RPN 1900) for PGE2 assay (Amersham RPA 530) following acidification, ethanol addition and centrifugation of conditioned and blank medium. The radioimmunoassay was performed in duplicate, after conversion of extracted PGE2 by methyl oximation.
Three days of indomethacin treatment decreased both basal PGE2 levels (36-40 versus 16-17 pg/106 cells) and LPS-stimulated levels (124-151 versus 62-83 pg/106 cells).
Tumor and colon tissue material
Tumor tissue and normal colon tissue samples (down to the serosa layer) were collected at surgery, snap frozen in liquid nitrogen and stored at -70˚C until analysis. Recent samples were kept in RNA later (Ambion) for 24 hours at 4˚C and then kept at -20˚C until analysis of RNA expression. For immunohistochemistry, biopsies were kept in 4% buffered formaldehyde solution for 3 days at 4˚C, washed and kept in 70% ethanol until dehydration and paraffin embedding.
RNA extraction and cDNA synthesis
Total RNA was extracted with the RNeasy Fibrous Tissue Midi Kit (Qiagen), and DNase treatment was included according to the kit protocol. Quality and quantity of RNA were checked in an Agilent 2100 BioAnalyzer with the RNA 6000 Nano Assay Kit (Agilent Technologies). The concentration of RNA was also measured in a Nano Drop ND-1000A spectrophotometer (Nano Drop Technologies, Inc.). Aliquots of total RNA were used for real-time PCR, where 1 µg total RNA was reverse transcribed with ClonTech 1st STRAND™ cDNA Synthesis Kit (Becton Dickinson) and incubated for 1 hour at 42˚C, followed by 5 minutes at 94˚C. Each sample was then diluted to a final volume of 100 µl. Reactions were run in parallel, with the reverse transcriptase being omitted in the control for DNA contamination. Poly(A+) RNA was purified using the mRNA Purification Kit (Amersham Biosciences) for microarray analysis. Purified poly(A+) mRNA fractions were also checked in the BioAnalyzer and quantified in the NanoDrop.
Tumor mRNA for microarray analysis was pooled from six indomethacin-treated patients and from six sham-treated controls respectively (indomethacin-treated, 71 ± 11 (SD) years; controls, 74 ± 5 (SD) years; 2 males and 4 females in each group; 1 Dukes A, 2 Dukes B, and 3 Dukes C in each group). Similar principles were used for normal colon tissue from the same patients.
Microarray expression profiling
Four hundred nanograms of pooled mRNA from indomethacin-treated patients (test) were labeled with Cyanine 3-dCTP (Amersham BioSciences) in a cDNA synthesis reaction using the Agilent Fluorescent Direct Label Kit. Four hundred nanograms of pooled mRNA from control patients were labeled with Cyanine 5-dCTP in parallel. An expression array (Whole Human Genome Oligo Microarray, Agilent Technologies) containing 44290 features, including positive and negative control spots, was used. Hybridization was performed during 18 hours with test versus control cDNA in a dual-color experiment, followed by post-hybridization washes according to "in situ Hybridization Kit Plus" (Agilent Technologies) instructions. Microarrays were dried with nitrogen gas in a laminar flow bench and images were quantified on Agilent G2565 AA microarray scanner and fluorescence intensities were extracted using the Feature Extraction software program (Agilent Technologies). Dye-normalized, outlier- and background-subtracted values were analyzed using the GeneSpring software program, imported with the FE Plug-in (Agilent). Three technical replicates of tumor tissue were run, including dye-swap and two replicates of normal colon. Informative features from pooled tumor RNA were 41059 out of the 44290 features analyzed. Hands-on variation was checked in a "yellow experiment" where the same tumor RNA was labeled with both dyes competing for the same targets.
Quantitative real-time PCR
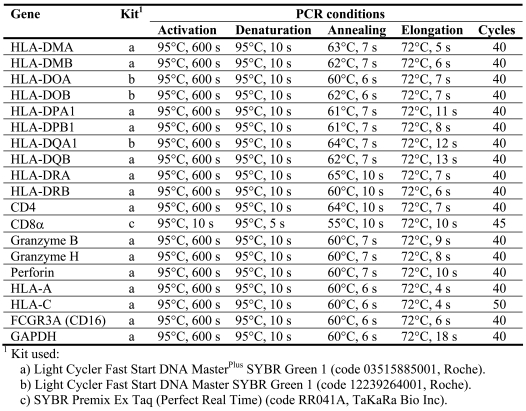
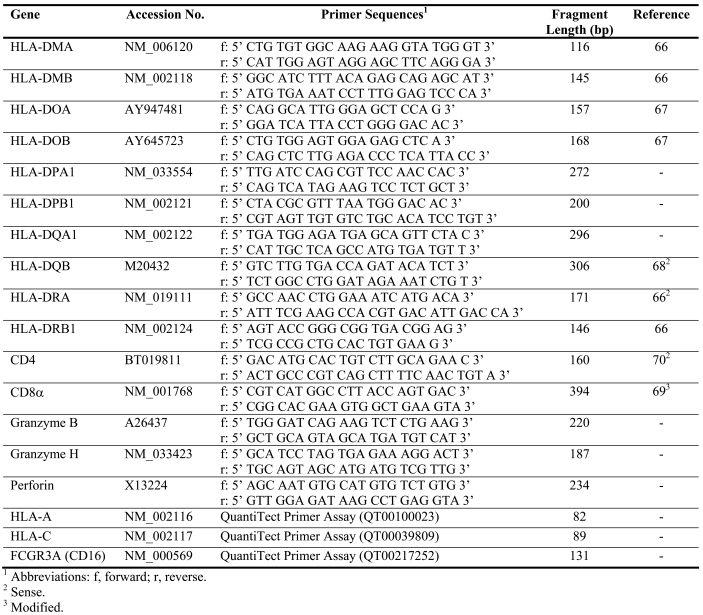
Real-time PCR was performed in a LightCycler 1.5 with LightCycler Fast Start DNA Master, LightCycler Fast Start DNA MasterPlus (Roche) or SYBR Premix Ex Taq Perfect Real Time (TaKaRa Bio Inc.). PCR conditions are given in Table 4. Primers for eighteen target genes, alpha and beta chain were included when applicable (except for CD8) and added to each capillary at a final concentration of 0.5 µM. Primer sequences, fragment length and gene accession number are given in Table 5 (66-70). Two microliters of cDNA were used for each amplification. All samples were analyzed in duplicate and compared to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Control Amplimer Set, 639003, BD Biosciences) which was the housekeeping gene which was the least variable of eleven candidates tested (17). Quantitative results were derived using the relative standard curve method, where the standard specimen was cDNA from an untreated human colon tumor (intermediate differentiation, Dukes C) resected at Sahlgrenska University Hospital. For CD8 alpha, granzymes and perforin, the expression level was too low in the untreated control tumor standard to obtain a standard curve. Instead pooled cDNA from five tumors from indomethacin-treated patients was used. All PCR products had the expected size, as analyzed with the Agilents BioAnalyzer in DNA 1000 Chip, although MHC genes may be polymorphic. All reactions were confirmed using both positive and negative controls (one dilution of standard curve cDNA and water substituted for cDNA, respectively).
Table 4.
PCR conditions and kits used for quantitative amplification.
Table 5.
Gene names and accession numbers, primer sequences and fragment lengths.
Immunohistochemistry
Formalin-fixed and paraffin embedded tissue sections (4 µm) were deparaffinized and rehydrated according to standard procedures and rinsed twice in 5 mM TBS, pH 7.8. Sections were microwave-irradiated in different target retrieval solutions. Eleven antibodies were used. Host species, final concentrations and suppliers are given in Table 6. Sections were mounted with Shandon Coverplates. Non-specific protein binding was initially blocked with TBS containing 5% fat-free dry milk, followed by the procedure described in EnVision Dual Link System-HRP (K4065, Dako Cytomation). Normal IgG from the same species as the primary antibody was used as the negative control and was incubated in parallel. Diaminobenzidine (DAB), included in the EnVision kit, was used as chromogen. Counterstaining was performed in Mayer´s hematoxylin and mounting was done in Mountex following dehydration (Histolab Products, Gothenburg, Sweden). The EnVision Doublestain Kit (K1395, Dako Cytomation) was used according to the manufacturer's instructions for dual labeling of CD8 and perforin respective to CD16 and CD56, where perforin and CD16 stained red (Fast Red) and CD8 and CD56 stained brown (Dab+). Sections were mounted in aqueous medium (Faramount, Dako Cytomation) following counterstaining.
Table 6.
Antibodies, host species, final concentrations and target retrieval solutions used for immunohistochemistry, with suppliers and product numbers.
Observations of protein occurrence and distribution on antibody-stained tissue sections were performed using a Nikon eclipse E400 microscope and Digital HyperHAD Color Video Camera (Sony) using the Easy Image analysis software (Tekno Optik AB, Sweden). Cells were counted manually, distinguishing between tumor epithelium and tumor stroma, in the presence of numerous infiltrating cells (CD4, CD8, CD20, CD68) in ten representative fields of view. Similarly, cells were counted in sections, also with tumor epithelium/tumor stroma distinction, with or without adjustment to equalize areas when cells and granule were sparse (CD83, granzyme B, perforin, CD16, C56). A semiquantitative scoring system was used for the evaluation of MHC immunostaining, where the protein distribution area (expressed as a percentage) was multiplied by an intensity score ranging from 0 to 5 (maximum score = 100 x 5).
Statistics
Primary variables were significantly altered gene transcript levels indicative of functional groups of molecules, such as the HLA locus. Patients were randomized to receive either sham treatment or study drugs (indomethacin, celebrex) in a 2:1 relationship by a computer-based algorithm (71). Power estimates on tumor tissue transcripts (α < 0.05, β = 0.80) indicated that 10-12 patients in the NSAID-treated and control groups would be enough to identify significantly altered gene expression according to our earlier experience (17).
The ratio between expressed transcripts in tumors from NSAID-treated patients versus tumors from placebo-treated patients was calculated using the GeneSpring software program for microarray evaluation. Filtering on flags and filtering on fold change (two-fold up and down) were used. Real-time PCR results were calculated as gene expression values relative to GAPDH expression obtained from the LightCycler® data files. Differences between study and control groups were tested for statistical significance using the non-parametric Mann-Whitney U-test, with P < 0.05 considered as significant in two-tailed tests and P < 0.10 a trend to significance.