Abstract
Mouse monoclonal antibody MX35 was developed against ovarian cancer. The antibody showed homogeneous reactivity with approximately 90% of human ovarian epithelial cancers and with a limited number of normal tissues by immunohistochemistry. Although mAb MX35 has been used in a number of clinical trials in ovarian cancer, it has been difficult to define the molecular identity of MX35. We report here that mAb MX35 recognizes the sodium-dependent phosphate transport protein 2b (NaPi2b) in human cancer cells. This conclusion is based on several lines of experimental evidence, including 1) the identification of SLC34A2, the gene coding for NaPi2b, by immunoscreening an ovarian cancer cell line cDNA expression library with mAb MX35; 2) mass spectrometry sequencing of peptides obtained by fragmentation from mAb MX35 affinity-purified antigen, which show complete sequence homology to amino acid sequences in NaPi2b; 3) selective down-regulation of SLC34A2 gene expression by RNA interference and the resulting loss of mAb MX35 binding to MX35-expressing human cancer cells; and 4) the demonstration of specific mAb MX35 reactivity with recombinant fusion proteins and with synthetic peptides of the putative largest extracellular loop of NaPi2b. We further show that NaPi2b in cancer cells is expressed on the cell surface as a heavily N-glycosylated protein, with evidence of additional post-translational modifications such as palmitoylation and the formation of disulfide bridges in the major extracellular loop. Membrane transporter molecules, such as NaPi2b, represent a new family of potential cell surface targets for the immunotherapy of cancer with monoclonal antibodies.
Keywords: ovarian cancer, monoclonal antibody, MX35, protein sequence analysis, NaPi2b, tumor antigen, cancer immunotherapy
Introduction
Epithelial ovarian cancer is one of the most common gynecologic malignancies and the fifth most frequent cause of cancer death in women, with an estimated incidence of about 15,000 in 2006 in the US alone. Most patients present with advanced disease and the mortality rate is approximately 65% of the incidence rate. Debulking surgery and platinum-based combination chemotherapy (including taxanes) are current treatment modalities; however, the majority of patients with relapsed epithelial ovarian cancer eventually succumb to the disease. Thus, there is a need for novel treatment modalities in ovarian cancer, including targeted therapies such as immunotherapy with monoclonal antibodies or cancer vaccine-based approaches.
Monoclonal antibody MX35 was generated from mice immunized with a cocktail of human ovarian carcinoma cells prepared from four different surgical specimens. Reactivity by immunohistochemistry with cryostat sections of a panel of frozen human tissues was used as the major hybridoma selection criteria (1). Monoclonal antibody MX35 showed homogeneous reactivity with approximately 90% of human ovarian epithelial cancers and a limited number of normal tissues. Subsequently, the localization and biodistribution of radiolabeled murine antibody was studied in patients with ovarian carcinoma in phase I clinical trials. Intact MX35 antibody targeted well to tumors in patients with ovarian cancer (2) and F(ab')2 of MX35 was shown to localize to micrometastatic ovarian carcinoma deposits within the peritoneal cavity (3). In preparation for the use of the antibody for radioimmunotherapy, e.g. as a targeted carrier of radionuclides in patients with ovarian carcinoma, the murine antibody and its fragments are currently being investigated in preclinical models (4, 5) and in a phase I clinical trial in patients with ovarian cancer as the carrier of the alpha-particle-emitting astatine-211 (6).
Despite the use of mAb MX35 in clinical trials, the detailed molecular nature of the antigen recognized by mAb MX35 has not yet been identified. Initial immunochemical characterization has described the MX35 antigen as a 95 kDa cell surface glycoprotein with the antigenic epitope stabilized by disulfide bonds (7). We now report on the identification of the MX35 antigen as the sodium-dependent phosphate transport protein 2b (NaPi2b), representing a new family of potential cell surface targets for the immunotherapy of cancer with monoclonal antibodies.
Results
Molecular cloning of the MX35 antigen by immunoscreening of an OVCAR-3 cDNA expression library
Screening of a cDNA library generated from the ovarian cancer cell line OVCAR-3, which expresses MX35 antigen on its cell surface, identified three distinct mAb MX35-reactive clones (N1, N4 and N6). Reactivity of the three clones was confirmed in a secondary screening. Subsequent cDNA restriction analysis and sequencing identified the strongest mAb MX35-reactive clone N1 as encoding an N-terminally truncated form of the sodium-dependent phosphate transport protein 2b (gene SLC34A2, size 3394 bp; fragment 742 bp - 4135 bp), clone N4 as an N-terminally truncated form of Zinc-finger protein 638 (ZN638, size 4637 bp; fragment 1798 bp - 6434 bp), and clone N6 as an N-terminally truncated form of the same Zinc-finger protein 638 (size 3585 bp; fragment 2850 bp - 6434 bp).
Confirmation of the identity of MX35 antigen as the sodium-dependent phosphate transport protein 2b encoded by the SLC34A2 gene
Co-typing of mRNA expression and MX35 antigen cell surface expression
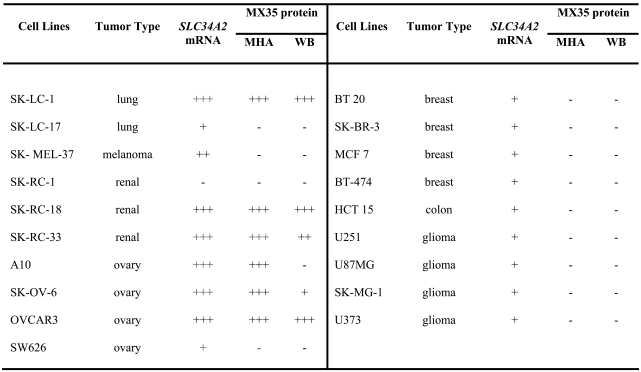
A panel of cancer cell lines was co-typed for SLC34A2 mRNA expression by RT-PCR and for cell surface expression of MX35 protein antigen in a mixed hemadsorption assay (MHA) using mAb MX35 as probe. In addition, cell lysates were probed by Western blot (WB) analysis for MX35 expression. A panel of cancer cell lines with known expression of the MX35 antigen was included. Strong expression of SLC34A2 mRNA correlated with MX35 antigen cell surface expression in all cells analyzed (Table 1). No such correlation was found for the Zinc-finger protein 638 (data not shown).
Table 1.
SLC34A2 mRNA expression and MX35 protein expression in a panel of different human cancer cell lines.
Mass spectrometry sequencing by fragmentation of peptides
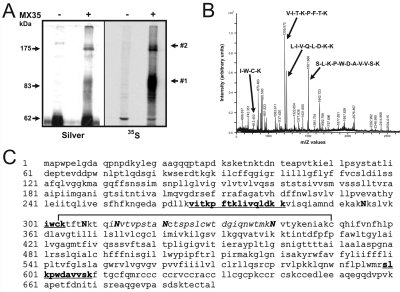
Immunoprecipitation of the MX35 antigen from two different MX35 antigen-positive cell lines, OVCAR-3 and SK-RC-18, following metabolic labeling of proteins with [35S] methionine and [35S] cysteine showed one major (approx. 90 kDa) and one minor (approx. 180 kDa) band on SDS-PAGE (Figure 1A). Subsequently, preparative quantities of the MX35 immune complexes were separated by SDS-PAGE and 35S-labeled protein bands were excised and subjected to tryptic digestion, followed by sequencing by fragmentation of peptides using mass spectrometry. Fragmentation of four selected peptides provided amino acid sequences VITKPFTK, LIVQLDKK, IWCK and SLKPWDAVVSK (Figure 1B), which showed complete alignment to amino acids 266-273, 274-281, 301-304 and 599-609 in the NaPi2b protein sequence (Figure 1C). The NaPi2b peptides were identified in both protein bands. This identifies sodium-dependent phosphate transport protein 2b as the MX35 antigen, rather than the Zn-finger protein also identified in the initial molecular screen.
Figure 1.
Mass spectrometry sequencing by fragmentation of peptides. Cell surface expressed MX35 antigen was isolated from two different MX35 antigen-positive cell lines, OVCAR-3 and SK-RC-18, by immunoprecipitation following metabolic labeling of proteins with 35S methionine and 35S cysteine. (A) SDS-PAGE of MX35 immune complexes labeled with 35S and visualized by autoradiofluorography or by silver staining. Monoclonal antibody MX35 precipitated the antigen in two forms, band #1 and #2, differing in size. (B) Peptide mass fingerprinting of trypsin digested Lys-tagged and sulfonated protein. (C) Sequence of the sodium-dependent phosphate transporter 2b protein. Peptides detected by mass spectrometry are shown in bold. The putative disulfide-bonded loop (aa 303-350) is also shown. The region containing the epitope recognized by mAb MX35 is shown in italics. Asparagine (N) residues that are probable N-linked glycosylation sites are shown in capital letters.
RNA interference
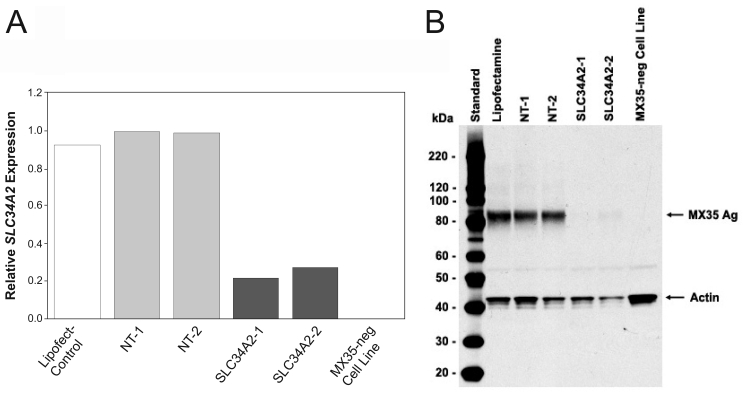
Two different sets of SLC34A2-specific "on-target" siRNAs were prepared to analyze if selective down-regulation of SLC34A2 gene expression by RNA interference would result in the loss or reduced binding of MX35 antibody in MX35-expressing cancer cells. Both SLC34A2-specific siRNA sets selectively down-regulated SLC34A2 mRNA in SK-RC-18 and OVCAR-3 cells as determined by real-time RT-PCR (Figure 2A). Binding of MX35 antibody to cell surface expressed MX35 antigen was significantly reduced as determined in MHA (data not shown). Specific down-regulation of MX35 protein antigen levels was confirmed by Western blot analysis (Figure 2B). "Non-targeting" siRNA had no effect on SLC34A2 mRNA and MX35 protein antigen expression levels in both cell lines. These results further validate NaPi2b as the MX35 antigen.
Figure 2.
Effects of siRNA interference on the level of SLC34A2 mRNA and MX35 protein expression in SK-RC-18 cells. Cells were transfected with SLC34A2 siRNA or control siRNA (NT1 and NT2) in the presence of Lipofectamine 2000. Cells were assayed 72 hours after transfection. (A) Total RNA was extracted and SLC34A2 mRNA levels were determined by real-time RT-PCR. (B) Cells were lysed and the level of protein expression was analyzed by SDS-PAGE and Western blotting using mAb MX35 and an anti-actin antibody.
Mapping of the antibody binding site in NaPi2b
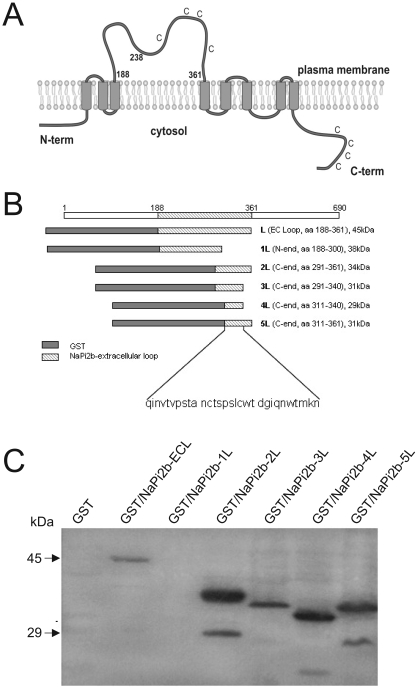
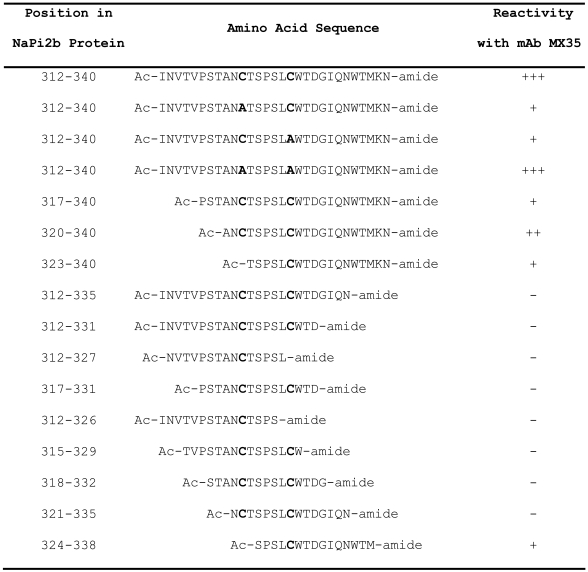
Bioinformatic analysis suggested that the protein encoded by SLC34A2 has at least 8 potential transmembrane domains, 5 putative intracellular domain sites and 4 putative extracellular domain (ECD) loops, with both the N- and C-terminal regions facing the cytoplasm (Figure 3A). Taking into account that mAb MX35 recognizes an epitope expressed on the cell surface, the potentially largest potential ECD loop was expressed as a glutathione S-transferase (GST) fusion protein (covering aa 188-361 of NaPi2b) (Figure 3B) in E. coli and as a His-tagged protein in Sf9 insect cells using a baculovirus expression system, and then probed for reactivity with mAb MX35 in Western blots. Both fusion proteins were recognized by mAb MX35. Preincubation of mAb MX35 with the bacterial fusion protein could selectively block binding of mAb MX35 to naturally expressed MX35 antigen in ovarian cancer tissue by immunocytochemistry (data not shown). Subsequently, shorter fusion proteins truncated from the N- and the C-terminus (Figure 3B) were studied and the mAb MX35-binding epitope was narrowed down to a fusion protein containing amino acids 311-340 of the NaPi2b protein sequence (Figure 3C). Binding of mAb MX35 to this peptide sequence was further confirmed by ELISA and dot blot immune staining using a synthetic 29-mer peptide representing amino acids 312-340 of the NaPi2b protein as the antigen. This peptide was further truncated from the N- and the C-terminus and the MX35-reactive epitope was narrowed to amino acids 324-338, as determined in a peptide spot analysis (Table 2). Within this region, the sequence WTM (aa 336-338) seems to be highly critical for antibody recognition. Although the amino acid region 324-338 (SPSLCWTDGIQNWTM) of the human protein is highly homologous to its murine counterpart (SPSYCWTDGIQNWTI), the murine protein was not recognized by mAb MX35 in a Western blot analysis of various mouse tissues, further confirming WTM as critical for MX35 antibody recognition (data not shown). Interestingly, the cysteine residues at position 322 and 328 are not required, as they could be replaced with alanine residues, even though antibody reactivity with the full-length wild type NaPi2b is reduction sensitive. These amino acids are probably not involved in the cysteine loop postulated to stabilize the epitope; this loop probably spans amino acids 303 to 350 (Figure 1C; UniProtKB/Swiss-Prot entry O95436).
Figure 3.
MX35 epitope mapping. (A) Schematic diagram of the predicted membrane topology of the sodium-dependent phosphate transporter 2b protein. (B) Schematic presentation of the NaPi2b protein and fusion proteins derived from the potentially largest extracellular region (aa 188-361) and the location of the MX35 epitope. (C) NaPi2b fusion proteins expressed in bacteria and immunoblotted with mAb MX35 after separation by SDS-PAGE.
Table 2.
Monoclonal antibody MX35 epitope mapping using a series of synthetic peptides located in a putative extracellular domain of the NaPi2b protein.
Further biochemical characterization of the MX35 antigen (NaPi2b)
General characteristics
Western blot analysis and radioimmunoprecipitation experiments with mAb MX35 showed that the protein is expressed in OVCAR-3 and SK-RC-18 cells in at least two major forms, one migrating in SDS-PAGE at approx. 90 kDa and a second migrating at about 180 kDa (Figure 1A); these could be monomeric and dimeric forms of the protein. While the recognition by mAb MX35 of its antigen is sensitive to reduction, immunoprecipitation of metabolically-labeled MX35 antigen showed no obvious difference in the SDS-PAGE migration pattern between reducing and non-reducing conditions. Extraction of protein from OVCAR-3 cells with Triton-X114 detergent showed that almost all of the mAb MX35-reactive protein partitioned into the lower, detergent enriched fraction, confirming that NaPi2b is an integral membrane protein (data not shown).
Glycosylation characteristics
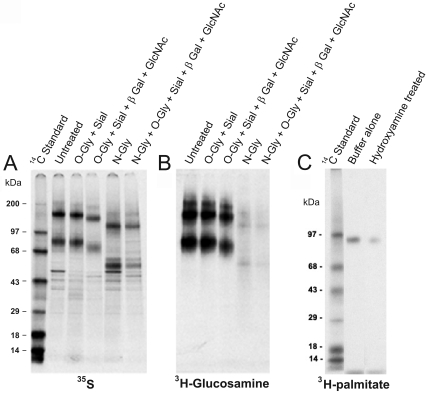
Sequence analysis of the SLC34A2 gene product predicts 5 potential N-glycosylation sites at N295, N308, N313, N321 and N340 (Figure 1C). Interestingly, all 5 putative glycosylation sites appear clustered within a rather short peptide region (aa 295-340) located in the largest predicted extracellular domain loop to which the mAb MX35 epitope was also mapped (Figure 1C). Metabolic labeling of SK-RC-18 cells with 3H-glucosamine, followed by immune precipitation with mAb MX35, showed that NaPi2b is indeed heavily glycosylated (Figure 4B) and treatment with N-glycanase but not O-glycanase removed most of this label. Treatment of [35S] Met- and Cys-labeled immunoprecipitates with various glycosidases showed that the glycan chains were sensitive to N-glycosidase, confirming that almost all sugars in the MX35 antigen were N-linked as predicted. Only a minor proportion of the carbohydrate was sensitive to O-glycosidase and sialidase or to a mixture of O-glycosidase, sialidase, β-galactosidase and N-acetylglucosaminidase. Treatment with a combination of N- and O-glycosidases resulted in a migration shift of the MX35 reactive bands in SDS-PAGE from approx. 90 kDa to 55-60 kDa and from approx. 180 kDa to 130-140 kDa. The former figure is much lower than the peptide size predicted for the unprocessed protein based on its cDNA sequence (75.7 kDa) but the protein may migrate anomalously on SDS-PAGE because of its many hydrophobic regions.
Figure 4.
NaPi2b/MX35 antigen biochemical characterization. Monoclonal antibody MX35 immune precipitations from metabolically-labeled SK-RC-18 cell lysates separated by SDS-PAGE under reducing conditions and visualized by autoradiography. (A) Enzymatic deglycosylation analysis of 35S-labeled immune complexes. (B) Enzymatic deglycosylation analysis of immune complexes derived from 3H-glucosamine labeled cells. (C) Acylation analysis of immune complexes from 3H-palmitate labeled cells.
Acylation characteristics
Bioinformatic studies revealed a cysteine-rich region at the C-terminal end, which could encompass potential sites for palmitoylation. Indeed, metabolic labeling of SK-RC-18 cells with 3H-palmitic acid followed by immune precipitation with mAb MX35 and SDS-PAGE analysis, suggests that the MX35 protein is acylated (Figure 4C). Treatment with base showed that acylation is partially reversible.
Discussion
Monoclonal antibody MX35 was developed as a therapeutic reagent for the treatment of ovarian cancer. In phase I clinical trials radiolabeled murine antibody MX35 targeted well to tumors in patients with ovarian cancer (2, 3) and the antibody is currently being studied in a phase I clinical trial as the carrier of alpha-particle-emitting radionuclide astatine-211 for radioimmunotherapy of ovarian cancer (6). Although there has been this clinical exploration of mAb MX35, it has been difficult to define the molecular identity of the MX35 antigen. We now have unambiguously identified the sodium-dependent phosphate transport protein 2b (NaPi2b) as the molecular target recognized by mAb MX35. This conclusion is based on the following experimental evidence:
A clone encoding for an N-terminally truncated form of NaPi2b (gene SLC34A2, size 3394 bp; fragment 742 bp - 4135 bp) was isolated from a cDNA library generated from the ovarian cancer cell line OVCAR-3 which expresses MX35 on its cell surface.
Co-typing of a panel of cancer cell lines showed a good correlation between SLC34A2 RNA expression by RT-PCR and MX35 cell surface expression, as determined by mixed hemadsorption assays.
Isolation of MX35 from the MX35-expressing cancer cell lines OVCAR-3 and SK-RC-18 and subsequent protein sequencing by fragmentation of peptides using mass spectrometry provided amino acid sequences that showed complete alignment to amino acid sequences in NaPi2b.
Selective down-regulation of SLC34A2 gene expression in MX35-expressing cancer cells by RNA interference resulted in the loss or reduced binding of mAb MX35.
The largest putative extracellular loop of the multi-transmembrane protein NaPi2b was expressed in E. coli and in insect cells and both expression products were recognized by mAb MX35 in Western blot assays and blocked binding of mAb MX35 to ovarian cancer tissues in immunohistochemistry.
A small synthetic peptide with 100% sequence homology to amino acids 312-340 of the major extracellular loop of NaPi2b was reactive with mAb MX35 in dot blot assays and by ELISA.
SLC34A2 is a member of the solute carrier gene family (8, 9) and the gene maps to chromosome 4p15.2. The SLC34A2 gene codes for a multi-pass membrane protein of 690 amino acids (Fig 3A). This protein has been reported to mediate transport of inorganic phosphate into epithelial cells via sodium ion co-transport and may have a role in phosphate homeostasis (for review, see 10 and 11) and in the synthesis of surfactants in lung alveoli (12). The human protein, sodium-dependent phosphate transport protein 2b (NaPi2b) or solute carrier family 34 member 2, also has several other synonyms. SLC34A2 cDNA was first isolated and cloned from a human small intestine and lung cDNA library (8, 9), and subsequent gene expression analysis by Northern blot showed that SLC34A2 was highly expressed in the lung and at low to moderate levels in several other normal human tissues including trachea, kidney, small intestine, ovary, placenta, uterus, testis, prostate, pancreas, mammary gland, thyroid gland, and salivary gland. An EST database search confirmed expression of the SLC34A2 gene in thyroid, ovary, lung, trachea and mammary gland, but showed only a few EST counts for kidney, small intestine, placenta, uterus, testis and prostate. Expression in the lung was further confirmed by genome scans (13, 14) and mRNA visualization by in situ hybridization showed the highest expression in alveolar type II cells (13). Increased SLC34A2 gene expression has been reported for ovarian cancer (15) and papillary thyroid cancer (16).
So far, the human SLC34A2 has been characterized largely on the gene level and NaPi2b transporter activities were generally studied with recombinant protein expressed in Xenopus oocytes (8, 9, 13, 17, 18). Little has been reported on the biochemistry of the naturally expressed protein product of SLC34A2. In this report, we provide experimental evidence that NaPi2b is naturally expressed as a heavily glycosylated plasma membrane protein in cancer cells. We could show that the predicted, potentially largest ECD (aa 188-361) of NaPi2b is indeed expressed on the cell surface, or at least the major part of it, as the antigenic epitope recognized by mAb MX35 (aa 324-338) is located within the predicted ECD and mAb MX35 bound to NaPi2b-expressing cancer cells in a MHA cell surface binding assay. We also provide experimental evidence that the predicted disulfide bridge in the ECD of NaPi2b is formed between C303 and C350 and does not involve C322 and C328. This is based on the observation that mAb MX35 can only react with non-reduced, naturally expressed NaPi2b, but recognized the synthetic peptide consisting of amino acids 311-340 (containing C322 and C328) in both reduced and non-reduced form. NaPi2b is heavily glycosylated and we have shown by exoglycosidase cleavage that the vast majority of the carbohydrates are N-linked. Predicted N-glycosylation sites are located in the ECD at amino acids N295, N308, N313, N321 and N340. It is of interest that all predicted glycosylations sites are within or in close proximity to the amino acid epitope recognized by mAb MX35 (aa 324-338) but do not interfere with binding of the antibody, as both glycosylated and non-glycosylated NaPi2b bound equally well to mAb MX35 in Western blot assays and immune precipitation assays. We also provide experimental evidence for further post-translational modifications of NaPi2b, showing by metabolic labeling that palmitate was incorporated into the protein, indicating that NaPi2b is likely to be naturally palmitoylated. Potential palmitoylation sites may be located within the C-terminal cytoplasmic domain which contains a cysteine rich stretch of 17 cysteines within amino acids 613-645. Acylation of NaPi2b was partially reversible by treatment with hydroxylamine, suggesting that NaPi2b may be palmitoylated both transiently and permanently. The protein appears to be expressed in the plasma membrane in at least two major forms distinguishable by their apparent molecular weight (90 kDa and 180 kDa) by SDS-PAGE, and which probably represent monomeric and dimeric forms of the protein as determined by mass spectrometry. Presence of homodimeric forms of NaPi2b have been proposed based on freeze fracture microphotograph analysis of oocytes expressing recombinant flounder NaPi2b (11).
With the finding that mAb MX35 recognizes a protein product of SLC34A2, it has now become possible to link mRNA expression profiles of the SLC34A2 gene with its gene products on the protein level and to study NaPi2b expression in normal tissues and in cancer. Whether the SLC34A2 gene products in cancer and in normal tissues are the same or are different will need to be determined, as anomalous SLC34A2 gene expression has recently been reported. Exon mutations in SLC34A2 (the gene contains 13 exons of which 12 are coding exons) have recently been described to occur in patients with pulmonary alveolar microlithiasis (13, 14). These mutations were predicted to affect translation, potentially resulting in various altered gene products including truncated proteins or proteins with amino acid substitutions, and aberrant splicing or an inability to express the protein. Several of those aberrant translations resulted in a functional loss of the NaPi2b protein, when expressed as recombinant protein in vitro. Since SLC34A2 is apparently the only phosphate transporter known to be highly expressed in the lung, inactivating mutations of the SLC34A2 gene were associated with accumulations of calcium phosphate microliths in the alveolar space and SLC34A2 has been reported to be the causative gene for pulmonary alveolar microlithiasis in two independent studies (13, 14). We have now initiated a mutational analysis of the SLC34A2 gene in various MX35 antigen-expressing cancer types, including ovarian, lung and kidney cancer, to investigate if mutated forms of the gene may also be present in cancer.
The MX35 antibody, which we now have shown to recognize NaPi2b, had initially been developed against ovarian cancer and the antibody showed homogeneous reactivity with approximately 90% of human ovarian epithelial cancers, but with only a limited number of normal tissues, by immunohistochemistry. In frozen tissue sections, mAb MX35 reactivity was detected with epithelial cells of normal lung, bronchus, thyroid, uterus, cervix, Fallopian tube, sweat glands and the collecting ducts in the kidney (1). Although this tissue expression profile of MX35 on the protein level appears to correlates well with the gene expression profile reported for SLC34A2, it is obvious that the NaPi2b protein expression analysis in human tissue will need to be revisited with more advanced immunohistochemical methodologies. We have now embarked on a comprehensive study investigating NaPi2b tissue expression in normal human tissue and in a wide range of different human cancer types, employing novel antigen retrieval techniques which facilitate the reactivity of MX35 in archival material.
What are the prospects for NaPi2b as a target for antibody-based cancer therapies in humans? The main concern would be expression in normal tissue, particularly the lung. However, in the initial biodistribution/pharmacokinetics analysis of mAb MX35 in humans, there was good tumor localization with no evidence for accumulation in the lung or other normal tissues. From other studies with monoclonal antibody targeting in clinical trials, in vitro expression of antigen by immunohistochemistry does not necessarily predict antibody localization in vivo, and this is best explained by inaccessibility of antigen-expressing cells to blood-borne antibody. The other issue that needs to be addressed is the relative impact of this phosphate pump and other related SLC family members in cancer cells compared to normal cells with regard to viability and proliferation. Resolving these questions of in vivo accessibility and the role of these genes in cancer cells will be critical in determining the optimal therapeutic strategies for mAb MX35. With the identification of NaPi2b as the molecular target of mAb MX35, it became evident that the MX35 antigen is not only expressed in ovarian cancers but also in a series of other epithelial cancers, including lung cancer, thyroid cancer and renal cancer, potentially expanding the therapeutic application of the MX35 antibody to these and other types of cancer. NaPi2b is the first example of a phosphate transporter that can be targeted as a cancer antigen with a monoclonal antibody. It is conceivable that other members of the large solute transporter family, most of them like SLC34A2/NaPi2b under tight regulatory control, also become abnormally expressed in cancer and thus turn into potential targets for immunotherapy of cancer with antibodies and vaccines.
Abbreviations
- ECD
extracellular domain
- GST
glutathione S-transferase
- MHA
mixed hemadsorption assay
- NaPi2b
sodium-dependent phosphate transport protein 2b
Acknowledgements
This work was conducted as part of the Atlantic Philanthropies/Ludwig Institute for Cancer Research Program. R. Kiyamova was supported by short term fellowships from the European Association for Cancer Research (EACR) and the International Union against Cancer (UICC).
References
- 1.Mattes MJ, Look K, Furukawa K, Pierce VK, Old LJ, Lewis JL Jr, Lloyd KO. Mouse monoclonal antibodies to human epithelial differentiation antigens expressed on the surface of ovarian carcinoma ascites cells. Cancer Res. 1987;47:6741–6750. [PubMed] [Google Scholar]
- 2.Rubin SC, Kostakoglu L, Divgi C, Federici MG, Finstad CL, Lloyd KO, Larson SM, Hoskins WJ. Biodistribution and intraoperative evaluation of radiolabeled monoclonal antibody MX35 in patients with epithelial ovarian cancer. Gynecol Oncol. 1993;51:61–66. doi: 10.1006/gyno.1993.1247. [DOI] [PubMed] [Google Scholar]
- 3.Finstad CL, Lloyd KO, Federici MG, Divgi C, Venkatraman E, Barakat RR, Finn RD, Larson SM, Hoskins WJ, Humm JL. Distribution of radiolabeled monoclonal antibody MX35 F(ab')2 in tissue samples by storage phosphor screen image analysis: evaluation of antibody localization to micrometastatic disease in epithelial ovarian cancer. Clin Cancer Res. 1997;3:1433–1442. [PubMed] [Google Scholar]
- 4.Back T, Andersson H, Divgi CR, Hultborn R, Jensen H, Lindegren S, Palm S, Jacobsson L. 211At radioimmunotherapy of subcutaneous human ovarian cancer xenografts: evaluation of relative biologic effectiveness of an alpha-emitter in vivo. J Nucl Med. 2005;46:2061–2067. [PubMed] [Google Scholar]
- 5.Elgqvist J, Andersson H, Back T, Claesson I, Hultborn R, Jensen H, Lindegren S, Olsson M, Palm S, Warnhammar E, Jacobsson L. Fractionated radioimmunotherapy of intraperitoneally growing ovarian cancer in nude mice with 211At-MX35 F(ab')2: therapeutic efficacy and myelotoxicity. Nuclear Med Biol. 2006;33:1065–1072. doi: 10.1016/j.nucmedbio.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Hultborn RAH, Bäck T, Divgi C, Elgqvist J, Himmelman J, Horvath G, Jensen H, Lindegren S, Palm S, Jacobsson L. Pharmacokinetics and dosimetry of 211at-MX35 F(ab')2 in therapy of ovarian cancer-preliminary results from an ongoing phase I study [abstract].; Cancer Biother Radiopharm; 2006. pp. 373–381. [Google Scholar]
- 7.Welshinger M, Yin BW, Lloyd KO. Initial immunochemical characterization of MX35 ovarian cancer antigen. Gynecol Oncol. 1997;67:188–192. doi: 10.1006/gyno.1997.4846. [DOI] [PubMed] [Google Scholar]
- 8.Feild JA, Zhang L, Brun KA, Brooks DP, Edwards RM. Cloning and functional characterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem Biophys Res Commun. 1999;258:578–582. doi: 10.1006/bbrc.1999.0666. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Bai L, Collins JF, Ghishan FK. Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium-phosphate (Na+-Pi) transporter (SLC34A2). Genomics. 1999;62:281–284. doi: 10.1006/geno.1999.6009. [DOI] [PubMed] [Google Scholar]
- 10.Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 11.Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Intl. 2006;70:1548–1559. doi: 10.1038/sj.ki.5001813. [DOI] [PubMed] [Google Scholar]
- 12.Traebert M, Hattenhauer O, Murer H, Kaissling B, Biber J. Expression of type II Na-P(i) cotransporter in alveolar type II cells. Am J Physiol. 1999;277:L868–L873. doi: 10.1152/ajplung.1999.277.5.L868. [DOI] [PubMed] [Google Scholar]
- 13.Huqun, Izumi S, Miyazawa H, Ishii K, Uchiyama B, Ishida T, Tanaka S, Tazawa R, Fukuyama S, Tanaka T, Nagai Y, Yokote A, Takahashi H, Fukushima T, Kobayashi K, Chiba H, Nagata M, Sakamoto S, Nakata K, Takebayashi Y, Shimizu Y, Kaneko K, Shimizu M, Kanazawa M, Abe S, Inoue Y, Takenoshita S, Yoshimura K, Kudo K, Tachibana T, Nukiwa T, Hagiwara K. Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis. Am J Respi Crit Care Med. 2007;175:263–268. doi: 10.1164/rccm.200609-1274OC. [DOI] [PubMed] [Google Scholar]
- 14.Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, Yildirim Z, Gocmen A, Tolun A. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangel LB, Sherman-Baust CA, Wernyj RP, Schwartz DR, Cho KR, Morin PJ. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene. 2003;22:7225–7232. doi: 10.1038/sj.onc.1207008. [DOI] [PubMed] [Google Scholar]
- 16.Jarzab B, Wiench M, Fujarewicz K, Simek K, Jarzab M, Oczko-Wojciechowska M, Wloch J, Czarniecka A, Chmielik E, Lange D, Pawlaczek A, Szpak S, Gubala E, Swierniak A. Gene expression profile of papillary thyroid cancer: sources of variability and diagnostic implications. Cancer Res. 2005;65:1587–1597. doi: 10.1158/0008-5472.CAN-04-3078. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Uno JK, Inouye M, Xu L, Drees JB, Collins JF, Ghishan FK. Regulation of intestinal NaPi-IIb cotransporter gene expression by estrogen. Am J Physiol. 2003;285:G1317–G1324. doi: 10.1152/ajpgi.00172.2003. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo A, Negoro T, Seo T, Kitao Y, Shindo M, Segawa H, Miyamoto K. Inhibitory effect of JTP-59557, a new triazole derivative, on intestinal phosphate transport in vitro and in vivo. Eur J Pharmacol. 2005;517:111–119. doi: 10.1016/j.ejphar.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 20.Yin BW, Finstad CL, Kitamura K, Federici MG, Welshinger M, Kudryashov V, Hoskins WJ, Welt S, Lloyd KO. Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int J Cancer. 1996;65:406–412. doi: 10.1002/(SICI)1097-0215(19960208)65:4<406::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Ritter G, Cohen LS, Nice EC, Catimel B, Burgess AW, Moritz RL, Ji H, Heath JK, White SJ, Welt S, Old LJ, Simpson RJ. Characterization of posttranslational modifications of human A33 antigen, a novel palmitoylated surface glycoprotein of human gastrointestinal epithelium. Biochem Biophys Res Commun. 1997;236:682–686. doi: 10.1006/bbrc.1997.6966. [DOI] [PubMed] [Google Scholar]
- 22.Iwahana H, Yakymovych I, Dubrovska A, Hellman U, Souchelnytskyi S. Glycoproteome profiling of transforming growth factor-beta (TGFbeta) signaling: nonglycosylated cell death-inducing DFF-like effector A inhibits TGFbeta1-dependent apoptosis. Proteomics. 2006;6:6168–6180. doi: 10.1002/pmic.200600384. [DOI] [PubMed] [Google Scholar]
- 23.Peters EC, Horn DM, Tully DC, Brock A. A novel multifunctional labeling reagent for enhanced protein characterization with mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2387–2392. doi: 10.1002/rcm.517. [DOI] [PubMed] [Google Scholar]
- 24.Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ, Altorki NK. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 25.Pfreundschuh M, Shiku H, Takahashi T, Ueda R, Ransohoff J, Oettgen HF, Old LJ. Serological analysis of cell surface antigens of malignant human brain tumors. Proc Natl Acad Sci USA. 1978;75:5122–5126. doi: 10.1073/pnas.75.10.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Materials and methods
Cell lines and antibodies
All cell lines were obtained from the cell bank of the Ludwig Institute for Cancer Research, New York Branch at Memorial Sloan-Kettering Cancer Center. Murine mAb MX35 (IgG1) was purified from hybridoma supernatant by protein G chromatography.
Immunoscreening of OVCAR-3 cDNA library
The cDNA expression library from the OVCAR-3 cell line has been described (19). A total of 1x106 recombinants phages were screened with MX35 monoclonal antibody (5 µg/ml TBS with 0.2% non-fat dried milk) for 15 h at room temperature. MX35 positive clones were further confirmed by secondary and tertiary screening. Isolated positive phages were converted into pBK-CMV phagemid using the Stratagene (La Jolla, CA) in vivo excision protocol. Rescued plasmid DNAs were purified using a miniprep kit (Qiagen, Valencia, CA) and subjected to restriction analysis and DNA sequencing.
Metabolic radiolabeling of cultured cells
Cultured cells were metabolically labeled with the Tran 35S-label mixture (MP Biomedicals, Inc. Irvine, CA) and 3H-glucosamine (Amersham Biosciences UK, Buckinghamshire, UK) as described previously (20). Metabolic palmitoylation using 3H-palmitic acid was performed as described (21).
Radioimmunoprecipitation, SDS-PAGE, autoradiography and immunoblotting
Radioimmunoprecipitation, SDS-PAGE, autoradiography and immunoblotting were performed as described (21).
MALDI-TOF/TOF mass spectrometry
Metabolic labeling, immunoprecipitation, SDS-PAGE and detection of 35S-labeled proteins were performed as described earlier. In-gel tryptic digestion and peptide mass fingerprinting were performed as described (22) with additional treatment of excised protein bands with DTT before destaining and digestion. In parallel experiments, extracted peptides were subjected to the labeling of lysine amino acid residues using 2-methoxy-4,5 dihydro-1H-imidazole (23) and to sulphonation of the N-terminus of peptides to improve fragmentation (Ettan CAF MALDI sequencing kit, GE healthcare Amersham Biosciences AB, Uppsala, Sweden). Peptides were subjected to de novo sequencing by fragmentation using post-source decay (PSD) in a Bruker Ultraflex MALDI TOF/TOF instrument (Bruker Daltonics, Bremen, Germany). The PSD spectra were interpreted manually. Experiments were performed with two different cell lines, OVCAR-3 and SK-RC-18, and three repeats were carried out for each cell line.
RNA interference assay
Two different sets of SLC34A2-specific SMARTpool siRNA (ON-Targetplus™ and siGENOME) and nontargeting SMARTpool siRNA controls (ON-Targetplus™ siCONTROL and standard siCONTROL) were purchased from Dharmacon, Inc. (Lafayette, CO). The transfection of siRNA was performed in the presence of Lipofectamine 2000 and Opti-MEM media (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions at a final concentration of 40 nM. Cells treated with Lipofectamine 2000 only and non-targeting siRNA pools were used as controls. Seventy-two hours after the transfection, cells were processed for MHA, real-time PCR and Western blot analysis.
RNA isolation, reverse transcription (RT)-PCR analysis and real-time PCR
RNA isolation, reverse transcription (RT)-PCR analysis (24) and real-time PCR were performed using standard molecular biology techniques. The gene specific primers used to amplify SLC34A2 by RT-PCR were 5'-TCA GCC AAA TTG CAA TGA AC-3' and 3'-ATC ATG ATC AGG CAA CCA CA-5'.
Cloning, expression and purification of GST/NaPi2b fusion proteins
Various regions of the human NaPi2b large extracellular loop were PCR amplified and cloned into the pGEX4T1 vector in frame with GST. Expression of GST/NaPi2b fusions in BL21 DE3 cells was induced by 1 mM IPTG for 3 hours at 37˚C. Cell pellets were disrupted by sonication in lysis buffer [25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Triton and protease inhibitor cocktail (Boehringer Mannheim, Germany)] and GST fusion proteins were purified from clarified supernatants by affinity chromatography using GST-Sepharose as recommended by the manufacturer.
Mixed hemadsorption assays
The mixed hemadsorption assay (MHA), which detects surface-bound IgG by adherence of rabbit anti-mouse IgG-coated human red blood cells (blood group O) to target cells, was performed as described (25).
Synthetic peptide microarray
Membrane arrays of synthetic peptides overlapping amino acids 312-340 of the NaPi2b protein sequence were custom made (JPT Peptide Technologies, Inc., Springfield, VA). For epitope mapping, membranes were incubated for 3 hours with 1 µg/ml primary antibody, followed by incubation with HRP-labeled goat anti-mouse polyclonal antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hours. Bound antibody was visualized by chemiluminescence (Western Lightning Plus). For epitope mapping under reducing condition, membranes were incubated in 1 mM DTT for 1 hour at room temperature and then washed extensively prior to antibody incubation.
Peptide blocking and peptide ELISA
A peptide corresponding to amino acids 312-340 of NaPi2b was chemically synthesized (Bio-Synthesis, Inc. Lewisville, TX) and co-incubated at varying concentrations with mAb MX35 prior to assaying mAb MX35 binding to MX35-positive and MX35-negative cells by MHA. Binding of mAb MX35 to the synthetic peptide 312-340 was also assayed by ELISA using NUNC Maxisorp plates to which the peptide (10 µg/ml in water) was adsorbed for 2 hours at room temperature.
Triton X-114 extraction
Cells were lysed in 1% Triton X-114 (Sigma, St. Louis, MO, USA) in 15 mM Tris-HCl containing 0.0025% (w/v) of bromophenol blue (Bio-Rad Laboratories, Inc., Hercules, CA) and Complete Protease Inhibitor Cocktail (Roche Diagnostics GmbH, Mannheim, Germany) at 4˚C for 30 min. The lysate was centrifuged twice at 16,000 x g for 10 minutes at 4˚C. The solubilized cell extract was loaded onto 6% sucrose in 15 mM Tris-HCl, pH 7.5, containing 0.06% Triton X-114 and protease inhibitors and incubated at 37˚C for 30 min, followed by centrifugation at 5,000 x g for 30 min at room temperature. The separated phases were then subjected to analysis.
Glycosidase treatments and acylation analysis
Aliquots of 35S-labeled or 3H-glucosamine-labeled immune complexes were subjected to treatment with various glycosidases: N-Glycanase; PNGaseF (5 mU), Sialidase A (5 mU), O-Glycanase (1.25 mU), β(1-4) Galactosidase (3 mU), and β-N-Acetylglucosaminidase (40 mU) using the Prozyme Enzymatic Deglycosylation Kit (Glyko, San Leandro, CA) according to manufacturer's instruction. Deglycosylation was monitored by SDS-PAGE, autoradiography and Western blot analysis as described above. 3H-palmitoyl-labeled NaPi2b was obtained by immunoprecipitation from SK-RC-18 cells cultured in the presence of 3H-palmitic acid, separated by 4-12% Bis-Tris SDS-PAGE gel, and visualized by autoradiofluorography. To test for thioester-bound palmitic acid, SDS-PAGE gels were preincubated with 0.2 M potassium hydroxide in methanol, or 1 M hydroxylamine-hydrochloric acid, pH 7.5, or 1 M Tris, pH 7.5, followed by autoradiography (21).