Abstract
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a negative regulator of T-cell mediated immune responses and is the target of new anti-tumor immunotherapy strategies. Ipilimumab is a fully human, antagonistic monoclonal antibody directed against CTLA-4. Results from preclinical and early clinical trials support currents phase II/III testing of ipilimumab as first- and second-line therapy for metastatic melanoma. Ipilimumab promotes durable objective responses and/or stable disease in patients with metastatic melanoma. Adverse events are medically manageable, largely immune-related, and presumably linked to the drug's mechanism of action. As more patients are treated with ipilimumab, it is becoming clear that the kinetics of responses are heterogeneous and significantly different from those of chemotherapy and other immunotherapy. Though objective response or stable disease is observed within 'conventional' time frames, responses have been observed weeks to months after therapy initiation. Response or stable disease may be preceded by apparent early disease progression, or may occur simultaneously with different progressing lesions within the same patient (a 'mixed' response). It is likely that the unique kinetics of response is a result of the time required to enhance and maintain an anti-tumor immune response to ipilimumab therapy. Consequently, patients may benefit from continued ipilimumab treatment through clinically known relevant disease progression or non-response during the full induction dosing schedule (12 weeks), without additional therapies. Understanding the kinetics of response to ipilimumab will help clinicians to manage patients who may benefit from treatment. In this article, several cases that illustrate the kinetics of response to ipilimumab are discussed.
Keywords: human, melanoma, ipilimumab, case reports
Introduction
Ipilimumab (formerly MDX-010) is a fully human, antagonistic monoclonal antibody directed against cytotoxic T lymphocyte antigen-4 (CTLA-4) (1), a T-cell surface receptor that negatively regulates T-cell mediated immune responses (2). The role of CTLA-4 in quelling normal T-cell responses was confirmed with the generation of CTLA-4 deficient mice that have a lethal lymphoproliferative phenotype (3, 4).
Unlike vaccines or other immunotherapies, ipilimumab specifically targets CTLA-4 to reduce T-cell suppression and allow effective anti-tumor immune response development (1). Results from early clinical trials of patients with metastatic melanoma showed that ipilimumab (either alone or in combination with other therapies) promoted objective response (OR) or stable disease (SD) with a tolerable safety profile (5-8). Ipilimumab is currently being tested in phase II/III trials for the treatment of metastatic melanoma, and in phase I/II studies of other cancer types, including prostate cancer.
In the authors' increasing experience with ipilimumab in the treatment of patients with metastatic melanoma, the time to response is quite heterogeneous. While response to chemotherapy is usually apparent after 1 or 2 cycles, OR in response to ipilimumab can occur soon after treatment initiation or after weeks or months, sometimes preceded by progressive disease (PD) or extended periods of SD. In a recent pooled analysis of 6 studies in 356 patients with stage III/IV melanoma, various ipilimumab doses and regimens were administered alone or in combination with dacarbazine, IL-2, or melanoma peptide vaccines to yield an overall OR rate of 12.9% (9). Of the patients, 28 first recorded OR after 12 weeks of ipilimumab therapy; 16 of them had SD with durations ranging from 2 to 16 months prior to achieving the OR, and 4 patients had apparent PD prior to response.
Since the patterns of response to ipilimumab are so strikingly different from those of standard cytotoxic chemotherapies, or even other approved immunotherapies, it is important to educate clinicians using ipilimumab and tremelimumab (Pfizer, Inc., New York, NY), another fully human anti-CTLA-4 antibody, about the need to appreciate the biology underlying this class of novel therapeutics. CTLA-4 blockade appears to be an effective cancer therapy, not because of a direct action on the tumor cells, but rather because antibody blockade of CTLA-4 allows T cells to respond more robustly to tumors. This kind of indirect anticancer effect may take much more time to produce a radiographically detectable change in tumor size.
Results
Case 1
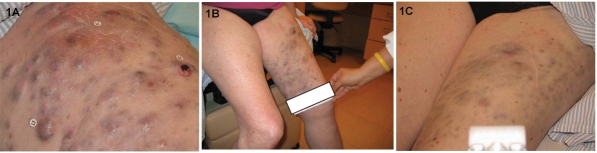
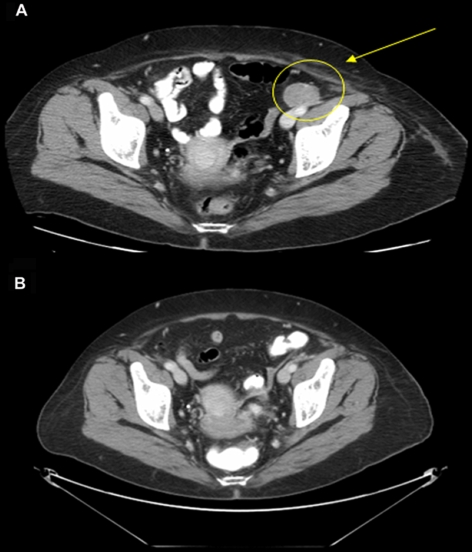
A female patient aged 62 years from study 1 had intrinsic soft tissue recurrence of metastatic melanoma in February 2006. She presented with PD of skin, soft tissue and lymph node metastases, and lymphopenia after prior treatments with sorafenib, anti-αvβ3 integrin monoclonal antibody, and temozolomide (75 mg/m2 for 6 weeks). The temozolomide regimen induced lymphopenia. At the start of ipilimumab therapy in November 2006 her absolute lymphocyte count (ALC) was 500 cells/mm3 and her lactate dehydrogenase (LDH) level was 1009 IU/L. Extensive disease was visible on her left leg (Figure 1A). At week 7 of ipilimumab administration she had complete resolution of her tumor-related pain and improvement in her functional status, as well as a decrease in the size of her visible lesions (Figure 1B). The only reported toxicity was an immune-related adverse event (irAE) in the form of a skin rash (grade 1), which did not require treatment. By week 10, her LDH level had dropped to within the normal range (105-333 IU/L) and she experienced lymphoid reconstitution. A biopsy of her skin lesion at week 10 showed no evidence of melanoma, only lymphocytes and macrophages laden with pigment. Radiographic tumor assessment at week 12 showed significant resolution of subcutaneous and cutaneous metastases in the thigh (Figure 2), and a pelvic lymph node (Figure 3). Visibly, we also significantly reduced the tumors (Figure 1C). The response continues to be durable at week 24. In this particular case, the kinetics of the response is reminiscent of standard cytotoxic chemotherapy.
Figure 1.
Response to ipilimumab therapy of patient case 1. Visible lesions at (A) baseline were reduced after (B) 7 and (C) 12 weeks of ipilimumab therapy. Palpable tumors flattened and disappeared over the course of therapy, leaving behind a "tattoo" of residual melanin pigment.
Figure 2.
Resolution of subcutaneous and cutaneous metastases in the thigh of patient case 1. Computed tomography (CT) scans showing that metastases at (A) baseline were reduced after (B) 12 weeks of ipilimumab therapy. These scans correlate with the photographs in Figure 1, revealing diminishing tumor burden.
Figure 3.
Resolution of a pelvic lymph node in patient case 1. CT images showing an enlarged pelvic lymph node at (A) baseline and its reduction after (B) 12 weeks of ipilimumab therapy. These images are from the same patient as Figures 1 and 2, showing a response to ipilimumab therapy.
Case 2
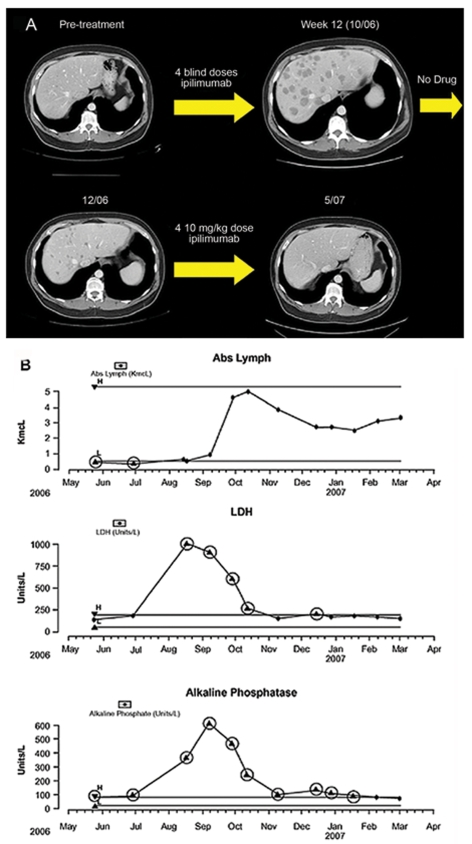
A male patient aged 50 years from study 2 presented with melanoma metastases to the liver, kidney, and mesenteric lymph nodes. During prior treatment with temozolomide (75 mg/m2 given continuously for 6 weeks out of an 8-week cycle) he experienced lymphopenia (ALC = 300 cells/mm3; CD4+ T-cell count = 89 cells/mm3) and PD after 10 months of treatment. In the induction phase of ipilimumab administration he had symptomatic and radiographic PD (until week 12; Figure 4A) with increased size and number of liver metastases, as well as increased visceral adenopathy and size of kidney mass, and new splenomegaly. Despite radiographic progression, however, around week 12 the patient began to note resolution of constitutional symptoms. In addition, the patient's lymphocyte count increased dramatically beginning at week 6, consistent with reconstitution of his immune system (Figure 4B). At week 16 he had improved performance status, stating that he no longer had abdominal pain and had recently enjoyed a short vacation with his friends. He had decreased hepatomegaly on exam, and SD on radiographic analyses (Figure 4A). The only reported irAE was a skin rash, which did not require treatment. The patient had, at that time, completed his 4 induction doses of ipilimumab. However, because the patient had his clinical response only on imaging performed after week 12, he was not eligible for maintenance therapy on study 2. He was therefore enrolled in study 3 where he received 4 doses of ipilimumab re-induction treatment beginning at week 24. This resulted in a gradual, near complete resolution of all measurable lesions including innumerable liver metastasis (Figure 4A) and the patient continues to have an excellent functional status and an ongoing complete response (CR) at 1 year after his first dose of ipilimumab. He continues to receive maintenance ipilimumab q12wk.
Figure 4.
Response to ipilimumab therapy of patient case 2. (A) Radiographic progression of disease at week 12, followed by a clinical response and near complete resolution of disease following another course of ipilimumab induction therapy. (B) Lymphocyte recovery consistent with immune reconstitution accompanied by a decline in LDH and alkaline phosphatase, reflective of decreasing tumor burden.
This patient is a graphic example of progression occurring before response. His description of constitutional symptom abatement, along with a sharp drop in LDH, were critical in our decision to keep him on study, despite imaging studies showing apparent disease progression.
Case 3
A male patient aged 52 years from study 2 had resection of an isolated retroperitoneal metastasis in 2004 and then developed recurrent disease in visceral lymph nodes and soft tissue in 2006. He was treated with high-dose IL-2, but unfortunately, after 2 cycles of therapy, a computed tomography (CT) scan performed in July revealed progression of disease. Ipilimumab was started in November 2006, at which time he had soft tissue disease of the chest wall and pelvis, retroperitoneal metastasis, and iliac nodal disease. The patient exhibited PD on clinical exam through week 10 of ipilimumab administration with discomfort due to enlargement of an axillary mass. Radiographic analysis at week 12 in fact revealed enlargement of multiple subcutaneous masses.
However, when the patient was examined a few days later at his week 12 visit, he reported shrinkage of his palpable tumors in the axilla and abdominal wall and this was confirmed on physical exam. These radiographic and clinical findings are consistent with an initial increase in tumor size through week 10 followed by a decrease in size. The initial tumor enlargement was suspected to be caused by inflammation. The only toxicity he experienced was a mild erythematous rash and discomfort at the site of the axillary mass, perhaps related to inflammation. The patient received 4 doses of induction on study 2, but was then taken off study at week 12 for progression of disease. He was then enrolled on study 3, where he has exhibited slow regression of palpable lesions through 4 additional doses of ipilimumab given as re-induction therapy, reached a partial response (PR) at week 31 and his PR is ongoing now at week 48 after initial ipilimumab therapy. He continues to receive maintenance dosing q12wk.
Case 4
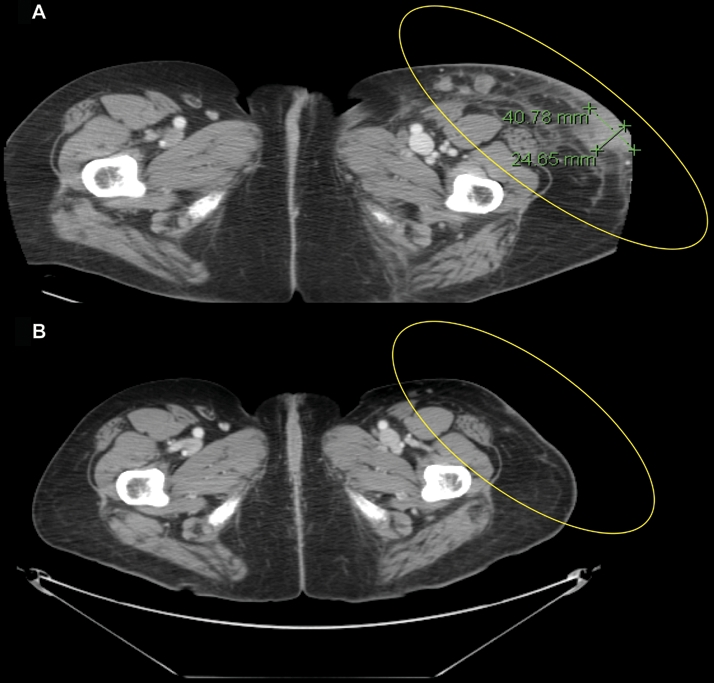
A male patient aged 60 years from study 1 was diagnosed with melanoma in late 2005 when he was found to have a large burden of disease in axillary lymph nodes and possible liver metastasis on computed tomography (CT) scans. He progressed on temozolomide and had a palliative lymph node dissection performed. He was then treated with temozolomide, cisplatin, and vinblastine and exhibited PD of his liver and abdominal lymph nodes. At week 12, after the full induction schedule of 4 doses of ipilimumab, radiologic measurements revealed that his liver lesions had regressed and his other lesions were stable or smaller. However, a new 2 cm axillary lymph node lesion was also noted on the same scan. The only toxicity was a mildly pruritic rash. The patient experienced slow improvement/SD through week 24. At week 24 he received his first dose of ipilimumab maintenance therapy (10 mg/kg) and continues to receive maintenance therapy q12wk. The axillary lymph node initially detected at the week 12 scan was confirmed to contain metastatic melanoma by needle biopsy. However, imaging showed it regressed beginning at week 39, with the most recent dimensions being 0.9 cm x 2.3 cm, decreased from a peak size of 1.6 cm x 2.7 cm. He has now received 2 doses of maintenance therapy at weeks 28 and 39 after initial ipilimumab treatment and continues to have SD at week 48. This pattern of response, with some lesions shrinking or stable in the presence of a single new lesion, is relatively rare with conventional therapies. It is in this type of patient that an appreciation of the biologic mechanism of action of ipilimumab is vital for decision making.
Case 5
A male patient aged 63 years from study 4 presented with a more than 15-year history of skin, soft tissue, and lymph node metastases. His history is complicated by prolonged lymphopenia due to long-term temozolomide use, which resulted in symptomatic Pneumocystis carinii pneumonia and 4 subsequent years of lymphopenia. He experienced PD when previously treated with vaccines, IL-2, temozolomide, and biochemotherapy. After approximately 12 weeks of 3 mg/kg ipilimumab (q3wk, plus gp100 peptides) treatment, he experienced a near complete response (CR) based on imaging and he also had an immune reconstitution syndrome consisting of a sarcoid-like reaction in his airways which lasted for 2 to 3 weeks and caused mild shortness of breath. These symptoms resolved without parenteral corticosteroids. This patient continues to be in near-CR, and, along with cases 1 and 2, demonstrates a correlation between lymphoid reconstitution and clinical response to ipilimumab.
Discussion
The cases in this report illustrate the variable kinetics of response that commonly appear with ipilimumab therapy. In the authors' experience, responses to ipilimumab have been observed prior to (case 1), at (cases 4 and 5), and after (cases 2 and 3) 12 weeks of induction dosing with ipilimumab.
The complexity of the immune response and the impact of individual patient status on the immune system creates challenges for the prediction of the time course of response. However, given the time required to produce a sufficient anti-tumor immune response, it is likely that the induction-dosing schedule (12 weeks) may need to be completed before clinical benefit is observed. This is in marked contrast to chemotherapy where response is usually detectable soon after treatment. This is probably because ipilimumab, unlike chemotherapy, does not have a direct effect on tumor cells, but exerts its effects through educating the immune system. Subsequently, the time taken to generate an anti-tumor immune response can result in a longer time to response. Indeed, the case studies described herein provide proof of this concept. It is reasonable to hypothesize that the immune system is activated during ipilimumab induction treatment between weeks 1 and 12. After week 12, the first time point for measuring ipilimumab efficacy and tumor assessment, some patients enter a maintenance phase and receive ipilimumab q12wk. Periodic re-inhibition of CTLA-4 through ipilimumab maintenance dosing should theoretically re-activate the anti-tumor immunologic activity established through the induction phase, ensuring continued activity, and help the immune system to recognize and respond to any new tumor antigens arising from the development of new lesions. Although week 12 is the protocol-defined initial assessment time point, it is clear from some of the cases described in this report that first response to ipilimumab may occur at different times.
Another clinical observation related to the unique kinetics of response is that apparent PD may occur prior to a response. This is demonstrated by cases 2 and 3, and has been reported previously (9, 10). Taken together, these findings suggest that patients undergoing anti-CTLA-4 therapy may benefit from completing their induction therapy and, if possible and appropriate, receiving maintenance therapy. Given the limited treatment options for patients with metastatic melanoma, this approach may be appropriate when response is lacking and in the absence of significant disease progression or during the initial weeks/months of treatment if the overall tumor burden is not increasing. These recommendations are based on clinical observations and phase II/III trials are in progress to evaluate fully the clinical use of ipilimumab and clarify how to best use this agent. Trials are also in progress to evaluate the benefit of maintenance therapy. A phase II study of patients with unresectable stage III or IV malignant melanoma is underway to identify potential markers/predictors of response and/or toxicity to ipilimumab (11). The effects of ipilimumab on lymphocyte recovery shown here suggest this could be one possible biomarker for clinical activity. It is anticipated that the results of the ongoing phase II study may assist clinicians in selecting the appropriate patients for ipilimumab therapy.
Another consideration is the true nature of the PD observed. We can hypothesize that the immune response elicited during the first weeks of ipilimumab treatment may be mistaken for PD: T-cell infiltration and immune-mediated inflammation as a result of ipilimumab activity would not be radiographically distinguishable from a growing tumor. In case 2, this could explain the PD observed during the first few weeks and months of ipilimumab treatment that was then followed by a response. Likewise, the apparent appearance of new lesions may perhaps reflect pre-existing, yet radiologically absent, micrometastases that could have increased in volume due to ipilimumab-mediated inflammation. Histopathological analysis of tumor biopsy samples will help us to define the nature of PD.
Disease progression may also occur as part of a 'mixed response', i.e., regression of some lesions and apparent progression of others, as observed in case 4. As noted above, the apparent PD may actually be inflammation, but it could also be because of the development of new, antigenically distinct lesions. In this event, PD would be evident until the ipilimumab-enhanced immune system has had time to identify and mount a response against this new tumor antigen. This reinforces the need to evaluate carefully when to stop treatment, even if there is evidence of new lesions. Furthermore, it raises an interesting question regarding the best way to evaluate response to ipilimumab, since, using Response Evaluation Criteria in Solid Tumors (RECIST) (12), case 4 would have been classed as PD despite the clear benefit ultimately obtained with ipilimumab treatment.
Early ipilimumab clinical trials were designed to study lower doses of ipilimumab (3 mg/kg or less, single or multiple doses), either alone and/or in combination with other therapies (6-8). Since these early development studies, clinical trials including the registrational phase II and III studies have used ipilimumab at 10 mg/kg for the induction and maintenance phases. Initial data from a dose-escalation trial indicate this higher dose is associated with improved response: the disease control (CR, PR, and SD) rate was 39% (9/23 patients) (13). The results of trials, study 2 in particular, will clarify the effect of dose on response, and it will be interesting to see which doses (0.3, 3, or 10 mg/kg) the patients in cases 2 and 3 received once study 2 is unblinded.
OR and SD resulting from ipilimumab therapy often appear to be of longer duration than those resulting from standard chemotherapy (6, 7, 9, 13). In study 4, the 5 reported ORs lasted from 25+ to 34+ months (6), while in the pooled analysis of 6 ipilimumab studies, the duration of OR ranged from 2+ to 60+ months (9). In the dose-escalating study, 7 patients treated with 10 mg/kg ipilimumab q3wk x 4 achieved SD as their best overall response with durations ranging from 3.5 to 13.5+ months (13). These data suggest that patients who achieve OR or SD on ipilimumab therapy may achieve long-term disease control with manageable irAEs with continued treatment. The durable SD, as observed in case 4 and prior trials, is a meaningful outcome in these patients with a poor prognosis and few other treatment options. Ongoing and future trials will assess the value of SD, the role of maintenance therapy to maintain responses, and provide information about how we should best evaluate the efficacy of these agents.
A completely novel observation first reported here is the occurrence of lymphoid reconstitution in patients with previously existing lymphopenia who were subsequently treated with ipilimumab. The timing of lymphocyte recovery generally immediately preceded or coincided with significant clinical anti-tumor response. Although we cannot conclusively prove that these are related occurrences, the timing and relationship to the mechanism of action of ipilimumab make this quite likely. The mechanism underlying lymphoid reconstitution, specifically in these patients who were previously treated with temozolomide remains a subject of current investigation. We speculate that checkpoint blockade with ipilimumab may allow for more robust homeostatic proliferation in patients who have pre-existing lymphopenia. This observation attests to the logic of combining ipilimumab with cytotoxic therapies.
In conclusion, the patients described in this paper illustrate the heterogeneity of the kinetics of response to ipilimumab therapy. These representative reports suggest that clinical improvement may occur some time after treatment initiation, which is contrary to traditional chemotherapy or immunotherapy regimens. PD, as determined by radiographic analysis, may be observed prior to clinical benefit and may be a function of the time required to mount an immune response to ipilimumab. The variable time to clinical benefit likely reflects the immune-related mechanism of action of ipilimumab, and may indicate that continued treatment/observation may be beneficial despite initial PD or SD. Ongoing and future trials will further investigate the best clinical use of this novel agent and the most appropriate method for assessing response.
Abbreviations
- CR
complete response
- CT
computed tomography
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- OR
objective response
- PD
progressive disease
- PR
partial response
- SD
stable disease
Acknowledgements
Editorial and writing assistance for this manuscript was provided by Leyla V. Smith, PhD. The authors would also like to thank Stephanie L. Terzulli, PhD for assistance in preparing this manuscript.
References
- 1.Morse MA. Technology evaluation: ipilimumab, Medarex/Bristol-Myers Squibb. Curr Opin Mol Ther. 2005;7:588–597. [PubMed] [Google Scholar]
- 2.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 5.Tchekmedyian S, Glasby J, Korman A, Keler T, Deo Y, Davis T. MDX-010 (human anti-CTLA4): a phase I trial in malignant melanoma [abstract].; J Clin Oncol; 2002. p. 56. [Google Scholar]
- 6.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischkoff SA, Hersh E, Weber J, Powderly J, Khan K, Pavlick A, Samlowski W, O'Day S, Nichol G, Yellin M. Durable responses and long-term progression-free survival observed in a phase II study of MDX-010 alone or in combination with dacarbazine (DTIC) in metastatic melanoma [abstract].; J Clin Oncol; 2005. p. 7525. [Google Scholar]
- 8.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, Kleiner D, Mavroukakis SA, Yellin M, Rosenberg SA. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamid O, Urba W, Yellin M, Nichol GM, Weber J, Hersh EM, Tchekmedyian S, Hodi FS, Weber R, O'Day S. Kinetics of response to ipilimumab (MDX-010) in patients with stage III/IV melanoma [abstract].; J Clin Oncol; 2007. p. 8525. [Google Scholar]
- 10.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bristol-Myers Squibb. ClinicalTrials.gov . Bethesda (MD): National Library of Medicine (US); 2000. Phase II Study to Determine Predictive Markers of Response to BMS-734016 (MDX-010).http://clinicaltrials.gov/show/NCT00261365 [cited September 21, 2007]. NLM Identifier: NCT00261365. [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Weber J, Hersh E, Yellin M, Nichol G, Urba W, Powderly J, O'Day S. The efficacy and safety of ipilimumab (MDX-010) in subjects with unresectable stage III or IV malignant melanoma [abstract].; J Clin Oncol; 2007. p. 8523. [Google Scholar]
- 14.Bristol-Myers Squibb; Medarex. ClinicalTrials.gov . Bethesda (MD): National Library of Medicine (US); 2000. A Single Arm Study of Ipilimumab Monotherapy in Patients With Previously Treated Unresectable Stage III or IV Melanoma.http://clinicaltrials.gov/show/NCT00289627 [cited September 21, 2007]. NLM Identifier: NCT00289627. [Google Scholar]
- 15.Bristol-Myers Squibb; Medarex. ClinicalTrials.gov . Bethesda (MD): National Library of Medicine (US); 2000. Study of Ipilimumab (MDX-010) Monotherapy in Patients With Previously Treated Unresectable Stage III or IV Melanoma.http://clinicaltrials.gov/show/NCT00289640 [cited September 21, 2007]. NLM Identifier: NCT00289640. [Google Scholar]
- 16.Bristol-Myers Squibb. ClinicalTrials.gov . Bethesda (MD): National Library of Medicine (US); 2000. A Companion Study for Patients Enrolled in Prior/Parent Ipilimumab Studies.http://clinicaltrials.gov/show/NCT00162123 [cited September 21, 2007]. NLM Identifier: NCT00162123. [Google Scholar]
- 17.National Cancer Institute (NCI) ClinicalTrials.gov . Bethesda (MD): National Library of Medicine (US); 2000. Monoclonal Antibody With or Without gp100 Peptides Plus Montanide ISA-51 in Treating Patients With Stage IV Melanoma.http://clinicaltrials.gov/show/NCT00077532 [cited September 21, 2007]. NLM Identifier: NCT00077532. [Google Scholar]
Materials and methods
Patients
The patients described in this article had stage III (unresectable) or stage IV melanoma according to modified World Health Organization (WHO) criteria and were treated in clinical trials conducted at Memorial Sloan Kettering Cancer Center in New York City between December 2004 and May 2007. To be eligible, patients had to be 16 years of age or older with a life expectancy of 16 weeks or more, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and a negative screen for HIV, and hepatitis B and C. Exclusion criteria included evidence of brain metastases on MRI or contrast computed tomography (CT), autoimmune disease, concomitant therapy with any anticancer agent and prior treatment with a CD137 agonist or an anti-CTLA-4 antibody. Eligible patients received induction treatment consisting of 4 separate 90-minute i.v. doses of ipilimumab every 3 weeks (q3wk x 4; until week 12). Subsequently, some patients (described below) received single maintenance doses of ipilimumab q12wk until disease progression, drug intolerance, withdrawal of consent or study closure. Tumors were assessed at baseline, week 12 (first efficacy assessment), and additional time points by MRI or CT, and OR and SD were evaluated. The studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice (ICH GCP); all patients provided written, informed consent.
Study 1 (CA184-008)
A phase II, open-label, single-arm, multicenter study (14) was recently completed in patients who had PD during or after at least one prior therapy, with at least one of the following: IL-2, dacarbazine, paclitaxel, carboplatin, fotemustine, or temozolomide. Eligible patients received induction ipilimumab at 10 mg/kg q3wk x 4. Patients with a tumor response or SD and tolerating treatment were eligible to receive single 10 mg/kg maintenance doses of ipilimumab q12wk. Patients with clinically detectable PD or who withdrew from the study continue to be followed.
Study 2 (CA184-022)
A phase II, randomized, double-blind, multi-arm, multicenter, dose-ranging study (15) in patients previously treated with other agents, but without a complete (CR) or partial response (PR), or poor tolerability, was recently completed. Eligible patients were randomized to receive induction ipilimumab at 0.3, 3.0, or 10 mg/kg q3wk x 4. Patients who tolerated ipilimumab and had SD, PR, or CR after induction were eligible to receive maintenance doses of ipilimumab q12wk at their respective dose.
Study 3 (CA184-025)
Some patients from studies 1 and 2 with SD, PR, or CR at week 12, but PD after week 12 entered a separate ongoing companion study (16) where they either: (i) receive re-induction treatment with ipilimumab at 10 mg/kg q3wk x 4, (ii) continue maintenance ipilimumab at 0.3, 3, or 10 mg/kg q12wk, or (iii) are followed without receiving additional study medication. Some patients from study 2 with early PD before or at week 12 may also be eligible for re-induction ipilimumab at 10 mg/kg in this study. Patients are assigned to one of these 3 groups based on specific eligibility criteria.
Study 4 (MDX010-19)
In a recently completed study (17), eligible patients with stage IV metastatic melanoma received escalating intra-patient doses of ipilimumab q3wk (2 each of 3, 5, and 9 mg/kg for a total of 6 to 8 doses, or 2 each of 5 and 9 mg/kg for a total of 4 to 6 doses). HLA-A*0201 negative patients received ipilimumab alone, while HLA-A*0201 positive patients received either ipilimumab alone or in concomitant combination with melanoma peptides (gp100209-217[210M] and gp100280-288 [288V], 1 mg each).