Abstract
Background
It has been proposed for many years that low-level laser (or light) therapy (LLLT) can ameliorate the pain, swelling, and inflammation associated with various forms of arthritis. Light is thought to be absorbed by mitochondrial chromophores leading to an increase in adenosine triphosphate (ATP), reactive oxygen species and/or cyclic AMP production and consequent gene transcription via activation of transcription factors. However, despite many reports about the positive effects of LLLT in arthritis and in medicine in general, its use remains controversial. For all indications (including arthritis) the optimum optical parameters have been difficult to establish and so far are unknown.
Methods
We tested LLLT on rats that had zymosan injected into their knee joints to induce inflammatory arthritis. We compared illumination regimens consisting of a high and low fluence (3 and 30 J/cm2), delivered at high and low irradiance (5 and 50 mW/cm2) using 810-nm laser light daily for 5 days, with the positive control of conventional corticosteroid (dexamethasone) therapy.
Results
Illumination with 810-nm laser was highly effective (almost as good as dexamethasone) at reducing swelling and a longer illumination time (10 or 100 minutes compared to 1 minute) was more important in determining effectiveness than either the total fluence delivered or the irradiance. LLLT induced reduction of joint swelling correlated with reduction in the inflammatory marker serum prostaglandin E2 (PGE2).
Conclusion
LLLT with 810-nm laser is highly effective in treating inflammatory arthritis in this model. Longer illumination times were more effective than short times regardless of total fluence or irradiance. These data will be of value in designing clinical trials of LLLT for various arthritides.
Keywords: biostimulation, low-level light therapy, zymosan-induced arthritis, photobiomodulation, cold laser, prostaglandin E2
INTRODUCTION
Rheumatic diseases are chronic, debilitating conditions. Varying degrees of severity and heterogeneity in clinical manifestations demand complex management and treatment strategies. Hundreds of millions of people in the world today are beset with a host of disabilities caused by trauma, aging, and degeneration and other affections of the musculoskeletal system. The economic burden of rheumatologic diseases has a considerable impact on our society [1]. In the last decade, musculoskeletal disease ranked number one in chronic impairments worldwide. Recognizing the enormous burden that is posed by musculoskeletal conditions, the United Nations and World Health Organization declared 2000–2010 the “bone and joint decade” with the aim to improve the outcomes and health-related quality of life (QOL) of patients who have rheumatologic disorders [2].
Pain and swelling characterize many conditions involving inflammatory and degenerative arthritides (osteoarthritis, rheumatoid arthritis, psoriatic arthritis, lupus arthritis, etc.). These conditions are among the most costly in terms of QOL affecting people of all ages, but the burden of degenerative diseases of bones and joints is very likely to increase substantially as the population ages. Limitation of movement is a serious problem in patients presenting with inflammatory arthropathies. Although pain relief is frequently the main goal in the acute treatment of these conditions, little is known about the mechanisms involved in pain development in arthritis. Drug and non-drug treatments are used to relieve pain and/or swelling. Non-steroidal anti-inflammatory agents (NSAIDs) and selective cyclooxygenase (COX-2) inhibitors (coxibs) [3] are commonly used as analgesic and anti-inflammatory agents. Although selective COX-2 inhibitors were designed to prevent the adverse effects in the gastrointestinal tract mediated by NSAID inhibition of COX-1 [4], prolonged use of COX-2-selective inhibitors may confer a risk of cardiovascular events, including myocardial infarction and stroke [5,6]. The cause of the cardiovascular adverse effects is uncertain but may include an imbalance in prostacyclin and thromboxane levels in the endothelium and blockade of prostanoid actions on renal function [7]. These considerations have prompted the search for alternative non-drug treatments for arthritis [8].
Low-level laser (or light) therapy, commonly known as LLLT, is a form of phototherapy which involves the application of low power light in the red or near infrared wavelengths to mitigate and treat various diseases and traumatic injuries. The light is frequently monochromatic and coherent as produced by lasers and is used to stimulate healing and regeneration. LLLT is used to increase the speed, quality, and tensile strength of tissue repair [9,10], resolve inflammation [11], reduce swelling [12], and give pain relief [13–15]. The technical term often used to describe this form of therapy is photobiomodulation or biostimulation [16]. Lasers or non-coherent light sources such as light-emitting diodes (LED) can be used for the stimulation of cell function. Their biological effect is not thermal, as is the case with surgical lasers. Visible or near-infrared photons are thought to be absorbed by chromophores within the cells such as cytochrome c oxidase located in the mitochondria [17]. Alterations in the activity of cytochrome c oxidase results in increased production of adenosine triphosphate (ATP), a major source of cellular energy, which leads to normalization of cell function, pain relief, and healing [18]. Many reports have investigated which are the optimal optical parameters such as wavelength, total fluence, irradiance (also known as fluence rate or power density), polarization state, coherence, and pulse structure for various experimental and therapeutic outcomes, but as yet there is little consensus on this complicated matter. There have also been many studies that have reported negative results [19].
In this study, we describe the effect of LLLT on the outcome of zymosan-induced arthritis (ZIA) in rats. Zymosan, a polysaccharide from the cell wall of Saccharomyces cerevisiae, is composed of glucan and mannan residues [20]. Injection of zymosan activates innate immune responses, stimulates inflammatory cytokine production [21] and can activate complement in the absence of immunoglobulins. Zymosan is recognized and phagocytosed principally by macrophages (TLR-2 mediated process) [22] leading to cellular activation. ZIA in mice was first described by Keystone in 1977 [23]. Using this model, the course of the inflammatory process is described as biphasic [24]. After an initial peak of inflammation at about day 3, inflammation subsides by day 7 but a chronic residual inflammation remains. There are gait disturbances in rats with ZIA starting at 2 hours, reaching a maximal value between 3 and 4 hours, after the injection of zymosan [25]. The purpose of this study was to analyze the effect of LLLT in the first acute phase of ZIA.
MATERIALS AND METHODS
Laboratory Animals
All animal experiments were approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital and were in accordance with NIH guidelines. Female Lewis rats (Charles River) weighing 180–200 g were housed in individual cages with free access to standard laboratory diet and drinking water. Animals were kept in a 12:12-hour light-dark cycle (lights on 6:00 AM to 6:00 PM) in a temperature-controlled room (26°C). All experiments were designed to minimize animal suffering and to use the minimum number associated with valid statistical evaluation.
Zymosan-Induced Arthritis
Rats received an intra-articular (ia) injection of 4-mg zymosan (Sigma Chemical Company, St. Louis, MO) dissolved in sterile saline, 50 μl total volume, into one rear knee (stifle) joint. The procedure was done under general anesthesia, using a mix of ketamine 80 mg/kg (Hospira, Inc.; Lake Forest, IL)/xylazine 20 mg/kg (Lloyd, Inc.; Shenandoah, IA) intramuscular. Before the zymosan injection, 5 hours after, and on a daily base during 6 days, the circumference of the knee was measured as the most accurate clinical parameter of swelling and inflammation. The circumference measurement of each rat knee at each time point was divided by the pre-zymosan circumference measurement of the knee of that particular rat to give the parameter termed “fraction of original circumference.”
Light Sources, Dosimetry, and Treatment
A diode laser (Model D030-MM-FCTS/B, Opto Power Corp., Tucson, AZ) was used. This laser was operated at 810-nm wavelength and had a maximum output power of 10 W, and the total power could be reduced to 50 mW by a power adjustment control on the laser. The homogeneous laser spot was produced by a lens and was approximately 45 mm in diameter and the distance between the surface of the rat knee and the lens was kept at 10 cm. These measurements were kept constant for all illumination regimens. The power output was controlled to give irradiances of either 5 mW/cm2 (total power 79 mW), or 50 mW/cm2 (total power 790 mW) as measured with a power meter (model DMM 199 with 201 Standard head, Coherent, Santa Clara, CA).
Five hours after zymosan injection rats were distributed in several treatment groups: using different fluence and irradiances all delivered from the 810-nm laser: 3 J/cm2 at 50 mW/cm2 (1 minute illumination); 3 J/cm2 at 5 mW/cm2 (10 minutes illumination); 30 J/cm2 at 50 mW/cm2 (10 minutes illumination); 30 J/cm2 at 5 mW/cm2 (100 minutes illumination). The treatments were repeated on a daily basis for 5 days. A group of rats were treated with dexamethasone as a positive control (Sigma Chemical Company). Rats received 0.01 mg/kg of dexamethasone dissolved in 100 μl sterile saline as an intra-articular injection into the affected joint starting 5 hours after the zymosan injection, and continuing daily for 5 days.
Prostaglandin E2 Assay
This was carried out using an enzyme immunoassay (EIA) kit for prostaglandin E2 (PGE2) metabolite, 13,14-dihydro-15-keto-PGE2 (PGEM), (Cayman Chemicals, Ann Arbor, MI). The PGEM assay was developed as a method of converting all of the immediate PGE2 metabolites to a single stable derivative that can be easily quantified by EIA. Known amounts of rabbit anti-PGE2 antisera bind to either the PGE2 in the sample or to the added acetylcholinesterase-linked PGE2 in a competitive assay. After purification and overnight derivatization of the samples (serum), they were plated in triplicates, the PGEM AChE Tracer and PGEM antiserum were added; following 18 hours of incubation at room temperature cholinesterase substrate was added and the plate was read at a wavelength of 405 nm.
Data Analysis and Statistics
Values are presented as means and standard deviations. The numbers representing the percent of original knee circumference at each time point were used for curve generation using Microcal Origin 6.1 program. Areas under the curve for each rat were calculated using the calculus integration function. The integrals were used for comparing the effects of different treatment regimens (irradiances, fluences). Differences in the areas under the curve between control and treatment groups and between different treatments groups were compared for statistical significance using a t-test for two samples assuming unequal variance in Microsoft Excel. The value of P<0.05 was considered significant.
RESULTS
Rat Model of Zymosan-Induced Arthritis and the Effect of Dexamethasone as a Positive Control Treatment
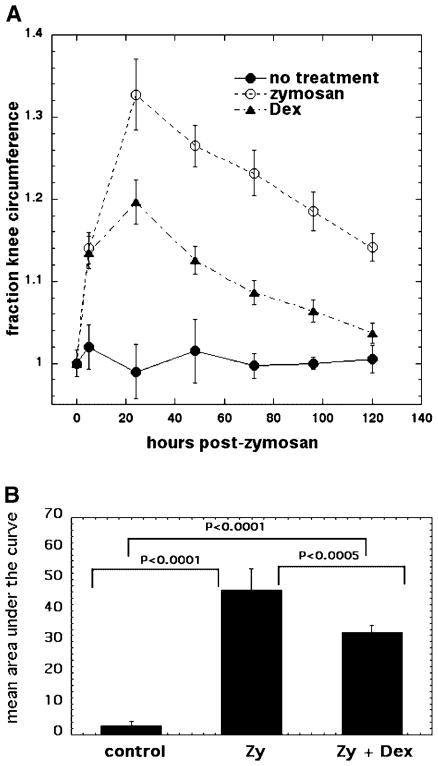
The rats with ZIA show increased inflammation and a predictable course of disease with the circumference of the knee rising to 15% more than the control knee at 5 hour, with a maximum swelling (34% increase in the circumference) 24 hours after the zymosan injection and then a gradual decline in swelling. Five days after ZIA there is a significant recovery but still a residual inflammatory process (16% increased knee circumference). Dexamethasone injected into the affected knee acts as a positive control therapy, initiated 5 hours after the zymosan injection and continued daily for 5 days. There is a significant reduction in the swelling compared to untreated ZIA after 24 hours (knee circumference 20% increase with dexamethasone treatment vs. 34% without treatment) and after 5 days there is almost complete recovery of the edema (residual 5% increase with dexamethasone vs. 16% without treatment) (Fig. 1A). There are highly significant differences in the mean areas under the curve between untreated rats and zymosan-treated rats, and between dexamethasone-treated zymosan rats (Fig. 1B).
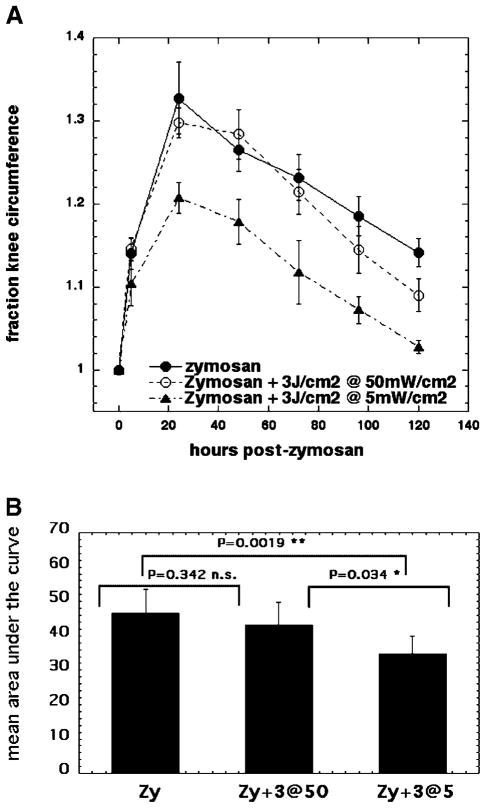
Fig. 1.
A: Curves showing time course of increase in relative circumference of rat knees that received either no treatment, injection of zymosan alone, or injection of zymosan with five daily IA injections of dexamethasone. B: Mean of areas under the curve for individual time courses of knee circumference.
LLLT Effect on Arthritis Depends on Total Fluence Delivered at 50 mW/cm2
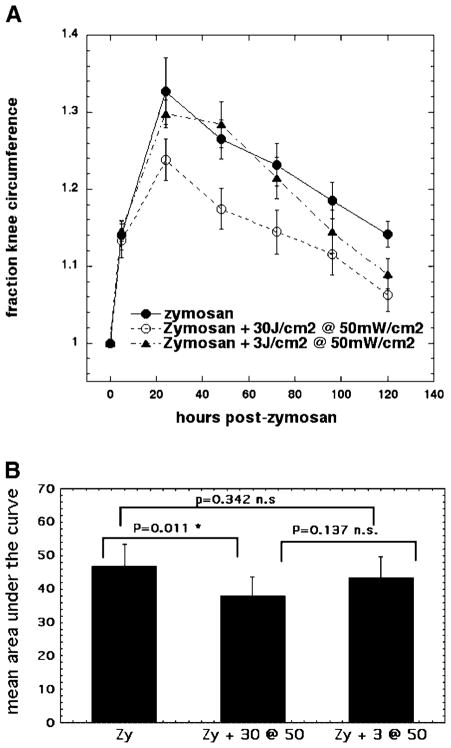
In Figure 2, we show the comparisons of the effect of 810-nm LLLT using two different fluences: 30 and 3 J/cm2 delivered at the same irradiance (50 mW/cm2). It is apparent that there is a significant reduction in the swelling seen with the 30 J/cm2 regimen especially at all timepoints starting 24 hours after zymosan injection (Fig. 2A). The lower fluence of 3 J/cm2 does not begin to have any positive effect until day 3 (72 hours) and then has a progressively greater positive effect at the 96 and 120-hour timepoints but still less than the 30-J/cm2 regimen (Fig. 2). There was a statistically significant difference between the mean area under the curve for zymosan and zymosan with 30 J/cm2 delivered at 50 mW/cm2 not seen between the other groups (Fig. 2B).
Fig. 2.
A: Curves showing time course of increase in relative circumference of rat knees that received either, injection of zymosan alone, or injection of zymosan with five daily illuminations with 810-nm laser light at fluences of either 3 J/cm2 or 30 J/cm2 both delivered at 50 mW/cm2. B: Mean of areas under the curve for individual time courses of knee circumference.
LLLT Effect on Arthritis Does Not Depend on Total Fluence Delivered at 5 mW/cm2
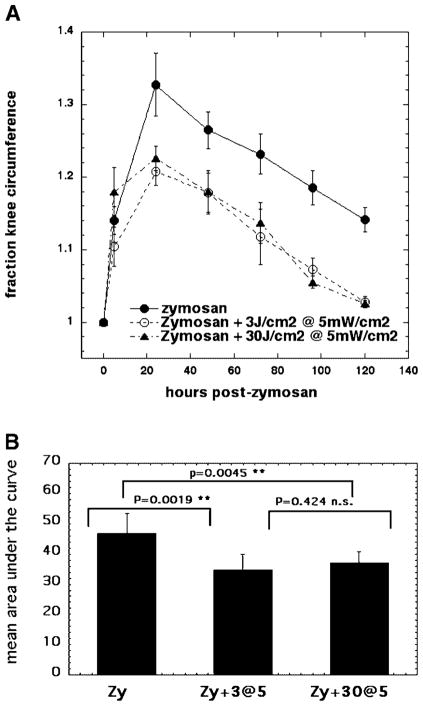
We then asked whether the observation described in the previous section, that a bigger fluence was more effective than a lower fluence in reducing ZIA knee swelling, would still apply when the light was delivered at a much lower irradiance of 5 mW/cm2. Using the identical previous fluences (3 and 30 J/cm2) there were equally positive benefits with both regimens that gave the same reduction in swelling of the knees on days 1, 2, 3, 4, and 5 after ZIA (Fig. 3A). Both the light treatment regimens gave statistically significant differences in areas under the curve compared to zymosan-treated knees, but the two light regimens were not significantly different from, each other (Fig. 3B). There therefore appears to be a contradiction with the previous results, in that the bigger fluence gives a better result than does the smaller fluence at 50 mW/cm2, but not at 5 mW/cm2. This discrepancy could be explained if the main factor to be considered in explaining the results was in fact the irradiance rather than the total fluence.
Fig. 3.
A: Curves showing time course of increase in relative circumference of rat knees that received either, injection of zymosan alone, or injection of zymosan with five daily illuminations with 810-nm laser light either 3 J/cm2 or 30 J/cm2 both delivered at 5 mW/cm2. B: Mean of areas under the curve for individual time courses of knee circumference. LLLT effect on arthritis does not depend on total fluence delivered at 5 mW/cm2.
LLLT Effect on Arthritis Does Not Depend on Irradiance When 30 J/cm2 Is Employed
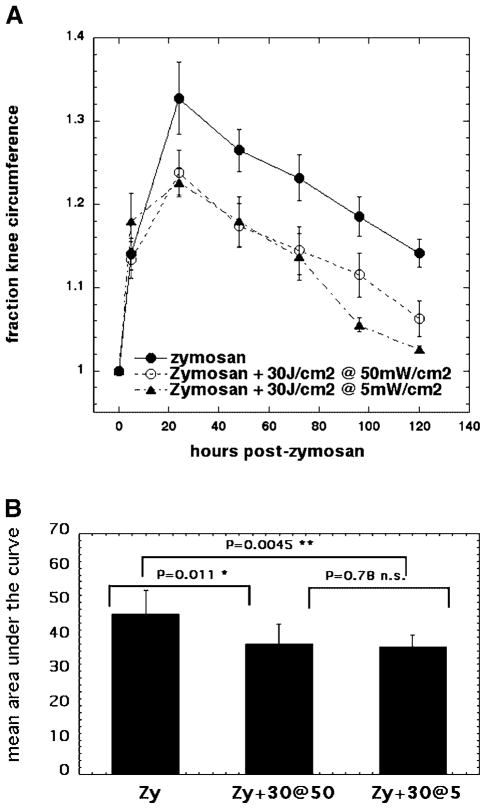
We therefore compared 30 J/cm2 delivered at either, an irradiance of 5 mW/cm2 or at an irradiance of 50 mW/cm2 (Fig. 4). As can be seen (Fig. 4A) these regimens were equally effective in reducing swelling at days 1, 2, and 3 post-zymosan injection. At days 4 and 5 the low irradiance of 5 mW/cm2 had a slight advantage in reducing swelling over the high irradiance of 50 mW/cm2. Both the light regimens gave significant differences in area under the curve compared to zymosan-treated knees, but were not significantly different from each other (Fig. 4B).
Fig. 4.
A: Curves showing time course of increase in relative circumference of rat knees that received either, injection of zymosan alone, or injection of zymosan with five daily illuminations with 810-nm laser light of 30 J/cm2 delivered at either 5 mW/cm2 or 50 mW/cm2. B: Mean of areas under the curve for individual time courses of knee circumference. LLLT effect on arthritis does not depend on irradiance at which 30 J/cm2 is delivered.
LLLT Effect on Arthritis Does Depend on Irradiance When 3 J/cm2 Is Employed
Since the previous results suggested that the irradiance did not have an effect on the LLLT reduction of swelling when the light dose was 30 J/cm2, we asked whether the same finding of independence of the effect on the irradiance would pertain when the fluence was 3 J/cm2. As can be seen in Figure 5A, this did not prove to be the case; 3 J/cm2 delivered at 50 mW/cm2 had only a slight effect in reduction of swelling at days 4 and 5, while the identical fluence delivered at 5 mW/cm2 had a positive effect in reducing swelling at all timepoints. The effective regimen of 3 J/cm2 delivered at 5 mW/cm2 gave statistically significant differences in area under the curve from the other two regimens (Fig. 5B).
Fig. 5.
A: Curves showing time course of increase in circumference of rat knees that received either, injection of zymosan alone, or injection of zymosan with five daily illuminations with 810-nm laser light of 30 J/cm2 both delivered at either 5 mW/cm2 or 50 mW/cm2. B: Mean of areas under the curve for individual time courses of knee circumference. LLLT effect is significantly better at low irradiance of 5 mW/cm2.
How can we explain these seemingly contradictory findings? We believe that the answer can be found in considering the length of time needed to deliver the indicated amount of light to the knee joint. The effective regimens were:
30 J/cm2 delivered at 5 mW/cm2 taking 100 minutes.
30 J/cm2 delivered at 50 mW/cm2 taking 10 minutes.
3 J/cm2 delivered at 5 mW/cm2 taking 10 minutes.
The ineffective regimen was
J/cm2 delivered at 50 mW/cm2 taking 1 minute.
It therefore seems as if a certain length of time of illumination such as 10 minutes is required to see a positive effect and that the effect is independent of the amount of energy delivered in that time, or the irradiance at which the light is delivered, and that moreover, employing an even longer illumination time gives no added benefit.
Prostaglandin E2 Assay
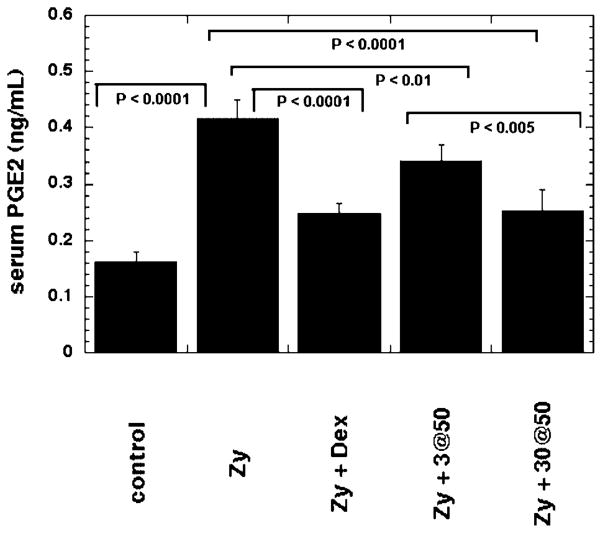
In Figure 6 we show the values determined from the serum PGE2 measurements carried out on serum isolated from blood samples taken from rats 24 hours after the injection of zymosan. The mean PGE2 concentration was more than doubled by the zymosan-induced inflammation and this elevated value was significantly reduced by almost 50% by the intra-articular injection of dexamethasone. The light regimen we found to be ineffective in reducing swelling (3 J/cm2 at 50 mW/cm2) did produce a significant reduction in serum PGE2 but the reduction was much less than the reduction seen with dexamethasone. By contrast the light regimen we found to be effective (30 J/cm2 at 50 mW/cm2) in reducing swelling produced a much greater reduction in serum PGE2, almost to the level obtained using dexamethasone.
Fig. 6.
Mean serum PGE2 levels taken from rats 24 hours after they received either: no treatment, zymosan alone, zymosan+dexamethasone, zymosan+an ineffective light regimen (3 J/cm2 at 50 mW/cm2), zymosan+an effective light regimen (3 J/cm2 at 50 mW/cm2). Values are means of six independent determinations of aliquots from serum pooled from three rats per group and bars are SD.
DISCUSSION
We investigated the use of LLLT for the treatment of ZIA in rats. Initially we evaluated the benefit of LLLT compared with the anti-inflammatory “gold-standard” treatment, dexamethasone. The progress of the inflammatory phase of the arthritis was conveniently followed by measuring the circumference of the rat hind knee or stifle joint. We saw a significant and progressive decrease in the circumference of rat knees, using five daily intra-articular injections of the anti-inflammatory corticosteroid, dexamethasone. Our initial experiments with five daily illuminations with 810-nm laser light suggested that the positive benefit in reduction of swelling was related to total fluence delivered, with 30 J/cm2 giving a significantly better reduction in circumference than did 3 J/cm2 when they were both delivered at an irradiance of 50 mW/cm2. However, the next set of experiments confounded this hypothesis because when we compared the same two fluences of 30 J/cm2 and 3 J/cm2, this time delivered at a low irradiance of 5 mW/cm2, there were no significant differences between the two fluences. We then hypothesized that perhaps the irradiance at which the light is delivered makes a difference to the outcome in LLLT for arthritis. Again in two experiments we obtained conflicting results. Thirty J/cm2 was highly effective whether delivered at 50 mW/cm2 or delivered at 5 mW/cm2, but 3 J/cm2 was only highly effective when delivered at 5 mW/cm2, and almost without any positive effect when delivered at 50 mW/cm2. Therefore we propose that the most important parameter in defining the optimum light delivery regimen is the illumination or time of exposure. The benefits in the reduction of swelling were significantly better when the time of exposure was 10 minutes (or 100 minutes) versus 1 minute.
Although LLLT has been used to treat several clinical conditions and has also been studied in many animal models and in cell culture systems, the mechanisms are still incompletely understood, and perhaps it will turn out to be the case that multiple mechanisms operate. On a molecular level, LLLT is known to stimulate ATP production, mitochondrial membrane potential, cytokine secretion, and cell proliferation [26]. There have been several reports that the enzyme cytochrome c oxidase, otherwise known as unit 4 of the respiratory chain located in the inner mitochondrial membrane, is an important chromophore in LLLT [16,17]. This protein has absorption bands that stretch from the visible into the near-infrared region of the spectrum (>900 nm). A recent commentary [27] proposed that the photons absorbed by cytochrome c oxidase led to dissociation of the inhibitory molecule, nitric oxide, from oxygen binding sites within the enzyme, thus increasing the enzyme activity in cellular respiration. If the primary cellular effect of LLLT is indeed to increase the activity of an enzyme, it makes sense to suppose that this increased activity should last for a sufficiently long time to have a real effect on cellular metabolism. In some photobiological processes such as photodynamic therapy and phototoxicity, the principal of reciprocity first described by Bunsen and Roscoe [28] is accepted to operate [29]. This principle states that the biological effect of light depends on the total number of photons absorbed (total fluence) rather than the irradiance or fluence rate. However, the photobiological processes in which reciprocity operates depend more on a photochemical effect (each photon has an equal chance to cause a chemical reaction) rather than on modifying the reactivity of an enzyme as proposed for LLLT.
The concept that the total delivered fluence is not the most important factor to be considered in analyzing the relationship between effectiveness and the optical parameters of LLLT has been suggested previously. In vitro experiments by Azevedo et al. [30] and by van Breugel et al. [31] both showed that the irradiance and illumination time were important in determining outcome, and that the relationship between total delivered fluence and response had a bell-shaped curve with a fluence value at which the observed response was maximal.
The correlation of the anti-inflammatory of LLLT in our ZIA model with reduction in serum PGE2 provides some hard biochemical evidence that there is a real effect on a cellular level. Reductions in inflammatory mediators such as PGE2 have previously been reported. Soriano et al. [11] found reduction in PGE2 in rats with microcrystalline arthropathies treated with LLLT, and this reduction correlated with beneficial effects of LLLT in patients with gout, pyrophosphate or hydroxyapatite arthropathies. In patients with Achilles tendonitis LLLT using infrared (904 nm, 5.4 J per point, power density 20 mW/cm2), it was reported that PGE2 concentrations significantly decreased after the treatment and this reduction correlated with decrease in pain and inflammation [32].
Brosseau [33] reported a meta-analysis, using the registries of the Cochrane Musculoskeletal Group and the field of Rehabilitation and Related Therapies as well as the Cochrane Central Register of Controlled Trials (CENTRAL). Only randomized controlled trials of LLLT for the treatment of patients with a clinical diagnosis of RA or OA were eligible. Thirteen trials were included, with 212 patients randomized to laser and 174 patients to placebo laser, and 68 patients received active laser on one hand and placebo on the opposite hand. In patients with RA, relative to a separate control group, LLLT reduced pain by 70% relative to placebo and reduced morning stiffness. There were no significant differences between subgroups based on LLLT dosage, wavelength, site of application, or treatment length. There are still more clinical trials required to establish the LLLT dosage and the treatment length.
The novel finding that the duration of light exposure is an important parameter in determining the effectiveness of LLLT, should of course be confirmed by more studies, but may go some way to explain some of the published negative studies that maybe did not use long enough illumination times. In addition, further studies will be required to better define the best optical parameters for LLLT and the complex relationship between disease state, total fluence, irradiance, illumination time, and number and frequency of treatments.
Acknowledgments
Contract grant sponsor: Palomar Medical Technologies Inc.; Contract grant sponsor: U.S. National Institutes of Health; Contract grant numbers: R01-CA/AI838801, R01-AI050875; Contract grant sponsor: Department of Defense CDMRP Breast Cancer Research; Contract grant number: W81XWH-04-1-0676.
This work was funded by Palomar Medical Technologies Inc. and by the U.S. National Institutes of Health (Grants R01-CA/AI838801 and R01-AI050875 to MRH). Ana P Castano was supported by Department of Defense CDMRP Breast Cancer Research Grant (W81XWH-04-1-0676). We are grateful to Tatiana N Demidova-Rice for assistance and a critical reading of the manuscript. We are grateful to R Rox Anderson for support.
Footnotes
The authors have disclosed potential conflicts of interests with this study.
References
- 1.Cooper NJ. Economic burden of rheumatoid arthritis: A systematic review. Rheumatology (Oxford) 2000;39(1):28–33. doi: 10.1093/rheumatology/39.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Harris ED., Jr The bone and joint decade: A catalyst for progress. Arthritis Rheum. 2001;44(9):1969–1970. doi: 10.1002/1529-0131(200109)44:9<1969::AID-ART342>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JA, Larkin S, Williams TJ. Cyclooxygenase-2: Regulation and relevance in inflammation. Biochem Pharmacol. 1995;50(10):1535–1542. doi: 10.1016/0006-2952(95)00212-x. [DOI] [PubMed] [Google Scholar]
- 4.Chan FK, Graham DY. Review article: Prevention of non-steroidal anti-inflammatory drug gastrointestinal complications—review and recommendations based on risk assessment. Aliment Pharmacol Ther. 2004;19(10):1051–1061. doi: 10.1111/j.1365-2036.2004.01935.x. [DOI] [PubMed] [Google Scholar]
- 5.White WB, Strand V, Roberts R, Whelton A. Effects of the cyclooxygenase-2 specific inhibitor valdecoxib versus non-steroidal antiinflammatory agents and placebo on cardiovascular thrombotic events in patients with arthritis. Am J Ther. 2004;11(4):244–250. doi: 10.1097/01.mjt.0000127360.23508.04. [DOI] [PubMed] [Google Scholar]
- 6.White WB, Faich G, Borer JS, Makuch RW. Cardiovascular thrombotic events in arthritis trials of the cyclooxygenase-2 inhibitor celecoxib. Am J Cardiol. 2003;92(4):411–418. doi: 10.1016/s0002-9149(03)00659-3. [DOI] [PubMed] [Google Scholar]
- 7.Zarraga IG, Schwarz ER. Coxibs and heart disease: What we have learned and what else we need to know. J Am Coll Cardiol. 2007;49(1):1–14. doi: 10.1016/j.jacc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Skelly MM, Hawkey CJ. Potential alternatives to COX 2 inhibitors. Bmj. 2002;324(7349):1289–1290. doi: 10.1136/bmj.324.7349.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci USA. 2003;100(6):3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003;21(2):67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- 11.Soriano F, Campana V, Moya M, Gavotto A, Simes J, Soriano M, Soriano R, Spitale L, Palma J. Photobiomodulation of pain and inflammation in microcrystalline arthropathies: Experimental and clinical results. Photomed Laser Surg. 2006;24(2):140–150. doi: 10.1089/pho.2006.24.140. [DOI] [PubMed] [Google Scholar]
- 12.Stergioulas A. Low-level laser treatment can reduce edema in second degree ankle sprains. J Clin Laser Med Surg. 2004;22(2):125–128. doi: 10.1089/104454704774076181. [DOI] [PubMed] [Google Scholar]
- 13.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: A meta-analysis study. Photomed Laser Surg. 2004;22(4):323–329. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- 14.Gur A, Sarac AJ, Cevik R, Altindag O, Sarac S. Efficacy of 904 nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: A double-blind and randomize-controlled trial. Lasers Surg Med. 2004;35(3):229–235. doi: 10.1002/lsm.20082. [DOI] [PubMed] [Google Scholar]
- 15.Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA. Photoradiation in acute pain: A systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 2006;24(2):158–168. doi: 10.1089/pho.2006.24.158. [DOI] [PubMed] [Google Scholar]
- 16.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 17.Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol. 2004;80(2):366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- 18.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 19.Tuner J, Hode L. It’s all in the parameters: A critical analysis of some well-known negative studies on low-level laser therapy. J Clin Laser Med Surg. 1998;16(5):245–248. [PubMed] [Google Scholar]
- 20.Di Carlo FJ, Fiore JV. On the composition of zymosan. Science. 1958;127(3301):756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- 21.de Hooge AS, van De Loo FA, Arntz OJ, van Den Berg WB. Involvement of IL-6, apart from its role in immunity, in mediating a chronic response during experimental arthritis. Am J Pathol. 2000;157(6):2081–2091. doi: 10.1016/S0002-9440(10)64846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755):811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 23.Keystone EC, Schorlemmer HU, Pope C, Allison AC. Zymosan-induced arthritis: A model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977;20(7):1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- 24.Frasnelli ME, Tarussio D, Chobaz-Peclat V, Busso N, So A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res Ther. 2005;7(2):R370–379. doi: 10.1186/ar1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da SRJC, Peixoto ME, Jancar S, de QCF, de ARR, da Rocha FA. Dual effect of nitric oxide in articular inflammatory pain in zymosan-induced arthritis in rats. Br J Pharmacol. 2002;136(4):588–596. doi: 10.1038/sj.bjp.0704755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavish L, Asher Y, Becker Y, Kleinman Y. Low level laser irradiation stimulates mitochondrial membrane potential and disperses subnuclear promyelocytic leukemia protein. Lasers Surg Med. 2004;35(5):369–376. doi: 10.1002/lsm.20108. [DOI] [PubMed] [Google Scholar]
- 27.Lane N. Cell biology: Power games. Nature. 2006;443(7114):901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 28.Loeb J, Northrop JH. Heliotropic animals as photometers on the basis of the validity of the Bunsen-Roscoe Law for heliotropic reactions. Proc Natl Acad Sci USA. 1917;3(9):539–544. doi: 10.1073/pnas.3.9.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindl A, Rosado-Schlosser B, Trautinger F. Reciprocity regulation in photobiology. An overview. Hautarzt. 2001;52(9):779–785. doi: 10.1007/s001050170065. [DOI] [PubMed] [Google Scholar]
- 30.Azevedo LH, de Paula Eduardo F, Moreira MS, de Paula Eduardo C, Marques MM. Influence of different power densities of LILT on cultured human fibroblast growth: A pilot study. Lasers Med Sci. 2006;21(2):86–89. doi: 10.1007/s10103-006-0379-9. [DOI] [PubMed] [Google Scholar]
- 31.van Breugel HH, Bar PR. Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med. 1992;12(5):528–537. doi: 10.1002/lsm.1900120512. [DOI] [PubMed] [Google Scholar]
- 32.Bjordal JM, Lopes-Martins RA, Iversen VV. A randomised, placebo controlled trial of low level laser therapy for activated Achilles tendinitis with microdialysis measurement of peri-tendinous prostaglandin E2 concentrations. Br J Sports Med. 2006;40(1):76–80. doi: 10.1136/bjsm.2005.020842. discussion 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brosseau L, Robinson V, Wells G, Debie R, Gam A, Harman K, Morin M, Shea B, Tugwell P. Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2005;(4):CD002049. doi: 10.1002/14651858.CD002049.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]