Abstract
Background and Objective
Phenothiazinium dyes have been reported to be effective photosensitizers inactivating a wide range of microorganisms in vitro after illumination with red light. However, their application in vivo has not extensively been explored. This study evaluates the bactericidal activity of phenothiazinium dyes against multidrug-resistant Acinetobacter baumannii both in vitro and in vivo.
Study Design/Materials and Methods
We report the investigation of toluidine blue O, methylene blue, 1,9-dimethylmethylene blue, and new methylene blue for photodynamic inactivation of multidrug-resistant A. baumannii in vitro. The most effective dye was selected to carry out in vivo studies using third-degree mouse burns infected with a bioluminescent A. baumannii strain, upon irradiation with a 652 nm noncoherent light source. The mice were imaged daily for 2 weeks to observe differences in the bioluminescence–time curve between the photodynamic therapy (PDT)-treated mice in comparison with untreated burns.
Results
All the dyes were effective in vitro against A. baumannii after 30 J/cm2 irradiation of 635 or 652 nm red light had been delivered, with more effective killing when the dye remained in solution. New methylene blue was the most effective of the four dyes, achieving a 3.2-log reduction of the bacterial luminescence during PDT in vivo after 360 J/cm2 and an 800 μM dye dose. Moreover, a statistically significant reduction of the area under the bioluminescence–time curve of PDT-treated mice was observed showing that the infection did not recur after PDT.
Conclusions
Phenothiazinium dyes, and especially new methylene blue, are potential photosensitizers for PDT to treat burns infected with multidrug-resistant A. baumannii in vivo.
Keywords: photodynamic inactivation, burn infection, new methylene blue, bioluminescence imaging
INTRODUCTION
The use of photodynamic therapy (PDT) [1] to treat localized infections generally involves the topical application of a photosensitizer (PS) into the infected tissue, followed by illumination with visible or near-infrared light [2,3]. In the presence of oxygen, light induces the formation of reactive oxygen species by energy or electron transfer from the PS excited state that are able to oxidize biomolecules and thereby kill cells [4]. The selectivity of the PS for bacteria over host tissue can be obtained by the appropriate chemical design [5–7] to ensure that the molecule will preferentially bind to bacterial cells rather than mammalian cells [8]. Three different classes of compounds have been used as PSs to inactivate bacteria: (1) phenothiazinium salts [9], (2) tetrapyrroles such as phthalocyanines [10] and porphyrins with cationic charges [11], and (3) conjugates between tetrapyrroles with cationic polymers [12–14].
Phenothiazinium-based PSs have been widely used against a range of microorganisms, demonstrating the efficacy of PDT to inactivate resistant forms of bacteria which are not easily killed by antibiotics such as methicillin [15] or vancomycin [16]. The substitution pattern of the phenothiazinium core has been varied to introduce important changes in the photochemical properties, like maximal absorption wavelength [15], or the lipophilicity [17], which affects the PS uptake and the location where the photo-damage will be produced.
Phoenix et al. [18] observed that for inactivation of a Gram-negative species, Escherichia coli, dimethylmethylene blue (DMMB) was the most phototoxic phenothiazinium dye, while Wainwright et al. [7] demonstrated that the inactivation of Pseudomonas aeruginosa was most efficient with toluidine blue O (TBO) that had the lowest minimal lethal concentration. Indeed, it has been observed that even for different strains of the same species, the minimum lethal concentration of a dye may vary [15] indicating that minor changes in the bacterial wall could produce differences in the affinity of a dye for bacterial cells.
However, neither in vitro nor in vivo studies have been done using phenothiazinium-based PS for inactivating Acinetobacter baumannii, a Gram-negative pathogenic bacterium that has recently attracted much attention due to its remarkable acquisition of multidrug resistance [19,20]. Indeed, a pandrug-resistant A. baumannii strain with a tremendous ability to develop synergistic resistance mechanisms and, subsequently, very persistent chronic infections has been found [21–23]. Thus, it is interesting to test the efficacy of PDT and phenothiazines against such new and hazardous pathogen.
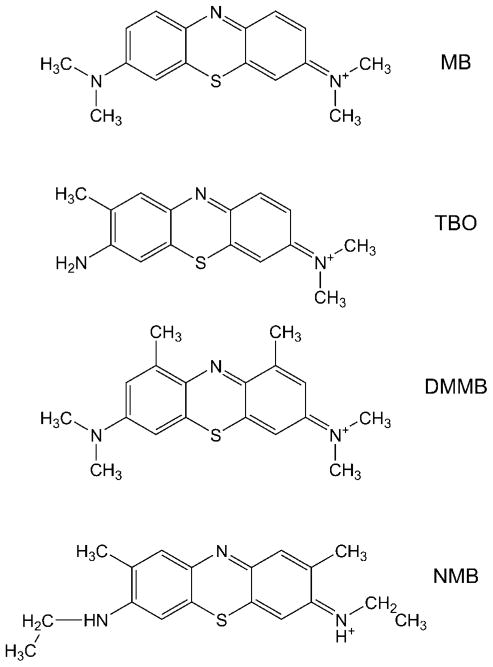
In this study we first performed in vitro studies using four different phenothiazinium dyes (Fig. 1) against a multidrug-resistant bioluminescent strain of A. baumannii in order to select the most phototoxic dye. Subsequently, we applied this selected PS and performed an in vivo experiment using mouse burns infected with bioluminescent A. baumannii.
Fig. 1.
Chemical structures of the four phenothiazinium dyes: methylene blue (MB), toluidine blue O (TBO), dimethylmethylene blue (DMMB), and new methylene blue N (NMB). Counterions are not shown.
MATERIALS AND METHODS
Photosensitizers and Light Source
TBO, methylene blue (MB), new methylene blue N (in the form of zinc chloride double salt; NMB), and 1,9-dimethylmethylene blue chloride (DMMB) were purchased from Sigma (St. Louis, MO). These PSs were dissolved in distilled water to give stock solutions with a dye concentration of 1 mM. All PS stock solutions were stored at 4°C in the dark for no more than 2 weeks, and immediately before experiments, were diluted in PBS without Ca2+ or Mg2+. Red light at 635 ± 15 or 652 ± 15 nm was delivered using a non-coherent light source with interchangeable fiber bundles (LumaCare, Newport Beach, CA). The range given corresponds to the full width at half maximum.
Bacterial Strains and Culture Conditions
A. baumannii ATCC BAA 747 was transduced with the lux CDABE operon (originally cloned from Photorhabdus luminescens) as described [24]. The bacteria were aerobically grown overnight at 37° in brain–heart infusion (BHI) broth (Fischer Scientific, Pittsburgh, PA) in the presence of 250 μg/ml of carbenicillin. Bacteria were then grown in new BHI medium with the antibiotic at 37°C in an orbital shaking incubator to an optical density at 600 nm (OD600) of 0.5, corresponding to ca. 108 colony forming unit (CFU)/ml. The suspensions were then centrifuged (5 minutes, 3,000 rpm) and resuspended with sterile PBS at pH 7.4 at the same concentration for photodynamic inactivation experiments.
In Vitro Experiments
Suspensions of bacteria (108 CFU/ml) in PBS were incubated in the dark at room temperature for 30 minutes with 0.1–20 μM of the PS in PBS. Centrifugation (3 minutes, 12,000 rpm) of 1-ml aliquots was used to remove the excess of PS that was not taken up by the bacteria when experiments required it.
Then 1-ml aliquots of the bacterial suspensions were placed in 24-well plates. The wells were illuminated from the top of the plates by the use of either 635 nm light for TBO and NMB or 652 nm light for MB and DMMB, an optical fiber, and a lens (to form a 2-cm diameter spot). Under these conditions, all PSs absorbed comparable amounts of incident photons, thereby allowing the assessment of their relative efficiencies. Fluences ranged from 0 to 30 J/cm2, using a fluence rate of 125 mW/cm2. At the time points when the requisite fluences had been delivered, 200 μl aliquots were taken from each well (the suspensions were thoroughly mixed before sampling to avoid the settlement of bacteria).
Light alone controls without PS were performed for all experimental conditions in order to rule out any inactivation effect due to the light.
For determination of CFUs, the aliquots were serially diluted, streaked on nutrient agar plates, and incubated in the dark for 18 hours at 37°C. Experiments were carried out in triplicate for each condition.
Bacterial luminescence was also measured on these 200 μl aliquots of bacterial suspensions in 96-well plates by the use of a luminescence plate reader.
In Vivo Burn Infection Model
Adult female BALB/c mice (Charles River Laboratories, Wilmington, MA), 6- to 8-week-old and weighing 17–21 g were used in the study. The animals were housed one per cage and maintained on a 12-hour light/dark cycle with access to food and water ad libitum. All animal procedures were approved by the Subcommittee on Research Animal Care (IACUC) of Massachusetts General Hospital and met the guidelines of National Institutes of Health.
In vivo experiments were performed by means of the protocol used by Dai et al. [24] fully described elsewhere. Briefly, burns were created by applying two pre-heated (≈95°C) brass blocks (Small Parts, Inc., Miami, FL) to the opposing sides of an elevated skin-fold on the dorsal surface of mice [25] for 10 seconds (nonlethal, full-thickness, third-degree burns). The combined brass block area was 15 mm × 10 mm giving an area of 150 mm2, corresponding to a 4% of total body surface area (TBSA) [26]. Immediately after the creation of burns the mice were resuscitated with intraperitoneal (IP) injections of 0.5 ml sterile saline (Phoenix Scientific, Inc., St. Joseph, MO) to prevent dehydration. Then, a bacterial suspension containing 108 cells in 50 μl sterile PBS was inoculated onto the surface of each burn with a pipette tip and then was smeared onto the burn surface with an inoculating loop. The mice were imaged with the luminescence camera immediately after applying the bacteria to ensure that the bacterial inoculum applied to each burn was consistent.
Bioluminescence Imaging
The bioluminescence imaging system (Hamamatsu Photonics KK, Bridgewater, NJ) has been described elsewhere in detail [27]. Briefly, an ICCD photon-counting camera (Model C2400-30H; Hamamatsu Photonics) was used. The camera was mounted in a light-tight specimen chamber, fitted with a light-emitting diode, a set up that allowed for a background gray-scale image of the entire mouse to be captured. By accumulating many images containing binary photon information (an integration time of 2 minutes was used), a pseudo-color luminescence image was generated. Superimposition of this image onto the gray-scale background image yielded information on the location and intensity in terms of photon number. The camera was also connected to a computer system through an image processor (Argus-50, Hamamatsu Photonics). Argus-50 control program (Hamamatsu Photonics) was used to acquire images and to process the image data collected.
Prior to imaging, mice were anesthetized by IP injections of ketamine–xylazine cocktail. Mice were then placed on an adjustable stage in the specimen chamber, and the wounds were positioned directly under the camera. A gray-scale background image of each wound was made, and this was followed by a photon count of the same region. This entire wound photon count was quantified as relative luminescence units (RLUs) and was displayed in a false color scale ranging from pink (most intense) to blue (least intense).
In Vivo PDT
Thirty minutes after application of the bacteria to the burns, NMB solution was applied. Three different aliquots of the PS were added to the PDT-treated burns and also to dark controls. Initially, 50 μl of the PS solution was added to the burn and then two more additional aliquots of 20 μl were added during PDT after 84 and 240 J/cm2 had been delivered. Thirty minutes after the first addition to allow the NMB to bind to and penetrate the bacteria, the mice were again imaged using luminescence camera to quantify any dark toxicity to the bacteria. Mice were then illuminated with 635 ± 15 nm light delivered by a noncoherent light source. The light fluence rate was routinely measured using a LaserMate™ power meter (Coherent, Portland, OR), and the fluence rate used was 100 mW/cm2. Mice were given total light doses of up to 360 J/cm2 in aliquots with luminescence imaging taking place after each aliquot of light. Immediately after PDT, the mice were resuscitated with a second IP injection of 0.5 ml sterile saline to prevent dehydration.
Mouse Follow-Up
The mice were anesthetized by IP injection of a ketamine–xylazine cocktail and the bacterial luminescence from the mouse burns was recorded daily until the bioluminescence disappeared or the animals were determined to be moribund and euthanized.
Statistical Methods
To compare the dose–response curves obtained using bioluminescence assay with colony formation assay, the slopes between neighboring points were calculated and compared for statistical significance using a Student’s t-test.
The time courses of bacterial luminescence of the burn were calculated by the use of numerical integration [28]. Differences in the areas under the curves between all the groups were compared for statistical significance by one-factor ANOVA. P-values of <0.05 were considered significant.
RESULTS AND DISCUSSION
In Vitro Experiments
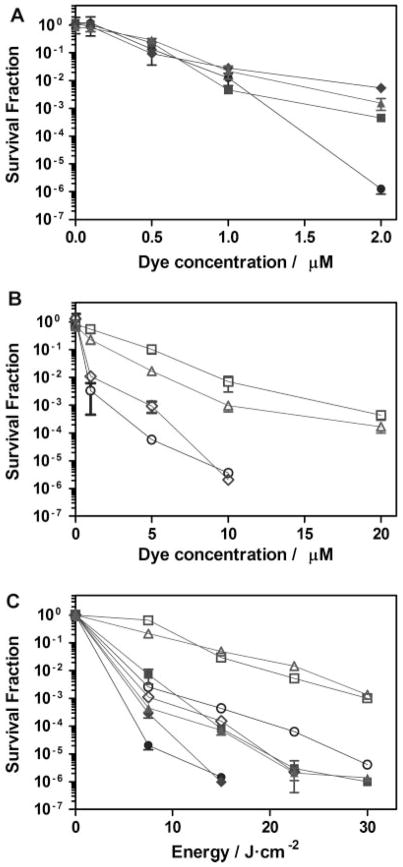
Phenothiazinium dyes have been commonly reported as lethal PSs for both Gram-negative and Gram-positive bacteria [18]. Four different phenothiazinium dyes were tested in this study in order to select the most phototoxic dye against A. baumannii for the in vivo experiments. NMB was found to be the most active with and without removing the excess of PS from the solution. Figure 2A shows the dye-concentration–responses of A. baumannii survival with respect to all the phenothiazinium dyes. As observed, after 22.5 J/cm2 irradiation with a dye concentration of only 2 μM, NMB was able to reduce the bacterial viability up to 6-log. DMMB, MB, and TBO could only achieve a reduction of A. baumannii between 2 and 3-log under the same conditions.
Fig. 2.
In vitro photodynamic inactivation of A. baumannii with methylene blue (MB; squares), toluidine blue (TBO; triangles), dimethylmethylene blue (DMMB; diamonds), and new methylene blue N (NMB; circles), removing the PS from the solution (open symbols) and without removing PS (filled symbols). The irradiation wavelength was 635 nm for TBO and NMB, and 652 nm for MB and DMMB. A,B: Dye-dose–response curves upon irradiation with 22.5 J/cm2. C: Energy-dose–response with a dye dose of 10 μM. Error bars are SEM and in some cases are smaller than the diameter of the symbols.
Dye-concentration–response after removing the excess of PS was also performed. The removal of the PS from the solution means that only the dye actually bound to the bacterial cells remains. As observed in Figure 2B, higher dye concentrations were needed to induce the same reduction to the bacterial viability compared with the concentrations needed without the removal of the excess PS. In that case, both NMB and DMMB were able to completely eliminate A. baumannii using a 10 μM concentration and 22.5 J/cm2 light irradiation. However, neither 20 μM of MB nor TBO could produce such an effect, inducing only a 3-log reduction of bacterial viability in both cases.
Those results can be explained by the different lipophilicity of the dyes. It is known that the log P values for MB and TBO are similar (−0.1 and −0.21, respectively) and lower than the ones for NMB and DMMB (1.2 and 1.01, respectively) [29]. Thus, MB and TBO should be more easily removed upon centrifugation than NMB and DMMB, as observed in our experiments.
Light-dose–response curves were performed as well using a 10 μM concentration of the dyes. As shown in Figure 2C, NMB and DMMB showed a similar behavior under these conditions. Both NMB and DMMB reduced 6-log of A. baumannii after 15 J/cm2 irradiation without removing the PS, while 30 J/cm2 irradiation was needed for MB and TBO to produce the same effect. After removing the PS, only NMB and DMMB were able to inactivate 6-log of bacteria after 30 and 22.5 J/cm2 light had been delivered, respectively. Only 3-log reduction was obtained for MB and TBO after 30 J/cm2 in the one-wash curves.
It is clear that the photobactericidal effect of the dyes is affected by the initial interaction between dyes and bacteria and, subsequently, by location of the dye and its strength of binding to the bacterial cell surface. Tegos and Hamblin [30] demonstrated that phenothiazinium dyes are substrates of microbial multidrug resistance pumps (MDRs). They used different wild-type, MDR-deficient, and MDR-overexpressing bacterial strains, observing a higher inactivation for the MDR-deficient mutants and a higher resistance for the MDR-overexpressing mutants, relative to the wild-type strains. Since in our experiments we used a wild-type multidrug-resistant strain of A. baumannii that will certainly possess MDRs, pumping out the PS from the outer structure of the Gram-negative bacteria might contribute to the results obtained.
Previously it has been shown that many phenothiazinium dyes, such as MB [31], TBO [31,32], and DMMB [33], interact with the bacterial lipopolysaccharides (LPS) that compose most of the outer structure of Gram-negative bacteria. If DMMB and NMB interact with the LPS of the bacterial cell surface leading to either a stronger binding or to a deeper location within the bacterial cell wall than MB and TBO, this would lead either to a higher amount of ROS formed or to a higher proximity of the ROS formed to the cell wall critical targets. Consequently, this would explain the higher photobactericidal effect after removing the excess of PS observed for DMMB and NMB than the killing observed for MB and TBO at the same concentrations.
According to the results for both the dye-concentration and light-dose–response curves in vitro, we decided to perform the in vivo experiments with NMB as PS.
Correlation Between Bacterial Luminescence and CFU
It has been previously demonstrated that A. baumannii CFUs quantified using serial dilutions of bacterial suspensions correlate linearly with the bioluminescence emitted by those bacteria [24]. In the present study, we confirmed that the bacterial bioluminescence reduction measured in real time during PDT correlated with the corresponding CFU reduction measured after serial dilution and colony formation (Fig. 3). As observed, the trend in a light-dose experiment with 0.5 μM of NMB was the same for both bioluminescence and CFU/ml. A Student’s t-test comparing the slopes in both curves indicated that there was no statistical significant difference between both detection methods, with a P-value of 0.65.
Fig. 3.
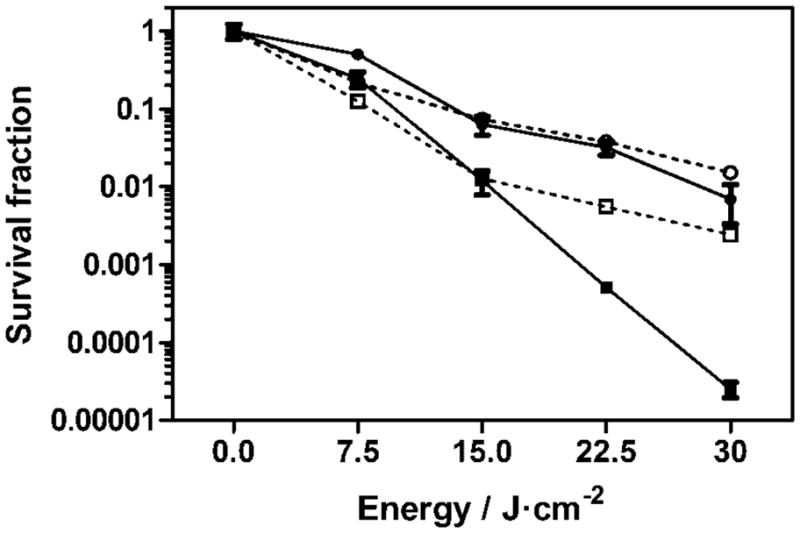
Light-dose–response of A. baumannii with 0.5 μM (circles) and 1 μM (squares) of new methylene blue N followed using a colony formation assay (solid line) and bacterial bioluminescence (dotted line).
However, a difference between these detection measurements was observed with a 1 μM concentration of NMB as a result of the lower sensitivity exhibited by the bioluminescence detection method. Similar survival fractions were observed after 7.5 and 15 J/cm2 irradiation (P = 0.97), while higher differences were observed after 22.5 and 30 J/cm2 (P = 0.02) irradiation, demonstrating the lower detection limit or in other words the dynamic range for the reduction in bioluminescence signal is ca. 3-log reduction.
These results allowed us to use the bioluminescence signal exhibited by A. baumannii for the infected burn in the in vivo experiments. However, higher light doses must be given to the infection, even after complete elimination of the bioluminescence signal, to assure the inactivation of the remaining bacteria during the PDT treatment in order to avoid a regrowth of the infection.
In Vivo PDT Treatment
Phenothiazinium dyes have not been extensively used in vivo to treat infections. TBO has been used in rats by Qin et al. [34] and Komerik et al. [35] in order to treat periodontitis by reducing the total bacterial flora or inactivating inoculated Porphyromonas gingivalis. Wong et al. [36] used TBO as well in order to treat wounds infected with Vibrio vulnificus in a mouse model, obtaining a 50% survival fraction of the PDT-treated mice.
MB has also been used in some in vivo experiments. Teichert et al. [37] used PDT with MB to treat oral candidiasis in an immunosuppressed murine model. Also Zolfaghari et al. [38] used MB as PS against methicillin-resistant Staphylococcus aureus in two different wound models obtaining a 1.4 and a 1.15-log reduction in the number of viable bacteria recovered from the wounds.
Interestingly, there is no report on the use of NMB as PS in an in vivo infection model.
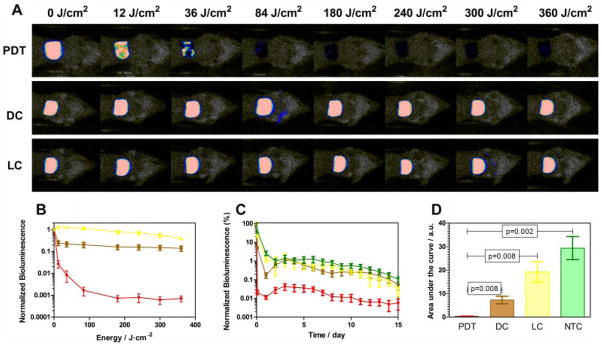
Figure 4A shows the successive bioluminescence images obtained from three representative mouse burns infected with A. baumannii. The PDT burn was treated with 800 μM of NMB and 635 nm light up to 360 J/cm2, the dark control with the same amount of NMB and no light, while the light control received 635 nm light up to 360 J/cm2. A complete elimination of the bioluminescence signal was observed after a light dose of 180 J/cm2 in the presence of NMB. After that, in order to inactivate the remaining bacteria that were not detectable by bioluminescence imaging and, subsequently, avoid the recurrence of infection, an additional 200 J/cm2 of light was given to each mouse.
Fig. 4.
A: Dose–response of bacterial luminescence from burns infected with luminescent A. baumannii and treated with 800 μM new methylene blue N, NMB, and light (PDT), with 800 μM of NMB only (DC) and with light (LC) only. B: Light-dose–response curves of the normalized bioluminescence for mice treated with photodynamic therapy (red), mice treated only with NMB (brown), and mice treated only with light (yellow). C: Time course of normalized bacterial luminescence values and (D) areas under the curves of the infected burns in mice treated with photodynamic therapy (red; PDT), mice treated only with NMB (brown; DC), mice treated only with light (yellow; LC), and nontreated mice (green; NTC).
It is known that phenothiazinium dyes can be photobleached after long exposures to red light, and the photobleaching can be magnified in the presence of either biomolecules [39] or bacteria [40]. In order to replace the PS destroyed by photobleaching, two additional aliquots of 20 μl of NMB were subsequently added during the irradiation process—after 180 J/cm2 and after 300 J/cm2.
The light-dose–responses of normalized bioluminescence of the different mouse groups are shown in Figure 4B. PDT induced a reduction of ca. 3.2-log of the bioluminescence while only 0.8- and 0.4-logs reduction were observed for the dark controls and the light controls, respectively. These results are consistent with those obtained by Dai et al. [24] using a conjugate between polyethylenimine and chlorine(e6) as PS, where a 3.6-log reduction of the bacterial luminescence was observed.
A key feature of a successful antimicrobial PS is that the damage inflicted to the bacterial cells in vivo is so extensive that regrowth of the microbial pathogens is effectively prevented [41]. Figure 4C shows the time courses of the mean bacterial luminescence from day 0 to day 15 for the PDT-treated group, the dark control group, the light control group, and a nontreated control group where neither light nor PS was applied. Six mice were used for each group. As shown in the graph, all the controls exhibited a similar time course of bioluminescence signal from day 2 until day 15, with a decrease of the signal at day 1 after the infection followed by an increase at day 2 observed in the PS alone dark control. This was presumably due to significant dark toxicity of the NMB to the bacteria. In contrast, the mean bioluminescence of the PDT-treated mice was 1.5–2 logs lower than the mean bioluminescence of all three control groups at every time point over the 2-week period.
The areas under the bioluminescence–time curves of each mouse group are represented in Figure 4D. Analysis using an ANOVA one-factor test showed statistically significant differences among the areas under the curves of all tested groups (P < 0.001). Student’s t-tests were performed to compare the areas under the curves between each control and the PDT-treated group, obtaining statistically significant differences between all the controls and the PDT-treated group (P < 0.008).
CONCLUSIONS
We have demonstrated that new methylene blue is the most powerful phenothiazinium dye tested for inactivating multidrug-resistant A. baumannii and is able to reduce more than 6-log the survival fraction of bacteria after an irradiation of 30 J/cm2 of red light with a dye concentration of 10 or 2 μM with and without removing the excess of PS, respectively.
In addition, we have shown that bacterial luminescence can be used as a real-time marker to monitor the survival fraction of bacteria during the initial 3-logs reduction of bacterial viability. There is no statistically significant difference between the light-dose–responses quantified by the bacterial luminescence of the A. baumannii strain and a colony formation assay.
Moreover, we have obtained more than 3-log reduction in the bacterial luminescence from the mouse burns infected with A. baumannii and demonstrated that there is a statistically significant difference between the areas under the bioluminescence–time curves for the normalized bioluminescence between all the controls and the PDT-treated mice.
In conclusion, new methylene blue is an effective antimicrobial PS for treating A. baumannii burn infections in vivo. As methylene blue and toluidine blue are already PSs in clinical practice, and based on the efficacy pattern obtained in this study, it may be interesting to clinically test NMB as a potential PS in a wide range of localized infections.
Acknowledgments
Contract grant sponsor: US NIH; Contract grant number: R01AI050875; Contract grant sponsor: Generalitat de Catalunya (DURSI); Contract grant sponsor: Fons Social Europeu.
This research was supported by US NIH grant R01AI050875 to M.R.H. X.R. was supported by the Generalitat de Catalunya (DURSI) and Fons Social Europeu with a predoctoral fellowship. T.D. was supported by the Bullock-Wellman Postdoctoral Fellowship Award. G.P.T. was supported by a Massachusetts Technology Transfer Center Award.
References
- 1.Bonnett R. Chemical aspects of photodynamic therapy. Amsterdam: Gordon and Breach Science Publishers; 2000. [Google Scholar]
- 2.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamblin MR, Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mroz P, Hamblin MR. Advances in photodynamic therapy: Basic, translational and clinical. Norwood, MA: Artech House; 2008. [Google Scholar]
- 5.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J Photochem Photobiol B. 1996;32:159–164. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 6.Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright M, Phoenix DA, Marland J, Wareing DRA, Bolton FJ. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol Med Microbiol. 1997;19:75–80. doi: 10.1111/j.1574-695X.1997.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 8.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romanova NA, Brovko LY, Moore L, Pometun E, Savitsky AP, Ugarova NN, Griffiths MW. Assessment of photodynamic destruction of Escherichia coli O157:H7 and Listeria monocytogenes by using ATP bioluminescence. Appl Environ Microbiol. 2003;69:6393–6398. doi: 10.1128/AEM.69.11.6393-6398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, Vanlier JE. Photosensitizing activity of water-soluble and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial-cells. Microbios. 1992;71:33–46. [PubMed] [Google Scholar]
- 11.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, Hasan T. Polycationic photosensitizer conjugates: Effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–951. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 13.Tegos GP, Anbe M, Yang CM, Demidova TN, Satti M, Mroz P, Janjua S, Gad F, Hamblin MR. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–1410. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polo L, Segalla A, Bertoloni G, Jori G, Schaffner K, Reddi E. Polylysine-porphycene conjugates as efficient photosensitizers for the inactivation of microbial pathogens. J Photochem Photobiol B. 2000;59:152–158. doi: 10.1016/s1011-1344(01)00114-2. [DOI] [PubMed] [Google Scholar]
- 15.Wainwright M, Phoenix DA, Laycock SL, Wareing DRA, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 16.Wainwright M, Phoenix DA, Gaskell M, Marshall B. Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp. J Antimicrob Chemother. 1999;44:823–825. doi: 10.1093/jac/44.6.823. [DOI] [PubMed] [Google Scholar]
- 17.Verma S, Sallum UW, Athar H, Rosenblum L, Foley JW, Hasan T. Antimicrobial photodynamic efficacy of side-chain functionalized benzo[a]phenothiazinium dyes. Photochem Photobiol. 2009;85:111–118. doi: 10.1111/j.1751-1097.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 18.Phoenix DA, Sayed Z, Hussain S, Harris F, Wainwright M. The phototoxicity of phenothiazinium derivatives against Escherichia coli and Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:17–22. doi: 10.1016/S0928-8244(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 19.Peleg AY. Optimizing therapy for Acinetobacter baumannii. Semin Respir Crit Care Med. 2007;28:662–671. doi: 10.1055/s-2007-996413. [DOI] [PubMed] [Google Scholar]
- 20.Perez F, Endimiani A, Bonomo RA. Why are we afraid of Acinetobacter baumannii? Expert Rev Anti-Infect Ther. 2008;6:269–271. doi: 10.1586/14787210.6.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: The dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;29:630–636. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Gootz TD, Marra A. Acinetobacter balumannii: An emerging multidrug-resistant threat. Expert Rev Anti-Infect Ther. 2008;6:309–325. doi: 10.1586/14787210.6.3.309. [DOI] [PubMed] [Google Scholar]
- 23.Doi Y, Husain S, Potoski BA, McCurry KR, Paterson DL. Extensively drug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2009;15:980–982. doi: 10.3201/eid1506.081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai TH, Tegos GP, Lu ZS, Huang LY, Zhiyentayev T, Franklin MJ, Baer DG, Hamblin MR. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–3934. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens EJ, Ryan CM, Friedberg JS, Barnhill RL, Yarmush ML, Tompkins RG. A quantitative model of invasive Pseudomonas infection in burn injury. J Burn Care Rehabil. 1994;15:232–235. doi: 10.1097/00004630-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Gilpin DA. Calculation of a new Meeh constant and experimental determination of burn size. Burns. 1996;22:607–611. doi: 10.1016/s0305-4179(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 27.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Davis PJ, Rabinowitz P. Methods of numerical integration. New York, NY: Academic Press; 1975. [Google Scholar]
- 29.Phoenix DA, Harris F. Phenothiazinium-based photosensitizers: Antibacterials of the future? Trends Mol Med. 2003;9:283–285. doi: 10.1016/s1471-4914(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 30.Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usacheva MN, Teichert MC, Biel MA. The interaction of lipopolysaccharides with phenothiazine dyes. Lasers Surg Med. 2003;33:311–319. doi: 10.1002/lsm.10226. [DOI] [PubMed] [Google Scholar]
- 32.Komerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem Photobiol. 2000;72:676–680. doi: 10.1562/0031-8655(2000)072<0676:teopao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Keler T, Nowotny A. Metachromatic assay for the quantitative-determination of bacterial-endotoxins. Anal Biochem. 1986;156:189–193. doi: 10.1016/0003-2697(86)90172-7. [DOI] [PubMed] [Google Scholar]
- 34.Qin YL, Luan XL, Bi LJ, Sheng YQ, Zhou CN, Zhang ZG. Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J Periodontal Res. 2008;43:162–167. doi: 10.1111/j.1600-0765.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 35.Komerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47:932–940. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong TW, Wang YY, Sheu HM, Chuang YC. Bactericidal effiects of toluidine blue-mediated photodynamic action on Vibrio vulnificus. Antimicrob Agents Chemother. 2005;49:895–902. doi: 10.1128/AAC.49.3.895-902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teichert MC, Jones JW, Usacheva MN, Biel MA. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:155–160. doi: 10.1067/moe.2002.120051. [DOI] [PubMed] [Google Scholar]
- 38.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang LZ, Tang GQ. The binding properties of photosensitizer methylene blue to herring sperm DNA: A spectroscopic study. J Photochem Photobiol B. 2004;74:119–125. doi: 10.1016/j.jphotobiol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Usacheva MN, Teichert MC, Sievert CE, Biel MA. Effect of Ca2+ on the photobactericidal efficacy of methylene blue and toluidine blue against gram-negative bacteria and the dye affinity for lipopolysaccharides. Lasers Surg Med. 2006;38:946–954. doi: 10.1002/lsm.20400. [DOI] [PubMed] [Google Scholar]
- 41.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]