Abstract
Background
Low levels of laser or non-coherent light, termed low-level light therapy (LLLT) have been reported to accelerate some phases of wound healing, but its clinical use remains controversial.
Methods
A full thickness dorsal excisional wound in mice was treated with a single exposure to light of various wavelengths and fluences 30 minutes after wounding. Wound areas were measured until complete healing and immunofluorescence staining of tissue samples was carried out.
Results
Wound healing was significantly stimulated in BALB/c and SKH1 hairless mice but not in C57BL/6 mice. Illuminated wounds started to contract while control wounds initially expanded for the first 24 hours. We found a biphasic dose–response curve for fluence of 635-nm light with a maximum positive effect at 2 J/cm2. Eight hundred twenty nanometer was found to be the best wavelength tested compared to 635, 670, and 720 nm. We found no difference between non-coherent 635 ± 15-nm light from a lamp and coherent 633-nm light from a He/Ne laser. LLLT increased the number of α-smooth muscle actin (SMA)-positive cells at the wound edge.
Conclusion
LLLT stimulates wound contraction in susceptible mouse strains but the mechanism remains uncertain.
Keywords: low-level laser, biostimulation, wound healing, mouse strain differences, smooth muscle actin, myofibroblasts
INTRODUCTION
The first publication about low-level laser therapy (LLLT) (then called laser biostimulation) appeared almost 40 years ago in Hungary [1]. Since then large numbers of studies demonstrating positive results of LLLT in cells in vitro, animal models, and clinical reports have been published, but the subject remains controversial [2]. Many negative studies have also been published further confounding the issue [3]. Nevertheless, the use of coherent-light sources (lasers) or non-coherent light sources (light-emitting diodes, LEDs) has become widespread by physical therapists [4] dentists [5], and practitioners of sports medicine [6]. In 2002, 510K FDA clearance was issued for an 830-nm diode laser for the treatment of carpal tunnel syndrome. There were several controlled trials reporting significant improvement in pain and some improvement in objective outcome measures [7–9].
The basic biological mechanism behind the effects of LLLT is thought to be absorption of red and near infrared light by chromophores contained in the protein components of the respiratory chain located in mitochondria, in particular cytochrome c oxidase [10,11]. It is thought that this absorption of energy may cause photodissociation of inhibitory nitric oxide from cytochrome c oxidase [12] leading to increased enzyme activity [13], increased electron transport [14], and increased production of ATP [15]. Moreover, low-level light was shown to stimulate the expression of multiple genes related to cellular migration, proliferation, and modulate the production of growth factors and cytokines [16].
The complexity of the parameters involved in LLLT such as wavelength, total fluence, fluence rate, coherence, pulse structure or continuous wave, and polarization state has meant that a number of negative studies of LLLT as well as many positive studies have been published [17]. However, one important point that has been demonstrated by multiple studies in cell culture [18], animal models [19], and in clinical studies is the concept of a biphasic dose response with the total delivered light energy density (fluence). The reason why the technique is termed low level is that there exists an optimal dose of light for any particular application, and doses lower than this optimum value, or more significantly, larger than the optimum value will have a diminished therapeutic outcome, or for high doses of light a negative outcome may result. While the studies exploring the effects of different power and energy densities on the outcome of the treatment are common, the studies of the wavelength dependency, especially in in vivo models are less widespread.
In this report, we describe the effects of LLLT with various optical parameters, including the wavelength, on the outcome of the treatment in a standardized model of full-thickness excisional wound healing in mice. A single exposure of the wound to light 30 minutes after wounding led to increased wound healing especially in the early time points 1–5 days post-injury. The likely mechanism involved in stimulation of wound healing by LLLT is enhancement of wound contraction by the dermal cells (fibroblasts and myofibroblasts) at the wound edge.
MATERIALS AND METHODS
Animal Model of Excisional Wound Healing
All animal experiments were approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital and were in accordance with NIH guidelines. Mice were housed five per cage and given access to food and water ad libitum. Male BALB/c, C57BL/6-1J, and SKH1 hairless mice were obtained form Charles River Laboratories (Wilmington, MA). A total of 139 mice between 6 and 9 weeks of age were used. The backs of BALB/c and C57BL/6 mice were depilated by the use of Nair cream (Carter-Wallace, New York, NY) 15–24 hours before wounding. The mice were anesthetized by an intraperitoneal injection of a ketamine–xylazine cocktail (90 mg/kg ketamine and 10 mg/kg xylazine) before wounding procedures and during treatments. Dorsal full thickness excisional wounds were made with sterile scissors and forceps, the wound was left uncovered during the whole period of experiments, that is, until fully healed. To ensure comparable wound size in all the mice, a 10×13 mm template was used. Single illuminations were performed 30 minutes after wounding; the area illuminated included the wound bed and intact skin at all four wound edges (approximately 5 mm). Non-illuminated control mice with wounds were kept anesthetized for the same length of time as the illuminated mice.
Light Sources, Dosimetry, and Treatment
Three light sources were used in this study. A non-coherent light source with interchangeable 30-nm band pass filters (LumaCare, London, UK) was used to deliver 635 ± 15-nm light in order to generate light dose–response curves. Fluences delivered were 1, 2, 10, and 50 J/cm2. Fluence rates used were 80–100 mW/cm2. Helium-Neon (632.8 nm) laser (Melles Griot, Carlsbad, CA) was used as a source of monochromatic coherent light. Two fluences 1 and 2 J/cm2 were used. Fluence rates of 2 and 1 mW/cm2 were used to deliver 1 and 2 J/cm2 of light, respectively. A monochromator coupled to a xenon arc lamp (Spectra Physics, Mountain View, CA) was used to produce 670 ± 15, 720 ± 15, and 820 ± 15-nm light. To create a homogeneous light spot with a diameter of 3 cm, the light was delivered using a ring light guide (Edmund Optics GmbH, Karlsruhe, Germany). Fluence of 1 J/cm2 was employed. Fluence rates were 0.59, 0.79, and 0.86 mW/cm2 for 670 ± 15, 720 ± 15, and 820 ± 15 nm, respectively. Power readings of He/Ne laser and LumaCare lamp were measured with the Lasermate power meter (Coherent, Inc., Santa Clara, CA). A power meter (Ophir Optronics, Inc., Wilmington, MA) was used for the xenon arc lamp.
Animal Follow-Up
General health of the animals was monitored daily. Adverse effects of wounding were not observed. Width and length of the wounds were measured with digital caliper (Control Company, Friendswood, TX) daily and the areas of the wounds were calculated. Wound images were acquired every other day using a digital camera. For all follow-up procedures, mice were anaesthetized with isoflurane solution (Baxter, Deerfield, IL). Mice were sacrificed by CO2 inhalation either 24 hours after wounding/treatment in order to obtain skin samples or after complete wound closure.
Immunohistochemistry
Identification of myofibroblasts was performed in skin samples taken after 24 hours from two BALB/c mice treated with 820 ± 15-nm light and two control BALB/c mice. Wounds together with underlying muscle layers were excised and fixed in 4% formalin, embedded in paraffin, and cut into 5 μm thick sections. Sections were rehydrated, subjected to the antigen retrieval procedure using Retrievagen A solution (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions, and rinsed with distilled water followed by incubation in phosphate buffered saline (PBS)/0.1% Triton X-100 for 5 minutes. In order to minimize non-specific binding, sections were treated with Mouse-on-Mouse blocking solution (Vector Labs, Burlingame, CA) for 1 hour according to the manufacturer’s instructions. Primary antibodies were mouse anti-α-smooth muscle actin (SMA) that labels myofibroblasts (Biogenex, San Ramon, CA) used at 1:200 dilution and pre-diluted rabbit polyclonal anti-CD-31 that labels endothelial cells (Abcam, Cambridge, MA). The incubation with primary antibodies was performed for 1 hour at room temperature. Sections were then washed and co-incubated with fluorescently labeled anti-mouse Alexa 488, anti-rabbit 546 secondary antibodies (Molecular Devices, Sunnyvale, CA) both at 1:250 dilution and 16.2 μM Hoechst 33342 (Molecular Probes, Invitrogen, Carlsbad, CA) for 1 hour at room temperature. After triple wash in PBS, stained sections were mounted with Fluorosave (CN Biosciences, San Diego, CA). Visualization of the slides was performed with Axiovert 200 M microscope (Carl Zeiss MicroImaging, Thornwood, NY) using 10× and 40× objective lenses.
Data Analysis and Statistics
The numbers representing the percent of original wound size were used for healing curve generation using KaleidaGraph program (Synergy Software, Reading, PA). The data between the experimental points were approximated using cubic spline interpolation technique. The areas under the curves were calculated by trapezoidal rule numerical integration. The number of the experimental points determined the number of the nodes employed for the numerical integration. The integrals were used for comparing the effects of different treatment regimens (wavelength, dose of light). Differences in the areas under the curve between control and treatment were used to evaluate the effects of treatment. Microcal Origin 6.1 software (Northampton, MA) was used for area under the curve calculations. All the data are presented as mean ± SEM. Statistical significance was analyzed by one-way ANOVA. The value of P < 0.05 was considered significant.
RESULTS
Low-Level Light Stimulates Wound Healing in BALB/c Mice
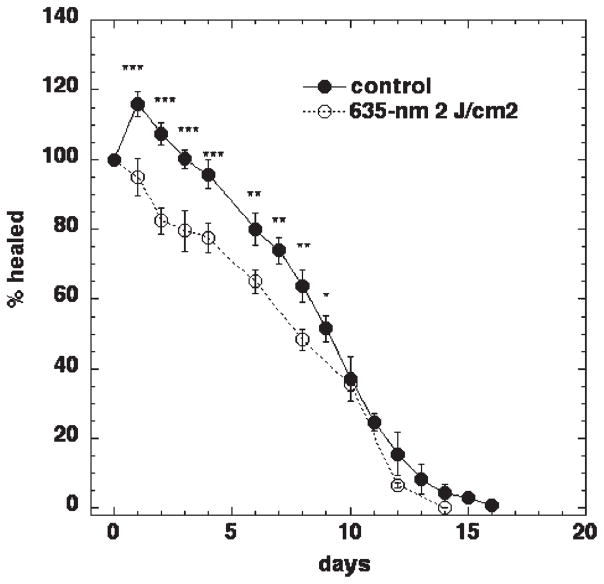
It has been shown previously that low-level red light induces acceleration of wound healing in experimental animals and patients [20]. Therefore, for initial experiments 2 J/cm2 of 635-nm non-coherent light was used on full thickness dorsal wounds in BALB/c mice. Healing curves generated for control mice demonstrated an initial increase in wound size from day 1 to day 4 post-wounding. At day 5, control wounds were the same size as the original size on day 0. From day 6 onwards, control wounds exhibited a gradual decrease in wound size until accomplishment of total healing at day 17 (Fig. 1). In marked contrast, the initial wound expansion seen in control mice was absent in mice treated with 2 J/cm2 of 635-nm light from a non-coherent light source 30 minutes after wounding. Instead of expanding, the wound started to contract immediately after illumination and 1 day later the mean wound area was highly significantly (P < 0.001) smaller than the mean wound area of the control wounds. This significant difference between control and illuminated wounds was maintained until day 9. Interestingly, at 10 days post-wounding the difference between control and treated wounds became non-significant and the wounds in both groups healed at approximately same rate until both groups achieved complete healing.
Fig. 1.
The effect of 635-nm light on excisional wound healing in BALB/c mice. Excisional wound healing in BALB/c mice with (n = 25) and without (n = 18) a single exposure to 2 J/cm2 of 635-nm light from a filtered lamp delivered 30 minutes after wounding. Data points are means and SEM. Significance was determined by one-way ANOVA and ***P <0.001; ** P <0.01; *P <0.05.
Biphasic Dose Response Between Fluence and Wound Healing
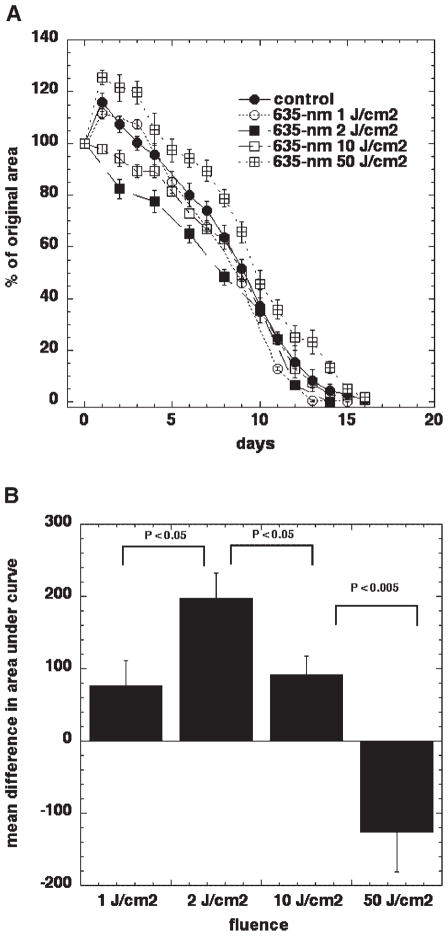
In order to establish the effect of varying the total energy density delivered on the stimulation of wound healing, in other words to construct a dose–response curve, we used different fluences of light from a 635-nm non-coherent light source. Fluence rates at which light was delivered varied between 80 and 100 mW/cm2 and the fluences were 1, 2, 10, and 50 J/cm2. Despite a small variation in fluence rates between different fluences, the comparison between the groups was performed.
Figure 2A shows significant differences between control and illuminated wounds in animals and between different total fluences delivered. As mentioned above, untreated wounds in BALB/c mice tended to expand 1–5 days after wounding. In mice treated with 1 J/cm2 and in animals treated with 50 J/cm2, this initial wound expansion was also present. Mice that received 50 J/cm2 showed a bigger expansion than that seen in control mice and the wound area did not return to its original size until 7–8 days after wounding. There was very little difference between the 1 J/cm2 group and no illumination control wounds. Mice treated with 2 or 10 J/cm2 demonstrate no increase in wound size the next day after treatment and 2 J/cm2 appears to be better than 10 J/cm2 (Fig. 2A). To show the effect of treatment, differences in the areas under the curves were calculated. Positive effects compared to control wounds were found for 1, 2, and 10 J/cm2; however, the maximal positive effect was found at 2 J/cm2. If 50 J/cm2 was used, the area under the control-healing curve was actually smaller that the area under experimental curve, thus the higher dose of 635-nm light—50 J/cm2 had a negative effect on the rate of mouse wound healing. Therefore, in our wound model, a biphasic dose response to 635-nm light from an incoherent light source was demonstrated: 2 J/cm2 has the largest positive effect, 1 and 10 J/cm2 improves healing to a lesser extent, while 50 J/cm2 has a negative effect on wound healing.
Fig. 2.
Biphasic dose response between fluence and wound healing. A: Excisional wound healing in BALB/c mice with no illumination (n = 18) and with a single exposure to 1 (n = 8), 2 (n = 25), 10 (n = 8), or 50 (n = 14) J/cm2 of 635-nm light from a filtered lamp delivered 30 minutes after wounding. Data points are means and SEM. B: The area under each individual mouse healing curve were subtracted from the area under the mean control (no illumination) healing curve and means of these differences in area are shown in Figure 2B with bars = SEM. Significance determined by one-way ANOVA.
820 nm Is the Most Effective Wavelength
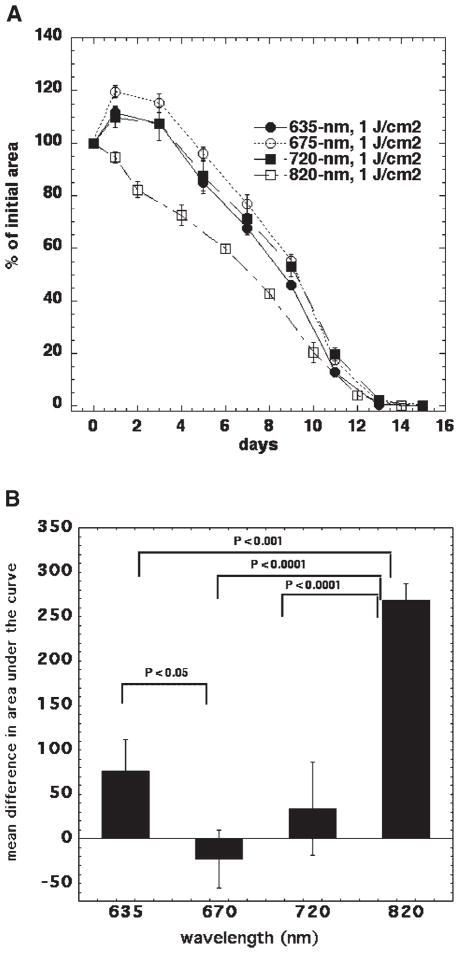
We compared four different wavelength ranges of red and near infrared light centered at: 635, 670, 720, and 820 nm delivered from the same non-coherent polychromatic light source to study the tissue response variation with wavelength. To minimize the number of variables that could interact with the effects of the light, a standardized wound model, as described above, was used. Irradiation was performed using low fluence rates that were similar for the different wavelength ranges with a fixed fluence of 1 J/cm2.
The most pronounced stimulation of wound healing by a considerable margin was obtained with 820-nm light (Fig. 3A,B). There was no expansion in wound size after wounding and illumination with 820 nm and contraction occurred approximately 3–4 days faster than with the other wavelengths. A noticeable difference between the groups was maintained until day 10 post-wounding. The other wavelengths had lesser effects, with the best one being 635 nm where a significant positive effect was also obtained. It should be noted that the action spectrum obtained in this experiment may be different if a different fluence is used. When the biphasic nature of the dose–response curve (Fig. 2B) is taken into account, this possibility becomes fairly likely.
Fig. 3.
The effect of the wavelength of light on mouse wound healing. A: Excisional wound healing in BALB/c mice with no illumination (n = 18) and with a single exposure to 1 J/cm2 of 635-nm (n = 8), 675-nm (n = 10). 720-nm (n = 5), or 810-nm (n = 17) light from a filtered lamp delivered 30 minutes after wounding. Data points are means and SEM. B: The area under each individual mouse healing curve were subtracted from the area under the mean control (no illumination) healing curve and means of these differences in area are shown in Figure 3B with bars = SEM. Significance determined by one-way ANOVA.
Wound Healing Stimulation Is Mouse Strain Dependent
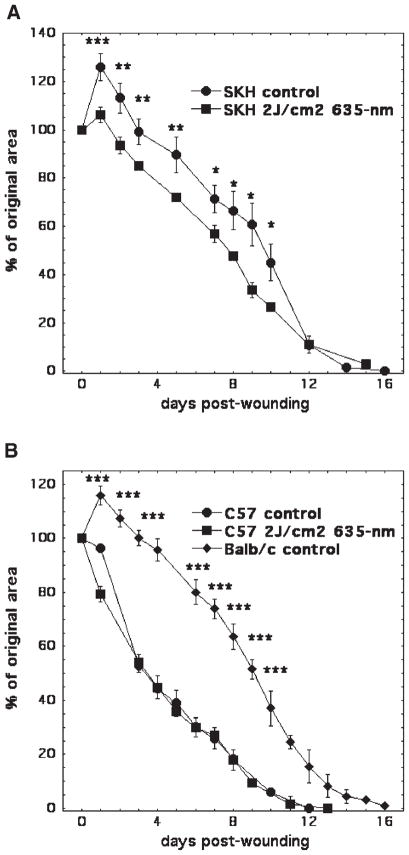
All experimental data presented above were obtained in BALB/c mice. However, significant differences in healing rates between mouse strains have been reported in the literature although this is not a well-studied area of research [21,22]. Therefore, we hypothesized that responses to treatment with light may also vary in different strains of mice. To test this hypothesis, we used C57BL/6 black mice and SKH1 hairless mice in addition to the previously studied BALB/c albino mice. Full thickness dorsal excisional wounds were employed. As expected from reports in the literature [21,22], in non-illuminated control mice, wounds healed at significantly different rates depending on the mouse strain. In BALB/c and SKH1-hairless mice, wound expansion was seen from day 1 to day 4–5 post-wounding. Wounds in C57BL/6 mice did not have any initial expansion and overall healed significantly faster; complete closure was achieved at day 12 in these mice and day 16–17 in both BALB/c and SKH1 mice (Fig. 4A,B). To investigate the effect of LLLT on wound healing in C57BL/6 and SKH1 mice, we used 2 J/cm2 635-nm non-coherent light delivered 30 minutes after wounding. SKH1 mice behaved in a similar fashion to BALB/c mice in their response to LLLT. It is possible that the response to light was not quite as pronounced in SKH1 compared to BALB/c mice, as there was still a small expansion at day 1 (Fig. 4A). By contrast, in C57BL/6 mice there was almost no effect of illumination (Fig. 4B).
Fig. 4.
The effect of LLLT on wound healing is mouse strain dependent. A: Excisional wound healing in SKH hairless mice with no illumination (n = 5) and with a single exposure (n = 5) to 2 J/cm2 of 635-nm light from a filtered lamp delivered 30 minutes after wounding. B: Excisional wound healing in C57BL/6 mice (n = 5) and BALB/c (n = 18) with no illumination and C57BL/6 mice (n = 7) with a single exposure to 2 J/cm2 of 635-nm light from a filtered lamp delivered 30 minutes after wounding. Data points are means and SEM. Significance determined by one-way ANOVA. ***P <0.001; **P <0.01; *P <0.05.
Laser and Non-Coherent Lamp Are Equivalent
There is a considerable debate in the literature about the differences between the cellular effects of monochromatic laser light and polychromatic light from non-laser light sources [23,24]. In order to investigate whether the effect of light on wound healing is independent of such properties of light as monochromaticity or coherency, but depends on only wavelength and intensity of light, we compared coherent monochromatic light from He/Ne laser (632.8 nm) and light from a broad band non-coherent light source (635 ± 15 nm). We used the same spot size and the same fluence rate, the differences were in the coherency and monochromaticity of the He/Ne laser light which were not present in the filtered broad-band light. BALB/c mice were used in this experiment. Illumination with 2 J/cm2 was performed 30 minutes after wounding. Improved wound healing was observed under both experimental conditions (He/Ne laser and 635 ± 15-nm lamp) as compared to controls; however, the difference between the light from different sources was not significant (Fig. 5). Therefore, we concluded that the wavelength is the main variable that defines the wound healing in response to light. However, more research needs to be done using different laser and polychromatic light sources.
Fig. 5.
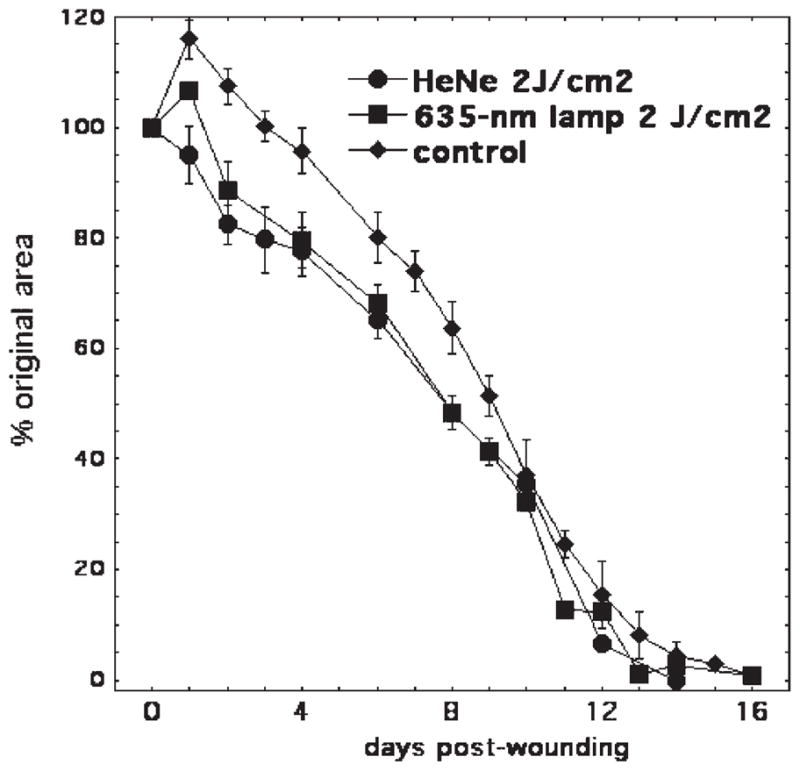
Laser and non-coherent light affect the excisional wound healing in mice. Excisional wound healing in BALB/c mice with (n = 25) and without (n = 18) a single exposure to 2 J/cm2 of 635-nm light from a filtered lamp, or 2 J/cm2 of 632.8-nm light from a He/Ne laser (n = 8) delivered 30 minutes after wounding. Data points are means and SEM.
Immunohistochemistry Demonstrates α-Smooth Muscle Actin-Positive Cells in Wound Edge
Figure 6 shows the immunofluorescence staining of tissue sections removed 24 hours after wounding from non-illuminated and illuminated mice. Nuclei are stained in blue by Hoechst 33342, SMA in green, and CD31 is stained in red. This triple staining allowed us to distinguish between SMA-positive cells that surround small blood vessels in the dermis from myofibroblastic cells situated in the dermis. SMA-positive cells localized in vicinity to CD31-positive endothelial cells represent smooth muscle cells and/or pericytes restricted to blood vessels; SMA-positive cells, not co-localized with CD31-positive cells and scattered throughout the dermis are identified as myofibroblasts. The number of green cells (labeled with white arrows) is higher in illuminated wounds.
Fig. 6.
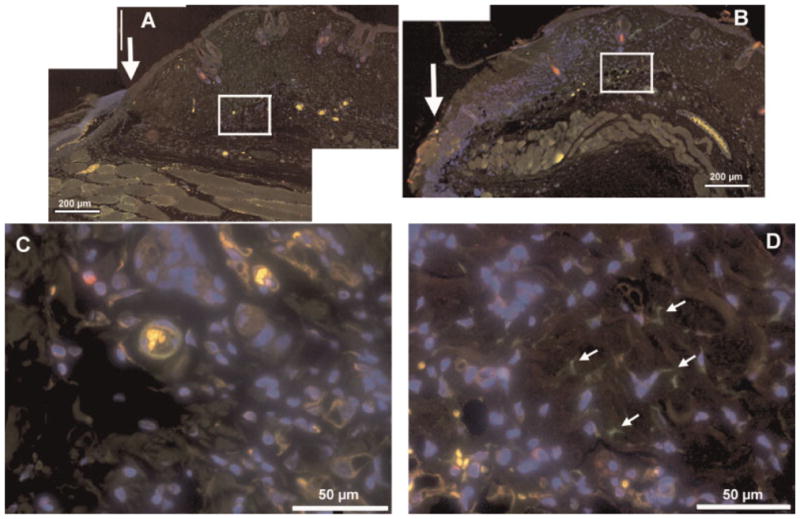
Immunofluorescence of tissue sections removed from control wounds and from illuminated wounds. Composite low magnification (10×) immunofluorescence images of mouse wounds 1 day after wounding; (A) control wound, (B) wound treated with 820-nm light as described. Arrow indicates the wound edge, scale bar is 1 mm. The areas in the white squares are shown in higher magnification (40×) for control (C), and illuminated (D) wounds, respectively. Scale bar is 200 μm for 10× and 50 μm for 40-X, arrows indicate green only stained cells proposed to be SMA-positive myofibroblasts in illuminated wounds (panel D).
DISCUSSION
There have been numerous reports in the literature over the last thirty years that LLLT with various optical parameters can stimulate wound healing in both small and large animals and also in humans. Even so LLLT for stimulation of wound healing remains controversial, and is not generally accepted as proven by either clinicians or by researchers alike [17,25]. The reasons for this enduring controversy in both the popular and scientific press is probably due to the high degree of complexity inherent in this technology. A large number of wavelengths of light in the red and near-infrared range of the electromagnetic spectrum have been tested. In addition, both the delivered fluence and the fluence rate have been varied quite widely. To further complicate matters, there is debate whether the coherent monochromatic light of a laser is better than non-coherent light in a defined wavelength range from a LED or a filtered lamp. Furthermore, the polarization state, pulse duration, and repetition rate are thought by some authors to be important. In addition to the large number of optical parameters that can logically be varied, there remains a large variety of conditions (even within the field of wound healing) that could be treated. A variety of non-healing ulcers occur mainly on the lower extremities that are caused by venous (and arterial) insufficiency, diabetes, prolonged pressure, and other comorbid conditions. These chronic wounds are often proposed as suitable disorders for LLLT. In order for some of these LLLT parameters to be subjected to scientific investigation, a reproducible and quantifiable animal model of LLLT effects would be desirable. We believe that an excisional wound-healing model on the mouse back fulfills some of the requirements of such an animal model.
There have been several previous reports on the use of LLLT in rodent wound healing studies. These reports can be separated into two broad groups depending on whether the animals used were normal, or whether they had been manipulated in some way to depress wound healing. These manipulations employed to impair normal wound healing include diabetes [26], ionizing radiation [27], treatment with corticosteroids [28], or malnourishment [29]. Indeed, it has been asserted that normal healthy rodents may not be a suitable model for studying LLLT effects on wound healing [30]. However, our data on healthy animals are useful for establishing treatment parameters that can be used for elucidation of the mechanisms underlying stimulation of wound healing by LLLT in transgenic and knockout mice [31] as well as in the models of impaired wound healing.
Our initial experiment in BALB/c mice showed that a single exposure of the excisional wound 30 minutes after wounding to 2 J/cm2 of 635 ± 15-nm light delivered at a fluence rate of 80 mW/cm2 resulted in statistically significantly smaller wounds between 1 and 10 days post-wounding (Fig. 1). Remarkably, the most noticeable difference between illuminated and control wounds was observed at 1 day post-wounding. Non-treated wounds showed a noticeable expansion the next day with the mean wound area being almost 20% bigger than it was immediately after wounding. By contrast, the illuminated wounds decreased in size after light delivery and by the next day the mean area was 5% less than it was immediately after wounding. The difference in wound areas between these two groups of mice at day 1 post-wounding was significantly different (P <0.001), and the difference remained significant although becoming increasingly less pronounced until day 9 post-wounding. On the tenth day after wounding, the wounds in the illuminated and control groups of mice had reached the same average area. Using the same light source, we were able to carry out an experiment allowing for evaluation of the effects of the dose of light on the healing of mouse wounds. Light was delivered at 1, 2, 10, or 50 J/cm2 from the same 635-nm light source and at the same fluence rate. The comparison of the area under the healing curves generated based on the wound healing rates in control and treated animals, led to a set of four values three of which (1, 2, and 10 J/cm2) showed positive effects on wound healing and the dose of 50 J/cm2 gave a negative effect, that is, illuminated wounds healed slower than controls (Fig. 2A,B). This biphasic dose–response (a little light is good but a lot of light is bad) has been reported for studies in LLLT in both cell culture [32] and animal models [33].
The effects of the light wavelength were evaluated by delivering 1 J/cm2 of light of four different wavelengths (635, 670, 720, and 820 nm) all at +/−15-nm band passes from a monochromator. Due to the nature of the light source, the fluence rate was low, less than 2 mW/cm2. This therefore meant that we were limited in the size of the total fluence that could be delivered and we therefore chose to deliver only 1 J/cm2. At this fluence, 820 nm had significant positive effect on wound healing and prevented the initial wound expansion observed in control wounds. There was a small significant effect of 635-nm light, neither of the other wavelengths had any effect. In retrospect, it might have been preferable to deliver several different fluences at each wavelength, as the action spectrum will probably be somewhat different at different absolute fluences. For instance, it is possible that although there was no effect of 1 J/cm2 675-nm light, a positive effect might have been obtained if 5 or 10 J/cm2 of 675-nm light had been delivered. Equally, it is possible that 720-nm (thought to be a non-effective wavelength) would have no effect regardless of the total fluence delivered. The most effective wavelengths correspond to the absorption peaks of the cytochrome C oxidase that is considered as the main chromophore responsible for cellular effects of LLLT [13]. The activation of this enzyme leads to an increase in the production of ATP that is required for numerous cellular functions providing both energy and phosphates required for the regulation of cellular functions. Moreover, addition of external ATP was shown to stimulate wound healing in an animal model [34]. Although no increase in contraction was found in mice treated with external ATP, in vitro observations suggest that ATP increases wound contraction by serving both as an energy source for generation of contractile force and motility, and as a phosphate donor for kinase reactions regulating contraction [35,36].
Generally, the studies of the effects of LLLT on wound healing are performed in a single strain of mice. Here, in addition to the BALB/c mice, responses to LLLT were tested in SKH1 and C57BL/6 mice. SKH1 is a hairless non-pedigreed immunocompetent mouse on an albino background, while C57BL/6 is an inbred pigmented strain widely used for transgenic and knockout models [37]. The differences in healing responses between BALB/c and C57BL/6 mice were described in the model of ear punch [38]. Similarly, we found large differences in healing of control non-illuminated wounds between the albino mice (BALB/c and SKH1) and the black C57BL/6 mice. The initial expansion in wound size observed in BALB/c mice was also seen in SKH1 hairless mice, but did not occur in C57BL/6 mice. The consequence of this difference in wound expansion was that excisional wounds in C57BL/6 mice healed significantly faster than the same wounds in BALB/c mice for the entire course of the healing process, and reached complete closure 4 days earlier (12 days vs. 16 days; Fig. 4B). The stimulating effect of 2 J/cm2 of 635-nm light was comparable in both mouse strains that exhibited the initial expansion in wound size, BALB/c and SKH1 (Fig. 4A), and resulted in the initial expansion being reduced or eliminated. By contrast, in C57BL/6 mice that showed no expansion in wound size and an overall faster healing process, LLLT had virtually no effect. It appears that wound healing in C57BL/6 is fast and the healing rate is hard to increase, while both BALB/c and SKH1 mice have sub-optimal wound healing that can be stimulated by light. The fact that the C57BL/6 mice did not show the same expansion as the other strains in the absence of light, suggest that the failure of these mice to respond to LLLT is not necessarily due to their increased skin pigmentation preferentially absorbing the light and thereby preventing the light reaching its site of action.
There has been considerable debate in the literature concerning the relative merits of laser and non-laser light for LLLT. Because LLLT was originally discovered using light delivered by lasers, for many years it was thought that certain properties of laser light, namely the fact that laser light is coherent and monochromatic, were important or even crucial in the LLLT effects. Since the advent of LEDs and other non-coherent light sources, this assumption has been questioned. Our data compared the effects of 2 J/cm2 of light delivered at comparable fluence rates originating from a He/Ne laser (monochromatic at 632.8 nm and long coherence length) with non-coherent light with a 30-nm band width centered at 635-nm produced by a filtered lamp and found the difference in mean healing curves was non-significant.
Our experimental data suggest that LLLT stimulates mouse wound healing by promoting contraction and/or preventing the expansion of mouse wounds. Wound contraction is thought to be facilitated by SMA expressing myofibroblasts in the dermis surrounding the injured area [39]. There is literature suggesting that LLLT induces the fibroblast-myofibroblast transformation both in vitro and in vivo [33,40,41]. Therefore, we hypothesized that the illumination delivered at 30-minutes post-wounding affects fibroblasts at the wound edge, inducing fibroblast–myofibroblast transition and, therefore wound contraction. In order to directly test this hypothesis, we performed immunohistochemistry on tissue sections of dermis that were removed from mice 24 hours after wounding and light exposure, using anti-SMA, and anti-CD31-specific antibodies. This revealed that there was a significant number of SMA positive cells in tissues surrounding LLLT-treated wounds, but not in non-illuminated control wounds. Unlike SMA-positive cells in untreated wounds, SMA-positive cells in wounds subjected to 820 ± 15-nm light were not co-localized with CD31-positive endothelial cells, suggesting that they were indeed myofibroblasts, rather than pericytes or smooth muscle cells associated with dermal blood vessels. The presence of contractile myofibroblasts at the edge of illuminated wounds could explain the lack of expansion of the wound area observed 1 day post-wounding in BALB/c and SKH1 mice. A recent report [42] has shown that LLLT (904-nm laser) both in vivo and in vitro can activate the secreted latent form of transforming growth factor (TGF)-β from its inactive state where the polypeptide homodimer is bound to latency associated peptide, into its active unbound state. TGF-β is the most important cytokine involved in the fibroblast–myofibroblast transition and induction of SMA expression [43]. The second mechanism by which wound contraction occurs is the locomotion of fibroblasts followed by the reorganization of the extracellular matrix at the edges of the wound [36]. The hypothesis that LLLT increases the rate of fibroblast migration was not tested in this study; however, it was shown previously that LLLT increases fibroblast motility [44]. Therefore, it is possible that LLLT stimulates wound contraction by both promoting fibroblast–myofibroblast differentiation and fibroblast motility.
In conclusion, we have shown that a single exposure to low levels of red or near infrared light significantly stimulates the healing of excisional wounds created in BALB/c and SKH1 mice. We have demonstrated a wavelength and dose dependence of LLLT-mediated wound closure, together with a light-induced alteration in dermal fibroblast phenotype. Understanding of the molecular mechanisms controlling these light-induced wound healing responses will reveal new cell- and tissue-based targets, together with opportunities for creating innovative wound healing therapeutics. Our future work will address the questions regarding the mechanisms of LLLT-induced wound contraction.
Acknowledgments
This work was funded by the U.S. National Institutes of Health (Grants CA/AI838801 and AI050875 to M.R.H., EY 15125 to I.M.H.) and by Abbott-Ross, Inc., (I.M.H.). Tatiana N. Demidova-Rice was supported by a Wellman Center Graduate Student Fellowship. We thank J. Michael Rukstalis, PhD for the help with immunohistochemistry. We are grateful to R. Rox Anderson for support.
Contract grant sponsor: U.S. National Institutes of Health; Contract grant numbers: CA/AI838801, AI050875, EY 15125; Contract grant sponsor: Abbott-Ross, Inc.; Contract grant sponsor: Wellman Center Graduate Student Fellowship.
Abbreviations used
- LLLT
low-level light therapy
- SMA
α-smooth muscle actin
References
- 1.Mester E, Ludany G, Sellyei M, Szende B, Gyenes G, Tota GJ. Studies on the inhibiting and activating effects of laser beams. Langenbecks Arch Chir. 1968;322:1022–1027. doi: 10.1007/BF02453990. [DOI] [PubMed] [Google Scholar]
- 2.Brosseau L, Welch V, Wells G, Tugwell P, de Bie R, Gam A, Harman K, Shea B, Morin M. Low level laser therapy for osteoarthritis and rheumatoid arthritis: A metaanalysis. J Rheumatol. 2000;27(8):1961–1969. [PubMed] [Google Scholar]
- 3.Tuner J, Hode L. It’s all in the parameters: A critical analysis of some well-known negative studies on low-level laser therapy. J Clin Laser Med Surg. 1998;16(5):245–248. [PubMed] [Google Scholar]
- 4.Chow RT, Barnsley L. Systematic review of the literature of low-level laser therapy (LLLT) in the management of neck pain. Lasers Surg Med. 2005;37(1):46–52. doi: 10.1002/lsm.20193. [DOI] [PubMed] [Google Scholar]
- 5.Sun G, Tuner J. Low-level laser therapy in dentistry. Dent Clin North Am. 2004;48(4):1061–1076. viii. doi: 10.1016/j.cden.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Bolin DJ. Transdermal approaches to pain in sports injury management. Curr Sports Med Rep. 2003;2(6):303–309. doi: 10.1249/00149619-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Irvine J, Chong SL, Amirjani N, Chan KM. Double-blind randomized controlled trial of low-level laser therapy in carpal tunnel syndrome. Muscle Nerve. 2004;30(2):182–187. doi: 10.1002/mus.20095. [DOI] [PubMed] [Google Scholar]
- 8.Naeser MA, Hahn KA, Lieberman BE, Branco KF. Carpal tunnel syndrome pain treated with low-level laser and microamperes transcutaneous electric nerve stimulation: A controlled study. Arch Phys Med Rehabil. 2002;83(7):978–988. doi: 10.1053/apmr.2002.33096. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub MI. Noninvasive laser neurolysis in carpal tunnel syndrome. Muscle Nerve. 1997;20(8):1029–1031. doi: 10.1002/(sici)1097-4598(199708)20:8<1029::aid-mus14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23(4):355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 11.Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photo-chem Photobiol Sci. 2004;3(2):211–216. doi: 10.1039/b306126d. [DOI] [PubMed] [Google Scholar]
- 12.Lane N. Cell biology: Power games. Nature. 2006;443 (7114):901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 13.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem. 2005;280 (6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 14.Pastore D, Greco M, Petragallo VA, Passarella S. Increase in < –H+/e− ratio of the cytochrome c oxidase reaction in mitochondria irradiated with helium-neon laser. Biochem Mol Biol Int. 1994;34(4):817–826. [PubMed] [Google Scholar]
- 15.Karu T, Pyatibrat L, Kalendo G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B. 1995;27(3):219–223. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Song S, Fong CC, Tsang CH, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol. 2003;120(5):849–857. doi: 10.1046/j.1523-1747.2003.12133.x. [DOI] [PubMed] [Google Scholar]
- 17.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. Low-level laser therapy for wound healing: Mechanism and efficacy. Dermatol Surg. 2005;31(3):334–340. doi: 10.1111/j.1524-4725.2005.31086. [DOI] [PubMed] [Google Scholar]
- 18.Skinner SM, Gage JP, Wilce PA, Shaw RM. A preliminary study of the effects of laser radiation on collagen metabolism in cell culture. Aust Dent J. 1996;41(3):188–192. doi: 10.1111/j.1834-7819.1996.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 19.Kana JS, Hutschenreiter G, Haina D, Waidelich W. Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg. 1981;116(3):293–296. doi: 10.1001/archsurg.1981.01380150021005. [DOI] [PubMed] [Google Scholar]
- 20.Lyons RF, Abergel RP, White RA, Dwyer RM, Castel JC, Uitto J. Biostimulation of wound healing in vivo by a helium-neon laser. Ann Plast Surg. 1987;18(1):47–50. doi: 10.1097/00000637-198701000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Gu W, Masinde G, Hamilton-Ulland M, Xu S, Mohan S, Baylink DJ. Genetic control of the rate of wound healing in mice. Heredity. 2001;86(Pt 6):668–674. doi: 10.1046/j.1365-2540.2001.00879.x. [DOI] [PubMed] [Google Scholar]
- 22.Masinde GL, Li R, Nguyen B, Yu H, Srivastava AK, Edderkaoui B, Wergedal JE, Baylink DJ, Mohan S. New quantitative trait loci that regulate wound healing in an intercross progeny from DBA/1J and 129×1/SvJ inbred strains of mice. Funct Integr Genomics. 2006;6(2):157–163. doi: 10.1007/s10142-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 23.Flemming KA, Cullum NA, Nelson EA. A systematic review of laser therapy for venous leg ulcers. J Wound Care. 1999;8(3):111–114. doi: 10.12968/jowc.1999.8.3.25848. [DOI] [PubMed] [Google Scholar]
- 24.Pontinen PJ, Aaltokallio T, Kolari PJ. Comparative effects of exposure to different light sources (He-Ne laser, InGaAl diode laser, a specific type of noncoherent LED) on skin blood flow for the head. Acupunct Electrother Res. 1996;21(2):105–118. [PubMed] [Google Scholar]
- 25.Kopera D, Kokol R, Berger C, Haas J. Low level laser: Does it influence wound healing in venous leg ulcers? A randomized, placebo-controlled, double-blind study. Br J Dermatol. 2005;152(6):1368–1370. doi: 10.1111/j.1365-2133.2005.06586.x. [DOI] [PubMed] [Google Scholar]
- 26.Byrnes KR, Barna L, Chenault VM, Waynant RW, Ilev IK, Longo L, Miracco C, Johnson B, Anders JJ. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed Laser Surg. 2004;22(4):281–290. doi: 10.1089/pho.2004.22.281. [DOI] [PubMed] [Google Scholar]
- 27.Lowe AS, Walker MD, O’Byrne M, Baxter GD, Hirst DG. Effect of low intensity monochromatic light therapy (890 nm) on a radiation-impaired, wound-healing model in murine skin. Lasers Surg Med. 1998;23(5):291–298. doi: 10.1002/(sici)1096-9101(1998)23:5<291::aid-lsm9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Pessoa ES, Melhado RM, Theodoro LH, Garcia VG. A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed Laser Surg. 2004;22(3):199–204. doi: 10.1089/1549541041438533. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro AL, Meireles GC, de Barros Vieira AL, Almeida D, Carvalho CM, dos Santos JN. Phototherapy improves healing of cutaneous wounds in nourished and undernourished Wistar rats. Braz Dent J. 2004;15(Spec No):SI21–SI28. [PubMed] [Google Scholar]
- 30.Karu TI. A suitable model for wound healing: How many times are we to stumble over the same block? Lasers Surg Med. 1999;25(4):283–284. doi: 10.1002/(sici)1096-9101(1999)25:4<283::aid-lsm1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Reid RR, Said HK, Mogford JE, Mustoe TA. The future of wound healing: Pursuing surgical models in transgenic and knockout mice. J Am Coll Surg. 2004;199(4):578–585. doi: 10.1016/j.jamcollsurg.2004.05.262. [DOI] [PubMed] [Google Scholar]
- 32.Pereira AN, Eduardo Cde P, Matson E, Marques MM. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med. 2002;31(4):263–267. doi: 10.1002/lsm.10107. [DOI] [PubMed] [Google Scholar]
- 33.Medrado AR, Pugliese LS, Reis SR, Andrade ZA. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med. 2003;32(3):239–244. doi: 10.1002/lsm.10126. [DOI] [PubMed] [Google Scholar]
- 34.Chiang B, Essick E, Ehringer W, Murphree S, Hauck MA, Li M, Chien S. Enhancing skin wound healing by direct delivery of intracellular adenosine triphosphate. Am J Surg. 2007;193(2):213–218. doi: 10.1016/j.amjsurg.2006.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreis TE, Birchmeier W. Stress fiber sarcomeres of fibroblasts are contractile. Cell. 1980;22(2 Pt 2):555–561. doi: 10.1016/0092-8674(80)90365-7. [DOI] [PubMed] [Google Scholar]
- 36.Ehrlich HP, Keefer KA, Myers RL, Passaniti A. Vanadate and the absence of myofibroblasts in wound contraction. Arch Surg. 1999;134(5):494–501. doi: 10.1001/archsurg.134.5.494. [DOI] [PubMed] [Google Scholar]
- 37.Kirchner LM, Meerbaum SO, Gruber BS, Knoll AK, Bulgrin J, Taylor RA, Schmidt SP. Effects of vascular endothelial growth factor on wound closure rates in the genetically diabetic mouse model. Wound Repair Regen. 2003;11(2):127–131. doi: 10.1046/j.1524-475x.2003.11208.x. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Mohan S, Gu W, Baylink DJ. Analysis of gene expression in the wound repair/regeneration process. Mamm Genome. 2001;12(1):52–59. doi: 10.1007/s003350010230. [DOI] [PubMed] [Google Scholar]
- 39.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 40.Pourreau-Schneider N, Ahmed A, Soudry M, Jacquemier J, Kopp F, Franquin JC, Martin PM. Helium-neon laser treatment transforms fibroblasts into myofibroblasts. Am J Pathol. 1990;137(1):171–178. [PMC free article] [PubMed] [Google Scholar]
- 41.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 42.Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Wound Repair Regen. 2007. Activation of latent TGF-β1 by low power laser in vitro correlates with increased TGF-β1 levels in laser enhanced oral wound healing. in press. [DOI] [PubMed] [Google Scholar]
- 43.Tamm ER, Siegner A, Baur A, Lutjen-Drecoll E. Transforming growth factor-beta 1 induces alpha-smooth muscle-actin expression in cultured human and monkey trabecular meshwork. Exp Eye Res. 1996;62(4):389–397. doi: 10.1006/exer.1996.0044. [DOI] [PubMed] [Google Scholar]
- 44.Hawkins D, Abrahamse H. Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg. 2006;24(6):705–714. doi: 10.1089/pho.2006.24.705. [DOI] [PubMed] [Google Scholar]