Abstract
Earlier extracellular recordings during natural sleep have shown that, during slow-wave sleep (SWS), neocortical neurons display long-lasting periods of silence, whereas they are tonically active and discharge at higher rates during waking and sleep with rapid eye movements (REMs). We analyzed the nature of long-lasting periods of neuronal silence in SWS and the changes in firing rates related to ocular movements during REM sleep and waking using intracellular recordings from electrophysiologically identified neocortical neurons in nonanesthetized and nonparalyzed cats. We found that the silent periods during SWS are associated with neuronal hyperpolarizations, which are due to a mixture of K+ currents and disfacilitation processes. Conventional fast-spiking neurons (presumably local inhibitory interneurons) increased their firing rates during REMs and eye movements in waking. During REMs, the firing rates of regular-spiking neurons from associative areas decreased and intracellular traces revealed numerous, short-lasting, low-amplitude inhibitory postsynaptic potentials (IPSPs), that were reversed after intracellular chloride infusion. In awake cats, regular-spiking neurons could either increase or decrease their firing rates during eye movements. The short-lasting IPSPs associated with eye movements were still present in waking; they preceded the spikes and affected their timing. We propose that there are two different forms of firing rate control: disfacilitation induces long-lasting periods of silence that occur spontaneously during SWS, whereas active inhibition, consisting of low-amplitude, short-lasting IPSPs, is prevalent during REMs and precisely controls the timing of action potentials in waking.
Inhibition is one of most powerful means of the nervous system to control the level of excitation in a population of active neurons (1, 2). The conventional point of view suggests that inhibition arises through the activation of different types of inhibitory interneurons (3, 4), followed by generation of inhibitory postsynaptic potentials (IPSPs) in target neurons (5). The firing of inhibitory interneurons activates biphasic γ-aminobutyric acid type A (GABAA)-GABAB IPSPs in postsynaptic cells, which prevent target neurons from firing (4, 6, 7). Less attention is commonly paid to disfacilitation, a form of inhibition during which neurons are hyperpolarized due to the temporal absence of excitatory synaptic activity (8–11). Here we provide direct evidence for the presence of two forms of inhibition in neocortical neurons, using intracellular recordings during the natural waking-sleep cycle of cats with chronically implanted electrodes. Periods of disfacilitation appeared as long-lasting hyperpolarizing potentials and were prominent during slow-wave sleep (SWS). On the other hand, active inhibition consisted of short-lasting, low-amplitude IPSPs, which were particularly numerous during eye movements. In rapid eye movement (REM) sleep, these IPSPs significantly suppressed neuronal firing, whereas in awake animals they precisely controlled the timing of spikes.
Methods
Preparation.
Preparation of chronic experiments was conducted under somnotol (35 mg/kg) anesthesia. The anesthesia was followed by i.m. injection of buprenorphine (0.03 mg/kg), every 12 h for 24 h, to prevent pain after surgery. Surgical procedures were performed in sterile conditions. In addition, cats were injected with 500,000 units of penicillin i.m. for 3 consecutive days. Under anesthesia, cats were implanted with 1–3 chambers, designed for intracellular recordings in chronic conditions, as well as with multiunit, field potential and stimulating electrodes. The chambers and electrodes were placed over various neocortical areas. Although all neurons illustrated in the figures of the present paper were recorded from suprasylvian areas 5 and 7, similar data were collected from other (sensory and motor) cortical fields. Stimulating and/or recording electrodes were placed in the cortex contralateral to the chamber and 1–3 electrodes were inserted around the chambers. Stimulating and recording electrodes were also placed in appropriate thalamic nuclei. In addition to the electroencephalogram (EEG) electrodes, pairs of electrodes were placed in ocular cavities and in neck muscles, to monitor the states of vigilance by recording the electro-oculogram and electro-myogram. A few bolts were cemented to the cranium to allow nonpainful fixation of the cat's head to a stereotaxic frame. After a recovery period (7–10 days), cats were adapted to be in the frame for 1–2 h. Usually, after 3–5 days of training, cats started to sleep in the frame and they displayed clearly identified states of waking, SWS, and REM sleep. At this time, intracellular recordings began. After focal lidocaine application, a small perforation was made in the dura to allow the insertion of micropipettes for intracellular recordings. After the pipette had been placed on the cortical surface, the chamber was filled with warm sterile 4% solution of agar. Usually, 2–3 recording sessions lasting for 1–2 h were performed daily in each animal, and 7–10 days of recording were performed from 1 chamber. The experimental procedure was approved by Comité pour la protection des animaux de l'Université Laval (Québec, Canada).
At the end of all experiments the cats were given a lethal dose of pentobarbitone.
Recording and Stimulation.
Field potential recordings and stimulation were obtained by using bipolar coaxial macroelectrodes inserted into the cortex and thalamus. The outer pole of the electrode was placed on the cortical surface or 0.1 mm deeper and the inner pole was placed at 0.8–1 mm of the cortical depth.
Intracellular recordings were obtained with sharp glass micropipettes. In the majority of cases, the pipettes were filled with a solution of 2.5–3 M K+ acetate (KAc). Several other recording solutions were used in some experiments. Cs+ acetate (2 M) was used to eliminate most of K+ currents and KCl 2–2.5 M to reverse Cl−-dependent IPSPs. The dc resistance of intracellular pipettes was 30–80 MΩ. A high-impedance amplifier (bandpass, 10 kHz) with an active bridge circuitry was used to record and inject currents into the cells. The signals were recorded on a tape with bandpass of at least 0–9 kHz and digitized at 10–20 kHz for off-line computer analysis. The indicated values of membrane potential were obtained from peaks of histogram of membrane potential distribution.
Results
To identify inhibitory potentials, we recorded intracellular activities from cortical neurons in nonanesthetized, nonparalyzed cats during the three major states of vigilance: wakefulness, SWS, and REM sleep. We recorded the activities of >700 neurons; of them, 34 neurons were recorded in three states of vigilance and 150 neurons in only two behavioral states of vigilance. Most neurons were electrophysiologically identified as regular-spiking (RS), fast-spiking (FS), intrinsically bursting, and fast-rhythmic-bursting (12–14), located in superficial and deep layers of sensory-motor, secondary visual, and associative areas. The states of vigilance were routinely characterized by simultaneous recordings of EEG, electro-myogram, and electro-oculogram (15). SWS was identified by high-amplitude, low-frequency cortical field potentials, and presence of muscle tone (Fig. 1). Intracellular recordings indicated that, during natural SWS, intracellular activities were highly synchronized with adjacent, but also remote, field potentials (Figs. 1 and 2). This finding is at variance with recent data on anesthetized cats (16). Similar to previous data from anesthetized animals (17), the depth-positive component of cortical EEG was associated in all recorded cortical neurons (n > 500) with cell hyperpolarization to −71.7 ± 0.7 mV (mean ± SE) and arrest of firing (arrowheads in Fig. 1 Left), whereas the depth-negative wave EEG was accompanied by cell depolarization (−62.1 ± 0.5 mV) and spiking (Fig. 1). REM sleep was associated with EEG activation, ocular saccades, and muscular atonia. Intracellular recordings showed that, during REM sleep, the membrane potential was quite stable with a mode at −60.8 ± 0.7 mV. During the ocular saccades of REM sleep, the RS neurons decreased their firing rates (Fig. 1 Center). The waking state (Fig. 1 Right) was characterized by an activated EEG, muscle tone, occasional eye movements, and stable membrane potential (−62.3 ± 0.6 mV).
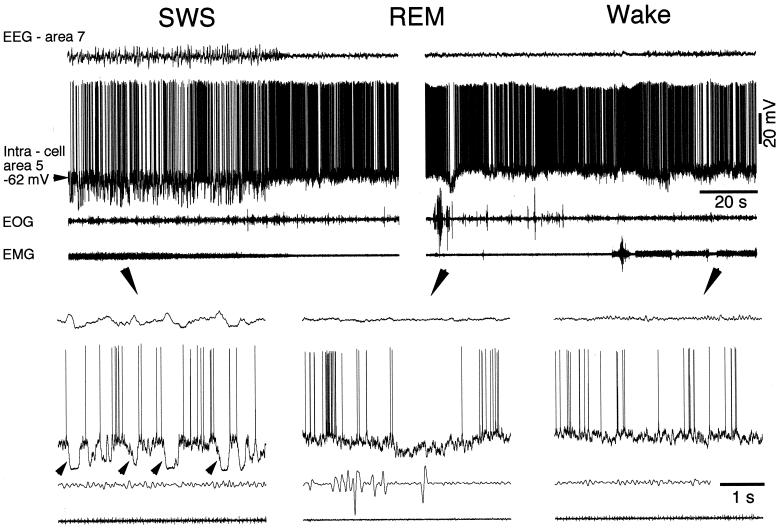
Figure 1.
Cortical intracellular correlates of natural SWS, REM sleep, and waking states. The four traces depict (from top to bottom): EEG from area 7, intracellular activity of area 5 RS neuron (membrane potential is indicated, −62 mV), electro-oculogram, and electro-myogram. High-amplitude and low-frequency field potentials, intracellular cyclic hyperpolarizing potentials, and stable muscle tone are distinctive features of SWS. Low-amplitude and high-frequency field potential oscillations, tonic neuronal firing with little fluctuations in the membrane potential, REMs, and muscle atonia are cardinal features of REM sleep. Low-amplitude and high-frequency field potential oscillations, tonic firing with little fluctuations in the membrane potential, and muscle tone with periodic contractions are characteristics of the waking state. Parts indicated by arrows are expanded below (arrows). Note cyclic hyperpolarizations in SWS and diminished firing rate during ocular saccade in REM sleep.
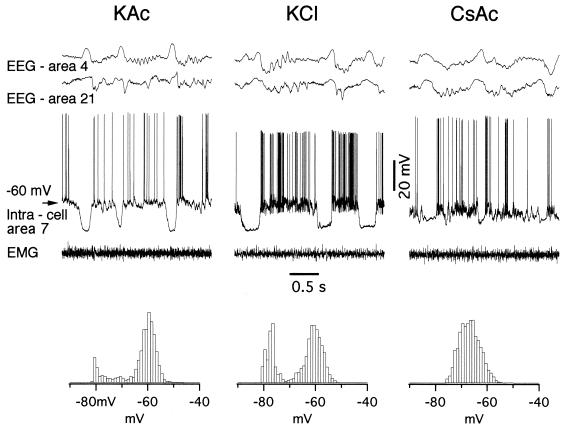
Figure 2.
Ionic nature of long-lasting hyperpolarizations during SWS. Periods of field potential and intracellular activities in three different cortical neurons are shown during SWS. In each case, intracellular activities of area 7 RS neurons are shown together with EEG from areas 4 and 21, and with electro-myogram. (Left) Control recordings with pipette filled with KAc. (Center) Pipette filled with KCl. (Right) Pipette filled with CsAc. Note that long-lasting hyperpolarizations are abolished by Cs+, a nonspecific blocker of K+ currents. Histograms represent membrane potential distribution. Note bimodal histograms in recordings with KAc and KCl, and unimodal histogram in recording with CsAc.
We investigated the nature of periods characterized by arrest of firing during SWS and REM sleep and we found two different underlying mechanisms. The first question we asked was: Are the long-lasting hyperpolarizations during SWS due to IPSPs or are they periods of disfacilitation? To answer this question, we compared simultaneously recorded EEG and intracellular activities from RS neurons to those recorded from FS cells. The conventional FS cell type is the electrophysiological signature of inhibitory interneurons (12, 18). In the present experiments, FS neurons fired with brief action potentials (half-width 0.27 ± 0.02), revealed monophasic afterhyperpolarizations and did not display spike-frequency adaptation. All of the FS neurons (n = 175) showed the same relationship to EEG as RS cells and other types of neurons, namely, they displayed long-lasting hyperpolarizing potentials during the depth-positive EEG waves and were depolarized during the depth-negative EEG waves. Also, FS neurons never showed an increased firing before or during the EEG depth positivity. This result suggests that cortical active inhibition does not account for the neuronal hyperpolarizations during SWS (see Discussion). To support the idea that the long-lasting hyperpolarizations during SWS are not IPSPs, we recorded with intracellular pipettes filled with KCl, which results in changes of Cl− reversal potential of GABAA IPSPs and in depolarizing potentials after activation of GABAA receptors. In all neurons (n = 57) recorded with KCl-filled pipettes, the long-lasting hyperpolarizations were virtually not affected by Cl− (Fig. 2 Center), thus indicating that these hyperpolarizations are not Cl−-dependent (GABAA-mediated) IPSPs. On the other hand, disfacilitation develops when synaptic currents are largely absent and the remaining “leak” currents dominate the membrane potential. The major part of the total leak current is maintained by K+. We hypothesized that the long-lasting SWS hyperpolarizations are due to the activity of K+ channels and therefore performed recordings with pipettes filled with Cs+ (n = 37), a nonspecific intracellular blocker of K+ currents (19). This resulted in reduction up to disappearance of SWS long-lasting hyperpolarizations (Fig. 2 Right). With Cs+-filled recording pipettes, the difference between the depolarizing and hyperpolarizing phases of the slow sleep oscillation was significantly decreased (P < 0.05, Student-Newman-Keuls test), compared to both KAc- and KCl-recording pipettes. A histogram of membrane potential distribution showed unimodal distribution (Fig. 2 Right). This finding indicates that K+ currents dominate during SWS long-lasting hyperpolarizations.
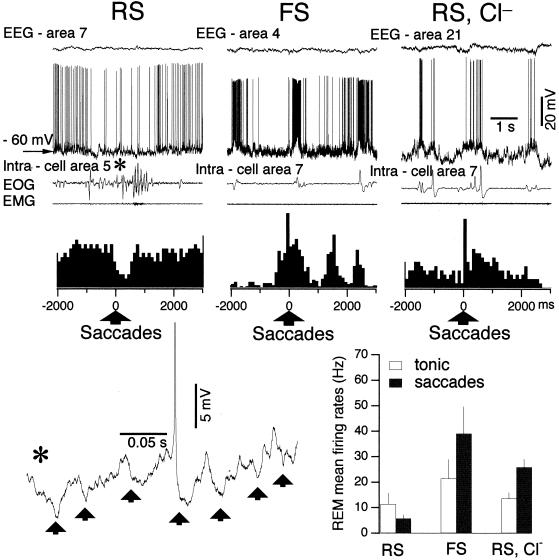
During the ocular saccades of REM sleep the arrest in firing of RS neurons was due to another mechanism. First, it occurred at only slightly hyperpolarized potentials (Fig. 1 Center) or at the same membrane potentials (Fig. 3 Left) as adjacent periods of neuronal activity. Second, detailed examination of intracellular traces showed the presence of multiple, low-amplitude (1–3 mV) and short (5–50 ms) hyperpolarizing potentials (Fig. 3, expanded at *). To some extent the amplitude and time course of these potentials resemble the single-axon IPSPs described in vitro (2, 4, 5, 20, 21). This led us to suppose that the decreased firing during ocular saccades was due to barrages of IPSPs imposed on pyramidal neurons by inhibitory interneurons. To test this hypothesis, we recorded from FS neurons (Fig. 3 Center). The mean firing rate of FS neurons during REM was significantly (P < 0.05) greater than the mean firing rate of RS neurons (Fig. 3, histogram). All FS neurons recorded in associative and visual areas during REM sleep (n = 14) displayed a statistically significant increase (32%) in firing rate (P < 0.01, paired comparison, t test) in association with ocular saccades (Fig. 3 Center and histogram), similarly to previous experiments using extracellular recordings of cortical putative interneurons during the natural sleep cycle (22). This result indicates a possible role of IPSPs in the decreased firing of RS neurons during REMs. To test this hypothesis, we recorded from RS neurons during REM sleep with pipettes filled with KCl (n = 7). In all these recordings, the firing rate of RS neurons increased almost twice during ocular saccades (P < 0.01, paired comparison, t test) (Fig. 3 Right and histogram), strongly suggesting the presence of Cl−-dependent IPSPs in RS neuron.
Figure 3.
Decreased firing in RS neurons during ocular saccades is associated with Cl-dependent IPSPs. (Left) RS neuron fired tonically and irregularly throughout REM. During ocular saccades the firing rate decreased. Below, peri-eye movement histogram of neuronal firing. An ocular saccade indicated by * in the upper trace is expanded at bottom. (Center) Ocular saccades during REM are associated with increased firing rate in conventional FS cortical neuron. (Right) Intracellular Cl− infusion results in depolarization of RS neuron during ocular saccades in REM sleep. Graph shows comparison in firing rates of RS neurons during tonic phases vs. ocular saccades in REM sleep (Left), FS neurons recorded with KAc-filled pipette (Center), and RS neurons recorded with KCl-filled pipette (Right).
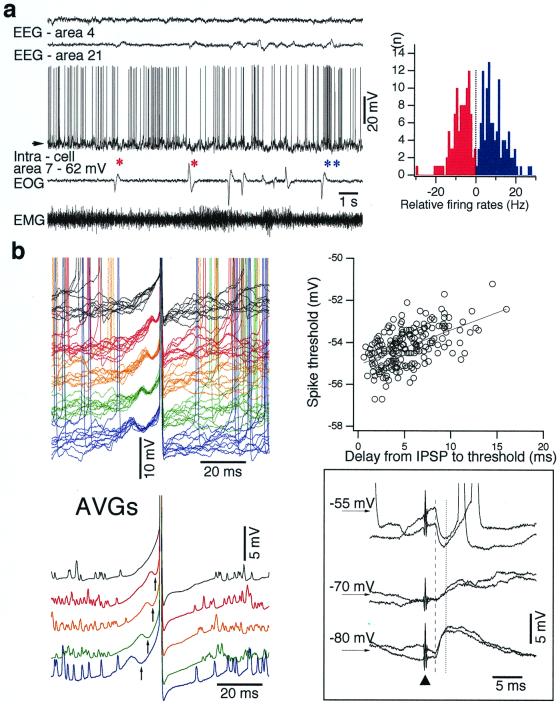
Similarly to REM sleep, the spontaneous long-lasting hyperpolarizing potentials were absent during quiet wakefulness. In the waking state, the same RS neuron could show increased or decreased firing accompanying eye movements (Fig. 4 a compare * and **). The histogram of firing rates related to eye movements (>200 individual saccades), relative to the mean firing rates of 15 RS neurons (Fig. 4a Right), showed a similar proportion of increase vs. decrease in spike frequency. Despite the fact that the firing of RS neurons could occur during eye movements in the waking state, short-lasting IPSPs associated with eye movements were still present in intracellular traces and were mainly preceding spikes. Fig. 4b Left depicts 50 individual traces (upper part) and their averages (lower part) selected by the relative timing of the spike. The preceding hyperpolarizing deflections were coded by different colors. During waking, the majority of spikes recorded in relation to eye movements (72.3% of 1,294 spikes) were preceded by such fast IPSPs, whereas outside eye movements only less than one-half (47.8% of 3,578 spikes) were associated with visible hyperpolarizing prepotentials. Fast hyperpolarizing prepotentials slightly delayed spikes and influenced the firing threshold. The shortest (2–5 ms) hyperpolarizations decreased the firing threshold by a few millivolts as compared to longer (10–20 ms) hyperpolarizations (Fig. 4b Right). Thus, spontaneous, short-lasting IPSPs set up precise timing for spikes to occur by overpowering the depolarization and by influencing the firing threshold (see Discussion). We suggest that these short-lasting spike-related hyperpolarizations are Cl−-dependent IPSPs. This suggestion is based on four facts. First, application of slightly subthreshold electrical shocks to corresponding thalamic nuclei (Fig. 4 Inset) or to the contralateral cortex induced short-lasting IPSPs with reversal potential between −65 mV and −72 mV (n = 43). Second, we did not find such hyperpolarizing potentials in recordings with pipettes filled with KCl (not shown). Third, recordings from neurons hyperpolarized below −70 mV showed tonic depolarizing potentials associated with eye movements. Fourth, similarly to REM sleep, FS neurons increased their firing rates during ocular saccades in waking state. The different actions of IPSPs in REM sleep and wakefulness may be related to a higher input resistance of neocortical neurons in awake animals compared to other states (23), which creates conditions in which shunting inhibition (24) is expressed differently in different states of vigilance.
Figure 4.
Spontaneous IPSPs control precise firing timing of neocortical neurons. (a) Epoch during the waking state is characterized by activated EEG, unimodal intracellular membrane potential, eye movements, and muscle tone. * indicate decrease in firing associated with eye movements and ** indicate increase in firing associated with eye movements. Histogram shows increase (blue) or decrease (red) saccade-related firing (±500 ms around saccade) for 15 neurons (>200 individual saccades), in relation to mean firing rates of the same neurons. To calculate abscissa the firing rate during ocular saccades was subtracted from mean firing rate. (b) More than one-half of the spikes during waking were associated with preceding short-lasting hyperpolarizing potentials (see superimposition of individual traces and below their averages). Longer-lasting hyperpolarizations slightly decrease firing threshold (plot). (Inset) Another neuron from motor area 4 recorded in waking, where short-lasting IPSPs were activated by stimulation of ventrolateral thalamic nucleus.
Discussion
The above results indicate that neuronal silence in naturally sleeping animals may be due to two dominating factors. (i) Long-lasting hyperpolarizing potentials, which occur spontaneously during SWS, are due to a dramatic decrease of synaptic activity (10, 25, 26). Their possible role is the reshaping of synaptic efficacy (1). The fact that conventional FS neurons never discharged during the prolonged hyperpolarizations in SWS is congruent to previous data recorded in anesthetized animals, in which FS neurons were formally identified as aspiny basket cells (27). Also, similarly to previous reports on anesthetized animals (10, 28), the apparent input resistance of neurons during the hyperpolarizing potentials in natural SWS was at least twice as high as the input resistance of neurons measured during the depolarizing phases of the SWS slow oscillation (23, 29). This rules out the possibility that GABAergic cortical inhibition operates during these prolonged hyperpolarizing potentials and, instead, suggests that the long-lasting hyperpolarizing potentials in SWS are periods of disfacilitation. Disfacilitation may be due to the relatively strong depression of intracortical and thalamocortical synapses under the condition of low cholinergic activity (29). Besides, disfacilitation may originate from the hyperpolarization of thalamocortical neurons during SWS (30), thus preventing thalamocortical neurons from firing and transmitting signals toward the cerebral cortex (11).
(ii) Spontaneous IPSPs are usually short-lasting (2–50 ms), clustered during ocular movements, and they result in firing pause during REM sleep and in setting up precise firing time in the waking state. It is well known that spikes are elicited by excitatory postsynaptic potentials with inverse relations between spike threshold and the rate of membrane depolarization preceding spikes. Each pyramidal cell receives 5,000 to 60,000 synapses (5, 31–33), and the effects of dendritic and somatic excitatory postsynaptic potentials are similarly powerful (34). The firing rate of cortical pyramidal neurons in awake animals range between 5 and 20 Hz (22, 35–37). These values indicate that each pyramidal cell receive 100–1,000 excitatory postsynaptic potentials in a time window as short as 1 ms, assuming no failure of synaptic transmission. The amplitude of the single-axon excitatory postsynaptic potentials is variable, in a range from 0.1 to 9 mV with a total mean ≈1 mV (38–42). In addition, our recent data suggest that a persistent sodium current (43–45) takes part in the maintenance of the depolarizing states of the membrane potential that are found during waking, REM and depolarizing phases of SWS (46). This indicates that very strong synaptic and intrinsic depolarizing pressure is experienced by neocortical neurons, which would result in extremely high firing rates. In neurons of visual cortex, the net synaptic depolarizing current increases during presentation of visual stimuli and reaches values close to 1 nA (47). In these conditions short-lasting IPSPs reported in the present paper would be in a perfect position to set up the timing of firing of cortical neurons. Slight hyperpolarization and increased conductance would prevent the neurons from firing and faster deinactivation of some Na+ channels, which would increase the firing probability at the end of IPSPs, because both persistent and transient Na+ currents are generated by the electrophysiologically uniform population of Na+ channels (48).
Our data support earlier (49, 50) ideas that the main physiological difference of REM and waking is ascribed to cortical inhibition. The spontaneous barrages of IPSPs during REM sleep are intimately grouped around ocular saccades to prevent firing of RS neurons, while they act selectively in waking state. The different functional role of IPSPs in waking and REM sleep could be attributed to dissimilar functioning of electrotonic vs. chemical connectivity (51, 52) in various states of vigilance or to different activation of inhibitory interneurons by muscarinic and nicotinic cholinoreceptors (53) in the two states. Because eye movements are implicated in cognitive guidance on where to look and when to look (54), our data suggest that different effects of active cortical inhibition may be a significant factor responsible for differences between the mental processing in REM sleep and wakefulness.
Acknowledgments
We thank P. Giguère and D. Drolet for excellent technical assistance. This work was supported by a grant from the Medical Research Council of Canada (to I.T.) and by grants from the Medical Research Council of Canada, Natural Science and Engineering Research Council of Canada, and Human Frontier Science Program (to M.S.). I.T. is a Scholar of the Fonds de recherche en santé du Québec. F.G. is a Ph.D. student and was partially supported by the Savoy Foundation.
Abbreviations
- SWS
slow-wave sleep
- REM
rapid eye movement
- RS
regular spiking
- FS
fast spiking
- IPSP
inhibitory postsynaptic potential
- EEG
electroencephalogram
- KAc
K+ acetate
- GABAA
γ-aminobutyric acid type A
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041430398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041430398
References
- 1.Galarreta M, Hestrin S. Nat Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Wang Y, Markram H. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi Y. J Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson A M, West D C, Hahn J, Deuchars J. J Physiol (London) 1996;496:81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somogyi P, Tamás G, Lujan R, Buhl E H. Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 6.Avoli M. Brain Res. 1986;370:165–170. doi: 10.1016/0006-8993(86)91118-2. [DOI] [PubMed] [Google Scholar]
- 7.Connors B W, Malenka R C, Silva L R. J Physiol (London) 1988;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llinás R R. J Neurophysiol. 1964;27:1117–1126. doi: 10.1152/jn.1964.27.6.1117. [DOI] [PubMed] [Google Scholar]
- 9.Wilson C, Chang H T, Kitai S T. Exp Brain Res. 1983;51:227–235. doi: 10.1007/BF00237198. [DOI] [PubMed] [Google Scholar]
- 10.Contreras D, Timofeev I, Steriade M. J Physiol (London) 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timofeev I, Contreras D, Steriade M. J Physiol (London) 1996;494:265–278. doi: 10.1113/jphysiol.1996.sp021489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connors B W, Gutnick M J. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- 13.Gray C M, McCormick D A. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- 14.Steriade M, Timofeev I, Dürmüller N, Grenier F. J Neurophysiol. 1998;79:483–490. doi: 10.1152/jn.1998.79.1.483. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M, McCarley R W. Brainstem Control of Wakefulness and Sleep. New York: Plenum; 1990. [Google Scholar]
- 16.Lampl I, Reichova I, Ferster D. Neuron. 1999;22:361–374. doi: 10.1016/s0896-6273(00)81096-x. [DOI] [PubMed] [Google Scholar]
- 17.Steriade M, Nuñez A, Amzica F. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azouz R, Gray C M, Nowak L G, McCormick D A. Cereb Cortex. 1997;7:534–545. doi: 10.1093/cercor/7.6.534. [DOI] [PubMed] [Google Scholar]
- 19.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 20.Tamás G, Buhl E H, Somogyi P. J Physiol (London) 1997;500:715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamás G, Somogyi P, Buhl E H. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steriade M. Behav Brain Sci. 1978;3:465–514. [Google Scholar]
- 23.Steriade, M., Timofeev, I. & Grenier, F. (2001) J. Neurophysiol., in press. [DOI] [PubMed]
- 24.Borg-Graham L J, Monier C, Frégnac Y. Nature (London) 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- 25.Paré D, Shink E, Gaudreau H, Destexhe A, Lang E J. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- 26.Destexhe A, Paré D. J Neurophysiol. 1999;81:1531–1547. doi: 10.1152/jn.1999.81.4.1531. [DOI] [PubMed] [Google Scholar]
- 27.Contreras D, Steriade M. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson C J, Kawaguchi Y. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gil Z, Connors B W, Amitai Y. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch J C, Fourment A, Marc M E. Brain Res. 1983;259:308–312. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- 31.Cragg B G. J Anat. 1967;101:639–654. [PMC free article] [PubMed] [Google Scholar]
- 32.DeFelipe J, Farinas I. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- 33.Mountcastle V B. Perceptual Neuroscience: The Cerebral Cortex. Cambridge, MA: Harvard Univ. Press; 1998. [Google Scholar]
- 34.Magee J C, Cook E P. Nat Neurosci. 2000;3:895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- 35.Hubel D H. J Physiol (London) 1959;147:226–238. doi: 10.1113/jphysiol.1959.sp006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evarts E V. J Neurophysiol. 1964;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- 37.Steriade M, Deschênes M, Oakson G. J Neurophysiol. 1974;37:1065–1092. doi: 10.1152/jn.1974.37.5.1065. [DOI] [PubMed] [Google Scholar]
- 38.Thomson A M, West D C, Deuchars J. Neuroscience. 1995;69:727–738. doi: 10.1016/0306-4522(95)00287-s. [DOI] [PubMed] [Google Scholar]
- 39.Stratford K J, Tarczy-Hornoch K, Martin K A, Bannister N J, Jack J J. Nature (London) 1996;382:258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- 40.Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. J Physiol (London) 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buhl E H, Tamãs G, Szilãgyi T, Stricker C, Paulsen O, Somogyi P. J Physiol (London) 1997;500:689–713. doi: 10.1113/jphysiol.1997.sp022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timofeev I, Grenier F, Bazhenov M, Sejnowski T J, Steriade M. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 43.Stafstrom C E, Schwindt P C, Crill W E. Brain Res. 1982;236:221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- 44.Llinás R R. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 45.Crill W E. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- 46.Timofeev I, Grenier F, Steriade M. J Physiol (Paris) 2000;94:343–355. doi: 10.1016/s0928-4257(00)01097-4. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed B, Anderson J C, Douglas R J, Martin K A, Whitteridge D. Cereb Cortex. 1998;8:462–476. doi: 10.1093/cercor/8.5.462. [DOI] [PubMed] [Google Scholar]
- 48.Alzheimer C, Schwindt P C, Crill W E. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steriade M, Kitsikis A, Oakson G. Sleep. 1979;1:339–355. doi: 10.1093/sleep/1.4.339. [DOI] [PubMed] [Google Scholar]
- 50.Llinás R R, Paré D. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 51.Galarreta M, Hestrin S. Nature (London) 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 52.Gibson J R, Beierlein M, Connors B W. Nature (London) 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 53.Xiang Z, Huguenard J R, Prince D A. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- 54.Schall J D, Thompson K G. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]