Abstract
Objective:
The diagnosis of tuberculosis (TB) ascites is problematic. Delay in the diagnosis and treatment of TB ascites are considered to be major factors that contribute to the high mortality of TB. This study identifies specific protein markers in ascitic fluid which will be useful in diagnosis of TB ascites.
Methods:
We used Two-Dimensional Electrophoresis, liquid chromatography-mass spectrometry/mass spectrometry, immunoblot analysis and Enzyme Linked Immunosorbent assay (ELISA) as a comprehensive quantitative proteomic screening system for the diagnosis of TB ascites.
Results:
The screen identified several antigens of interest: a 30-kilodalton (kDa) protein that demonstrated significant homology to the antigen 85B and 85C (Ag 85) complex; a 65-kDa protein that corresponded to Mycobacterium tuberculosis (MTB) heat shock protein 65 (65-kDa HSP), Rv0440; a 14-kDa protein and 71-kDa protein that exhibits an amino acid sequence identical to that of MTB heat shock protein 14 (14-kDa HSP), GroES; and MTB heat shock protein 71 (71-kDa HSP), Rv0350 respectively. ELISA confirmed that TB ascites patients were consistently positive for these antigens at higher rates than non-TB ascites patients.
Conclusion:
The 65-kDa HSP, 71-kDa HSP, 14-kDa HSP and Ag 85 complex proteins may serve as very useful diagnostic markers for TB ascites.
Keywords: TB ascites, heat shock proteins, M. tuberculosis antigens
Background
Tuberculosis (TB) is a serious infectious disease. India has far more TB cases than any other country, which is a significant problem on its own. Along with the increased incidence of TB, however, the incidence of extra-pulmonary TB [EPTB] has also recently increased.1,2 TB ascites is one of the clinical signs of abdominal TB. The clinical presentation of TB ascites is problematic, since it is non-specific and can mimic the symptoms of many other infectious diseases. As a result, diagnosis is often delayed.3 These delays in the diagnosis and treatment of TB ascites are considered to be major factors that contribute to the high mortality of TB.4 In most cases, diagnosis relies on clinical observations, imaging of the infected area and detection of Mycobacterium tuberculosis (MTB) in ascitic fluid by either acid-fast bacillus (AFB) staining or culturing. The sensitivity of the Ziehl-Neelsen staining test for direct AFB detection is quite low, and, thus, AFB culture takes a very long time to complete.5
Over the past few decades, analyses of TB biomarkers have attracted attention with respect to a variety of extra-pulmonary disorders.6,7 With the increased interest in and improved technical capabilities of clinical proteomics, comparative investigations with respect to differential protein expression has become more common than ever before for the diagnostic and prognostic assessment of disease states.8 In the present study, we used Two-Dimensional Polyacrylamide Gel Electrophoresis (2 DPGE), liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) and immunoblot analysis in the present study as a comprehensive quantitative proteomic screening system for the diagnosis of TB ascites. The identification of new biomarkers will be useful for the development of sensitive and specific tests for the prediction and/or early diagnosis of TB ascites in patients.
Material and Methods
Patients and samples
We prospectively selected ascitic fluid samples from 20 patients (13 male, 7 female), ranging in age from 6 to 72 years of age, who were suspected to have TB ascites based upon clinical symptoms and/or operative findings from the inpatient and outpatient services at the Central India Institute of Medical Sciences in, Nagpur. In addition, 21 control individuals were selected from among patients who were admitted to the hospital for acute or chronic defined, non-TB ascites diseases, including inflammatory bowel disease, various infectious disorders, malignancy, gastrointestinal symptoms, abdominal tenderness accompanied by non-specific fever, pneumonia, bronchitis, lung cancer and lung infection. All subjects were negative for HIV and have received BCG vaccination. For the collection of ascitic fluid, the patient was allowed to lay on his/her back with head at 45°–90° elevation. The area where the needle was to be inserted was cleaned with iodine or similar solution and drapped. The anesthetic was administered to numb the area. The paracentesis needle was carefully inserted into the abdomen. About 1000 to 1500 ml of fluid was removed. For diagnosis 50 ml of the fluid was sent to the laboratory for the analysis of different parameters. Samples were obtained from all patients before the initiation of anti-Koch treatment (AKT) and were stored at −20 °C until they were ready for experimental analysis. Patient consent was obtained for all samples that were collected from all study groups for use in this study.
The diagnosis of TB ascites was accomplished through a combination of several methodologies. First, a sputum microscopic examination was performed of two serial sputum samples that had been stained with Ziehl-Neelsen Stain, according to the guidelines of India’s Revised National Tuberculosis Control Programme. Out of the twenty TB ascites patients, however only one was AFB-positive based upon the initial results for the Ziehl-Neelsen Stained sputum samples. In the absence a positive result with the Ziehl-Neelsen test, tissue biopsies were taken. TB infection was diagnosed by the presence of a typical granuloma formation, which is composed of epithelioid histiocytes and mononuclear inflammatory cells, in the absence of other diagnoses. If both tests were negative, the patients were diagnosed by clinical symptoms. A clinical diagnosis of TB ascites was confirmed after a successful empiric therapeutic trial.
Cases were followed at regular interval over a period of nine months. Improvement in all subjects was judged based upon clinical criteria, including improvements in abdominal pain, cough, fever, appetite and, weight gain, as well as radiographic evidence. The Ethical Committee of Central India Institute of Medical Sciences, Nagpur, approved the study. All analyses were performed double blinded.
One- and two-dimensional PAGE
For the first dimension, 125 μl (150 ug protein) of each sample from the TB ascites and non- TB ascites groups was applied to a Bio-Rad IPG strip (pH 3–10, 7 cm) and then it was subjected to isoelectric focusing (IEF). Briefly, the IPG strips were rehydrated overnight and IEF was then carried out at 20 °C in a Protean IEF unit (Bio-Rad, USA). Prior to second dimension electrophoresis, the IPG gel strips were immersed in equilibration solution for 15 min. The second dimension separations were carried out at 10 °C using SDS slab gels (10%) without stacking gels and a mini-protean tetra cell electrophoresis system (Bio-Rad). The IPG strips were embedded on the top of the gels with 0.5% agarose, and electrophoresis was performed at 30 mA/gel for 1 h. The gels were fixed with a methanol:acetic acid:water (5:1:5) solution, stained with Coomassie brilliant blue Stain and destained in a solution of 10% methanol and 7% acetic acid. Gel images were taken using the gel documentation system (Bio-Rad) and were imported into the PD Quest (Bio-Rad) 2D gel analysis software package. For detection of spots a master gel image was created by combining all of the spots that were present in both the TB ascites and non-TB ascites groups and was used to match each spot.
Antibodies
Antibodies against the purified MTB 85B and 85C (Ag85) complex, CS-90, the MTB heat shock protein 65, (65-kDa HSP), Rv0440, and, the MTB heat shock protein 71, (71-kDa HSP), Rv0350, were obtained from Colorado State University through the TB Research Materials and Vaccine Testing Contract (NO1-AI-75320). A polyclonal antibody (PAb) against the MTB heat shock protein 14, (14-kDa HSP), GroES, was raised in rabbits [1:4000] (Bangalore Genei, Bangalore), and the corresponding IgG was purified from whole serum using column chromatography.
Immunoblot analysis
After two-dimensional electrophoresis, the gel was transferred to a nitrocellulose membrane by electroblotting at 100 V for 3 h. The membrane was incubated with the primary antibodies (PAbs) of interest [14-kDa HSP (1:4000); Ag 85 complex (1:2000); 65-kDa HSP; 71-kDa HSP] for 2 h at room temperature, followed by incubation with the secondary antibody, which was goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP). Antibody reactivity was visualized by the detection of HRP activity with tetramethylbenzidinehydrogen peroxide (TMB/H2O2) as a substrate.
Indirect enzyme linked immunosorbent assay (ELISA) protocol
Indirect ELISA was performed as previously described by Kashyap et al.9 Briefly, 100 μl (1:400) ascitic fluid samples from TB ascites patients and the individuals in the non-TB ascites group were added to the micro-titer wells and blocked with 0.5% bovine serum albumin (BSA) in phosphate buffered saline (PBS). After washing with PBS, the respective antibodies (Ag 85 complex at 1:5000, 14-kDa HSP at 1:2000, 65-kDa HSP at 1:5000 and 71-kDa HSP at 1:3000) were added and the plates were incubated at 37 °C for 60 min. The wells were washed, then secondary antibody (goat anti-rabbit IgG-HRP, 1:10000) was added and the samples were incubated for 60 min at 37 °C. After another wash with PBS, 100 μl of TMB/H2O2 substrate was added to the wells and incubated at room temperature for about 10 min. The reaction was then stopped with 100 μl of 2.5 N H2SO4. The absorbance of each well was read at 450 nm. Each sample was tested in triplicate.
LC-MS analysis
An excised protein band was sent to Pro-Tech, Australia for LC-MS/MS analysis. Pro-Tech, Australia characterized this protein using the following protocol: (1) each gel piece was destained and washed prior to in-gel digestion, (2) protein bands was excised, digested and treated with trypsin after reduction and (3) alkylation agents were added prior to the analysis on the LC-MS/MS.
In-gel digestion was carried out in 50 mM NH4HCO4 buffer, pH 8.5 at 37 °C for approximately 4 hours. An equal volume of the digestion buffer was added, depending on the volume of the gel piece, and usually ranged from 20 to 50 μl. The amount of proteolytic enzyme (Promega trypsin, modified, sequencing grade) that was used depended on both the size of the gel piece and the estimated amount of protein within the gel band. Typically, 200 ng to 1 μg trypsin was used per gel band. Acetonitrile (ACN), in a volume equal to 3–5 times the volume of the digestion buffer, was then added to the digestion mix to extract the peptides. The samples, were then centrifuged at high speed for five minutes. The supernatant was transferred to a clean microfuge tube with a gel-loading pipette tip and dried in a SpeedVac on medium heat. For the reduction step, alkylation agents were added prior to analysis with LC-MS/MS. The dried sample was dissolved in 0.5% acetic acid (HOAC) for LC-MS/MS analysis. A Finnigan (ThermoFinnigan, San Jose, CA) LCQ ion trap MS in-line coupled with a high pressure liquid chromatography (HPLC) system was used for LC-MS/MS. A 75 μm (ID) × 10 cm length, 3 μm packing C18 capillary column, which was packed in-house, was connected to a specially designed nanoSpray device, which is capable of delivering a stable electrospray at flow rates of 100 nl/min to 1500 nl/min. The mobile phases included Solvent A (2% ACN, 97.5% H2O, 0.1% formic acid) and Solvent B (90% ACN, 9.5% H2O, 0.1% formic acid). For this analysis, the ion trap MS was set to operate in a data-dependent mode with the Automatic Gain Control (AGC) on. The MS/MS data were first evaluated against several internal quality control (QC) standards. After passing the QC standards, the MS/MS data was loaded into the proprietary ProtQuest search engine to search the most recent non-redundant protein database. The results from the ProtQuest search were then manually analyzed. The endoproteinase trypsin (sequencing grade) was obtained from Promega or Roche (Indianapolis, IN). The ammonia bicarbonate (analytical grade) and HOAC (>99.8% purity) were obtained from Sigma. ACN, methanol (MeOH), and water were each HPLC grade and obtained from Sigma (St. Louis, MO).
Statistical analysis
The sensitivities and specificities of the ELISA test for the diagnoses in the TB and Non-TB groups were calculated. The positive and negative predictive values were calculated using different rates of TB prevalence. The receiver operating curve (ROC) was used to calculate the cut-off value, and comparisons between TB ascites and non-TB ascites groups were performed with the chi-square test.
Results
The sample populations included 20 TB ascites patients and 21 non-TB ascites patients. There were 13 males and 7 females, with a mean age of 51.34 years (range 6 to 72 years) in TB ascites group. In the non-TB ascites control group, there were 11 males and 10 females, with a mean age of 50.34 years (range 8 to 70 years). None of the patients were positive for anti-HIV antibodies, and none were receiving immunosuppressive drugs. None of the patients (except one case) experienced alternate constipation, diarrhea, weight loss or a doughy feeling on palpation of the abdomen.
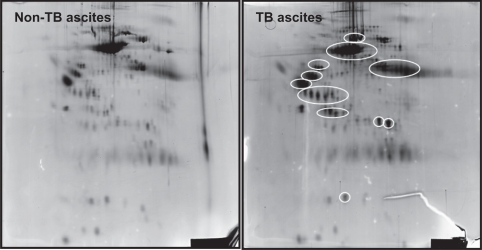
To generate a ascitic fluid protein signature that was associated with the diagnosis of TB ascites, we first randomly selected a set of ten samples (five TB ascites positive and five negative patients) for two dimensional electrophoresis. Detailed maps of the ascitic fluid proteins of TB ascites patients were generated by 2 DGPE, A total of 285 protein spots were observed. Proteins that were up-regulated in TB ascites patients, compared to their levels in non-TB ascites patients, are shown in Figure 1. Eight proteins that were highly expressed in the TB ascites cases were selected for further molecular characterization using LC-MS/MS. These eight proteins were also selected in order to identify ascitic fluid protein signature specific for TB ascites.
Figure 1.
Two dimensional gel electrophoresis of the ‘ascitic fluid’ collected from A) TB ascites (n = 5) and B) non- TB ascites (n = 5) patients. Proteins which are up regulated in the TB ascites patients are indicated with  .
.
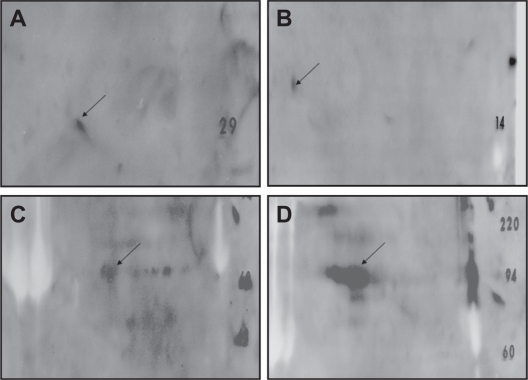
One of the eight proteins was a 30 kDa protein that demonstrated a significant homology to the Antigen 85 complex, another containing 65 and 71 kDa proteins that correspond to amino acid sequences of MTB 65-kDa HSP and 71-kDa HSP, respectively. Another was a 14-kDa protein that exhibited an amino acid sequence that was identical to that of the 14-kDa HSP (Table 1). Although amino acid sequences were also obtained for the four other selected proteins, none of these showed significant homology to the sequences of any known protein. These results were reconfirmed through immunoblot analysis with specific PAbs against the identified proteins (14, 30, 65, 71). When 2D PAGE protein profile was probed with the respective PAbs, the demonstrated reactivity was most strongly seen in the abdominal TB patients (Fig. 2).
Table 1.
MS identification of the protein(s) bands in TB ascites patients. The peptides were detected by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
| Protein | Peptide sequence | Amino acid positiona | % Totalb |
|---|---|---|---|
| 14 kDa | PEFSE | 16–20 | 3.47 |
| FDGR SEFAYGSFVR | 87–100 | 9.72 | |
| % coverage | 13.19 | ||
| Ag 85 complex | RRLMIGTA AA | 13–22 | 3.07 |
| PGLPVEY LQVP | 43–53 | 3.3 | |
| ELPQWLS ANRAVKPTGS AAIGLSM | 144–167 | 7.3 | |
| ALLDPSQGMG PSLIGLAMGD AGGYKA |
191–216 | 8 | |
| THSWEYWGAQ LNA | 301–313 | 4 | |
| % coverage | 25.67 | ||
| 65 kDa | EKIGA | 5–9 | 2.4 |
| VAAGA | 45–49 | 2.4 | |
| AKEVETKE | 73–80 | 3.9 | |
| ERQ | 145–148 | 1.9 | |
| STVKD | 165–170 | 2.4 | |
| IIAED | 186–190 | 2.4 | |
| % coverage | 15.4 | ||
| 70 kDa | RHMGSDWSIE IDGKKY | 71–86 | 2.56 |
| PYITV DADKNPLFLD | 256–270 | 2.4 | |
| RKRREEADVR NQAE | 501–604 | 2.24 | |
| % coverage | 7.2 |
Notes:
Amino acid position of the peptide in the mature protein;
Percentage the peptides contributes to the total mass of the mature protein.
Figure 2.
Immunoblot analysis of the identified proteins, demonstrating reactivity with the polyclonal antibodies against A) Ag 85 complex B) Mycobacterium tuberculosis heat shock protein 14 (14-kDa HSP) C) Mycobacterium tuberculosis heat shock protein 65 (65-kDa HSP) D) Mycobacterium tuberculosis heat shock protein 71 (71-kDa HSP).
After the identification of MTB proteins in the ascitic fluid of TB ascites patients using the combined system of 2D PAGE, LC-MS/MS and Immunoblot analysis. ELISA was performed to evaluate the protein levels in the ascitic fluid samples of suspected TB ascites patients by using antibodies against each of the identified proteins that were obtained from Colorado State University, USA.
The mean absorbance value of all the identified antigens (14-kDa HSP, Ag 85 complex, 65-kDa HSP, 71-kDa HSP) are shown in Tables 2–5. The antigen activity of all the antigens in the Tb ascites group is significantly higher than the non-TB ascites subjects.
Table 2.
Representation of Mycobacterium tuberculosis heat shock protein 14 (14-kDa HSP), in ascitic fluid from TB ascites and non-TB ascites patients using the antibodies generated against 14-kDa HSP by ELISA.
| Patients group | Positive for 14 kDa Hsp antigen (cut off 0.631) | Negative for 14 kDa Hsp antigen (cut off 0.631) | Absorbance (mean ± SD) | Range |
|---|---|---|---|---|
| TB ascites (n = 18) | 18 (100%) | 0 (00%) | 0.814 ± 0.134 | 0.637–1.029 |
| Non TB ascites (n = 21) | 5 (23.80%) | 16 (76.20%) | 0.571 ± 0.118 | 0.336–0.907 |
The mean 14-kDa HSP antigen value (with range) in the TB ascites group was significantly higher than that in the non-TB ascites group [p < 0.0001].
Table 5.
Representation of Mycobacterium tuberculosis heat shock protein 71 (71-kDa HSP), Rv0350 in ascitic fluid from TB ascites and non-TB ascites patients using the antibodies generated against 71-kDa HSP by ELISA.
| Patients group | Positive for 71 kDa Hsp antigen (cut off 0.860) | Negative for 71 kDa Hsp antigen (cut off 0.860) | Absorbance (mean ± SD) | Range |
|---|---|---|---|---|
| TB ascites (n = 18) | 15 (83.33%) | 3 (16.67%) | 0.968 ± 0.261 | 0.481–1.615 |
| non TB ascites (n = 21) | 5 (23.80%) | 16 (76.20%) | 0.644 ± 0.198 | 0.362–1.136 |
The mean 71-kDa HSP antigen level in the TB ascites group was significantly higher than that in the non-TB ascites group [p = 0.0005].
The identified diagnostic marker specific to TB ascites showed good sensitivity (72%–100%) and specificity (76%–95%) when tested with clinical samples of TB ascites and non-TB ascites subjects.
Discussion
This study, to the best of our knowledge, is the first detailed description of the protein profiles of a reasonably large panel of biomarkers, which were assessed simultaneously using 2 DPGE, LC-MS/MS and immunoblot analyses of the ascitic fluid and were compared between TB ascites patients and non-TB ascites patients. Our results indicate that the identifying antigen(s) might be useful for the development of diagnostic tool for the diagnosis of TB ascites.
First antigen, Antigen 85 Complex represents a family of fibronectin-binding proteins, which are most prominently represented by 85A and 85B. Members of this complex have been found to be both secreted and retained in the cell wall of MTB.10 These proteins have been found in the blood and sputum of pulmonary tuberculosis patients.11–13 We previously demonstrated the presence of an Antigen 85 complex in the tuberculous meningitis (TBM) and pulmonary tuberculosis patients.9,14,15
Another promising systemic marker identified in this study for tuberculosis ascites is the 14-kDa HSP (alpha crystallin family), Rv 3418c. It was originally identified by three MAbs that were generated in two separate laboratories16,17 and was previously assigned an apparent molecular weight of 16,000 Da.18 The 14-kDa HSP antigen has previously shown considerable promise as a serodiagnostic target in assay protocols that were based upon MAb competition and by ELISA assay.19,20
The MTB 65-kDa HSP was identified as a another potential ascitic fluid biomarker for TB ascites. This protein is produced in response to host reaction during infection, which is the reason that the more general term, stress protein, has been applied to this particular protein class.21 An earlier study in our laboratory appears to have been the first used of this antigen for the diagnosis of TBM and pulmonary tuberculosis.22,23
On the other hand, significant differences for the level of the 71-kDa HSP protein were also observed between TB ascites and controls. This particular antigen from mycobacteria has been identified as a target of the humoral and cellular immune responses during mycobacterial infection.24 In single study, using Immunohistochemical analysis Dubaniewicz A et al reported the expression of hsp70Mtb, hsp65Mtb, and hsp16Mtb in all 25 lymph node tissues and tuberculous granulomas from patients with sarcoidosis and in one non-specific lymphadenopathy case with only weak hsp70Mtb reactivity.25
In conclusion, we hypothesize that the 65-kDa HSP, 71-kDa HSP, 14-kDa HSP and Ag 85 complex proteins may have an important role in the diagnosis of TB ascites. Additional studies that included a high number of ascitic fluid samples will be required in order to determine the sensitivity and specificity of these potential proteins as biomarkers for TB ascites. The identification of these markers can contribute to the clinical diagnosis of TB ascites and may also provide additional insight into the pathogenesis of TB ascites.
Table 3.
Demonstration of the Antigen (Ag) 85 Complex antigens in ascitic fluid from TB ascites and non-TB ascites patients using the antibodies against the purified MTB B and C (Ag 85) complex, CS-90, received from Colorado State University, USA by ELISA.
| Patients group | Positive for antigen 85 complex (cut off 0.762) | Negative for antigen 85 complex (cut off 0.762) | Absorbance (mean ± SD) | Range |
|---|---|---|---|---|
| TB ascites (n = 18) | 13 (72.22%) | 5 (27.78%) | 0.824 ± 0.193 | 0.45–1.27 |
| Non TB ascites (n = 21) | 2 (9.52%) | 19 (90.48%) | 0.593 ± 0.172 | 0.374–1.31 |
The mean Ag 85 complex value in the TB ascites group was higher than in the non-TB ascites group [p < 0.0001].
Table 4.
Demonstration of the Mycobacterium tuberculosis heat shock protein 65 (65-kDa HSP), Rv0440; in ascitic fluid from TB ascites and non-TB ascites patients using the antibodies against the 65-kDa HSP, received from Colorado State University, USA by ELISA.
| Patients group | Positive for 65 kDa Hsp antigen (cut off 0.722) | Negative for 65 kDa Hsp antigen (cut off 0.722) | Absorbance (mean ± SD) | Range |
|---|---|---|---|---|
| TB ascites (n = 18) | 16 (88.88%) | 2 (11.12%) | 0.816 ± 0.129 | 0.453–0.975 |
| Non TB ascites (n = 21) | 1 (4.76%) | 20 (95.24%) | 0.561 ± 0.113 | 0.39–1.01 |
The mean absorbance value of the 65-kDa HSP antigen in TB ascites patients was significantly higher than in the non-TB ascites group [P < 0.001].
Acknowledgments
We would like to acknowledge the help of Colorado State University, USA for supplying tuberculosis research material (Contract No 1-A1-40091). We thank Prashant D Deoras for performing statistical analysis.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Das P, Shukla HS. Clinical diagnosis of abdominal tuberculosis. Br J Surg. 1976;63:941–6. doi: 10.1002/bjs.1800631213. [DOI] [PubMed] [Google Scholar]
- 2.Louie E, Rice LB, Holzman RS. Tuberculosis in non-Haitian patients with acquired immunodeficiency syndrome. Chest. 1986;90:542–5. doi: 10.1378/chest.90.4.542. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Tahir M, Mohan A, Smith-Rohrberg D, Mishra HK, Pandey RM. Diagnostic accuracy of ascitic fluid IFN-gamma and adenosine deaminase assays in the diagnosis of tuberculous ascites. J Interferon Cytokine Res. 2006;26:484–8. doi: 10.1089/jir.2006.26.484. [DOI] [PubMed] [Google Scholar]
- 4.Sood R. Diagnosis of Abdominal Tuberculosis: Role of Imaging. Indian Academy of Clinical Medicine. 2001;2:169–77. [Google Scholar]
- 5.Singh-Ranger D, Rockall T, Narward AH, Haldane M, Abrahams R, Mcdonald P. Abdominal tuberculosis: the problem of diagnostic delay. Scand J Infect Dis. 1999;31:517. doi: 10.1080/00365549950164111. [DOI] [PubMed] [Google Scholar]
- 6.Djoba Siawaya JF, Chegou NN, Heuvel MM, Heuvel MM, Diacon AH, Beyers N, et al. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine. 2009;47:132–6. doi: 10.1016/j.cyto.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Redman JE, Shaw MJ, Mallet AI, Santos AL, Roberts CA, Gernaey AM, et al. Mycocerosic acid biomarkers for the diagnosis of tuberculosis in the Coimbra Skeletal Collection. Tuberculosis. 2009;89:267–77. doi: 10.1016/j.tube.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Bell AW, Bergeron JJ, Yanofsky CM, Carrillo B, Beaudrie CE, et al. Methods for peptide identification by spectral comparison. Proteome Sci. 2007;5:3. doi: 10.1186/1477-5956-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashyap RS, Rajan AN, Ramteke SS, Kelkar SS, Purohit HJ, Taori GM, et al. Diagnosis of tuberculosis in an Indian population by an indirect ELISA protocol based on detection of Antigen 85 complex: a prospective cohort study. BMC Infect Dis. 2007;7:74. doi: 10.1186/1471-2334-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of. Mycobacterium tuberculosis Microbiol Rev. 1992;56:648–61. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miki K, Nagata T, Tanaka T, Kim YH, Uchijima M, Ohara N, et al. Induction of protective cellular immunity against Mycobacterium tuberculosis by recombinant attenuated self-destructing Listeria monocytogenes strains harboring eukaryotic expression plasmids for antigen 85 complex and MPB/MPT51. Infect Immun. 2004;72:2014–21. doi: 10.1128/IAI.72.4.2014-2021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senthil Kumar KS, Uma Devi KR, Alamelu R. Isolation and evaluation of diagnostic value of two major secreted proteins of. Mycobacterium tuberculosis Indian J Chest Dis Allied Sci. 2002;44:225–32. [PubMed] [Google Scholar]
- 13.Wallis RS, Perkins M, Phillips M, Phillips M, Joloba M, Demchuk B, et al. Induction of the antigen 85 complex of Mycobacterium tuberculosis in sputum: a determinant of outcome in pulmonary tuberculosis treatment. J Infect Dis. 1998;178:1115–21. doi: 10.1086/515701. [DOI] [PubMed] [Google Scholar]
- 14.Kashyap RS, Dobos KM, Belisle JT, Purohit HJ, Chandak NH, Taori GM, et al. Demonstration of components of antigen 85 complex in CSF of Tuberculous meningitis patients. Clin Diagn Lab Immunol. 2005;12:752–8. doi: 10.1128/CDLI.12.6.752-758.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashyap RS, Biswas SK, Purohit HJ, Chandak N, Agarwal N, Taori GM, et al. Significance of 30 kD protein as a diagnostic marker in CSF of Tuberculous meningitis. Annals of Indian academy of Neurology. 2001;4:197–201. [Google Scholar]
- 16.Coates ARM, Allen BW, Hewitt J, Ivanyi J, Mitchison DA. Antigen diversity of M. tuberculosis and M. bovis detected by means of monoclonal antibodies. Lancet. 1981;2:167–9. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- 17.Kolk AH, Ho ML, Klatser PR, Eggelte TA, Kuijper S, de Jonge S, et al. Production and characterization of monoclonal antibodies to M. tuberculosis, M. bovis BCG and M. leprae. Clin Exp Immunol. 1984;58:511–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Verstijnen CP, Schöningh R, Kuijper S, Bruins J, von Ketel RJ, Groothuis DG, et al. Rapid identification of cultured M. tuberculosis with a panel of monoclonal antibodies in Western blot and immunofluorescence. Res Microbiol. 1989;140:653–66. doi: 10.1016/0923-2508(89)90197-6. [DOI] [PubMed] [Google Scholar]
- 19.Chandramuki A, Bothamley GH, Brennan PJ, Ivanyi J. Levels of antibody to defined antigens of Mycobacterium tuberculosis in tuberculous meningitis. J Clin Microbiol. 1989;27:821–5. doi: 10.1128/jcm.27.5.821-825.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingston AE, Salgame PR, Mitchison NA, Colston MJ. Immunological activity of a 14-kilodalton recombinant protein of Mycobacterium tuberculosis H37Rv. Infect Immun. 1987;55:3149–54. doi: 10.1128/iai.55.12.3149-3154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 22.Mudaliar AV, Kashyap RS, Purohit HJ, Taori GM, Daginawala HF. Detection of 65kD heat shock protein in cerebrospinal fluid of tuberculous patients. BMC Neurol. 2006;6:34. doi: 10.1186/1471-2377-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajan AN, Kashyap RS, Purohit HJ, Taori GM, Daginawala HF. Serodiagnosis of tuberculosis based on the analysis of 65kD heat shock protein of Mycobacterium tuberculosis. Int J Tuberc Lund Dis. 2007;11:1–6. [PubMed] [Google Scholar]
- 24.Zügel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubaniewicz A, Dubaniewicz-Wybieralska M, Sternau A, Zwolska Z, Izycka-Swieszewska E, Augustynowicz-Kopec E, et al. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J Clin Microbiol. 2006;44:3448–51. doi: 10.1128/JCM.01433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]