Abstract
Objective
A recent 12-week controlled comparison demonstrated the superiority of clozapine to “high-dose” olanzapine in adolescents with treatment-refractory schizophrenia. In the present study, the authors conducted a 12-week, open-label, follow-up study to examine changes in lipid and glucose metabolism in youths maintained on clozapine and to determine whether patients who were previously randomized to high-dose olanzapine (up to 30 mg/day) responded to clozapine.
Method
Thirty three (14 clozapine, 19 olanzapine) (85%) of 39 patients were available for the present 12-week, open-label extension study. Extended safety data using an intention-to-treat analysis from the 14 subjects treated with clozapine for a total of 24 weeks are presented. In addition, we report the clinical outcomes for 10 of 19 olanzapine-treated patients who were switched after 12 weeks to clozapine due to treatment nonresponse. Clinical response was defined as a decrease of 30% or more in total Brief Psychiatric Rating score from week 12 and a Clinical Global Impression–Improvement rating of 1 (very much improved) or 2 (much improved).
Results
The incidence of hypertriglyceridemia (defined as fasting triglycerides >125 mg/dL) (10/14 = 71%) and the incidence of “prediabetes” (defined as fasting blood glucose ≥100) (4/14 = 29%) at week 24 in the clozapine-treated subjects were notable. Seven (70%) of 10 of young patients with schizophrenia who failed treatment with “high-dose” olanzapine were found to respond to a 12-week, open-label clozapine trial.
Conclusions
Clinicians and caregivers need to be aware of potential metabolic adverse events of long-term clozapine treatment. Adolescents with a poor response to olanzapine may do better on clozapine.
Introduction
An early-onset of schizophrenia (onset of psychosis by age 18 years) tends to result in a more symptomatically severe form of the disorder associated with chronic disability (Kranzler et al. 2006). Despite the availability of multiple pharmacologically unique first-line, second-generation antipsychotic (SGA) medications, substantial numbers of treatment refractory children and adolescents with schizophrenia continue to present for long-term treatment (Kranzler et al. 2005). Clozapine remains the only antipsychotic medication tested in a multiple double-blind trial that has consistently been found to be superior to other agents for treatment-refractory adults with schizophrenia. Clozapine has advantages over conventional antipsychotics and some atypical antipsychotics in terms of lower rates of extrapyramidal side effects and risk of tardive dyskinesia. However, clozapine therapy is restricted to patients with treatment-refractory schizophrenia due to concerns regarding agranulocytosis, seizures, myocarditis, substantive weight gain, and metabolic syndrome associated with its use.
Although not approved by the Food and Drug Administration (FDA) for use in children and adolescents, there are controlled treatment trial data indicating that clozapine may be effective for children and adolescents refractory to at least two typical antipsychotic medication trials (Findling et al. 2007). Naturalistic observational data suggest clozapine has benefits in terms of preventing rehospitalization and medication compliance for youngsters with psychotic disorders characterized by histories of multiple hospitalizations, extreme violence, alcohol and substance use, suicidality, and trauma (Kranzler et al. 2005). With careful surveillance and monitoring, adverse hematological side effects can be detected early and the development of long-term irreversible side effects can be minimized (Gerbino-Rosen et al., 2005). Together, these data suggest that a clozapine trial should be considered for treatment of a child who is suffering from severe, disabling psychopathology who does not respond to or cannot tolerate “first-line” antipsychotic medications to prevent negative outcomes associated with treatment-refractory schizophrenia, such as long-term institutionalization, incarceration, and/or early death from drug use, violence, or suicide (Findling et al. 2007).
For the treatment of adults with schizophrenia, two SGA drugs, olanzapine and clozapine, appear to be more effective than other currently available agents (Davis et al. 2003; Lieberman et al. 2005; McEvoy et al. 2006). On the basis of data from the recent Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial, it has been suggested that a physician consider an olanzapine or clozapine trial in “any patient with schizophrenia who has not had a full clinical remission of the illness, which includes the reversal of cognitive and psychosocial disabilities” (Freedman 2005). There are some data suggesting that olanzapine is effective for children and adolescents with schizophrenia compared to placebo (Kryzhanovskaya et al. 2006). Despite the possibility that clozapine monotherapy is considered to be effective in treatment-resistant cases (Kumra et al. 1996; Shaw et al. 2006; Kumra et al. 2008), published data on treatment-induced weight gain in children and adolescents and related metabolic adverse effects are primarily limited to data from short-term trials (<12 weeks) (Kumra et al. 1996; Shaw et al. 2006; Kumra et al. 2008).
Our group recently completed a 12-week double-blind, parallel group comparison of clozapine and “high-dose” olanzapine (up to 30 mg/day) in children and adolescents (ages, 10–18 years) with schizophrenia who failed treatment with at least two atypical antipsychotics (Kumra et al. 2008). Significantly more clozapine-treated adolescents met response criteria (66%) compared with olanzapine-treated subjects (33%). The response criteria reflected both a decrease of 30% or more in the total Brief Psychiatric Rating Scale (BPRS) score from baseline to end point and a Clinical Global Impression Scale–Improvement (CGI-I) rating of 1 (very much improved) or 2 (much improved). In that study, the upper limit for olanzapine (30 mg/day) was the same as what was employed in the CATIE trial for adults. However, at the conclusion of the 12-week, double-blind study, several patients (in both the clozapine and olanzapine-treated groups) remained significantly functionally impaired and continued to experience residual symptoms ranging from psychosis to aggression (Kumra et al. 2008). Similar findings demonstrating the superiority of clozapine to standard-dose olanzapine (up to 20 mg/day), particularly with respect to reduction of negative symptoms, were reported in a separate group of treatment-refractory children and adolescents with childhood-onset schizophrenia who were studied at the National Institute of Mental Health (Shaw et al. 2006).
After completion of the 12-week double-blind portion of the study (Kumra et al. 2008), subjects were offered participation in an open-label, 12-week continuation phase. To our knowledge, there are no systematic data regarding changes in body composition or glucose and lipid metabolism for periods of 6 months or longer in clozapine-treated children and adolescents. This is problematic because preliminary data suggest that pediatric populations may be at higher risk of SGA-induced weight gain than adults (Gothelf et al. 2002; Ratzoni et al. 2002; Bloch et al. 2003; Sikich et al. 2004; McClellan et al. 2007) and certain metabolic abnormalities, especially diabetes, emerge later in the course of antipsychotic treatment (Correll and Carlson 2006). Thus, the primary focus of this report was to describe the outcomes of a subgroup of patients (14 of 33) in this follow-up sample that received up to 24 weeks of treatment with clozapine. We hypothesized that there would be a high rate of adverse metabolic side effects in this group. Second, we examined the clinical outcomes of another subgroup of patients (10 of 33) who were deemed to be nonresponders to a double-blind comparison of clozapine versus “high-dose olanzapine” and who were administered open-label olanzapine trial during the present 12-week extension study.
Materials and Methods
This article reports the outcome of children and adolescents with schizophrenia, based on up to 24 weeks of observation, who participated in a 12-week double-blind comparison of clozapine and “high-dose” olanzapine (up to 30 mg/day) (Kumra et al. 2008), followed by an open-label continuation phase of an additional 12 weeks.
Study participants
The rationale, design, and methods of the trial have been described in detail elsewhere (Kumra et al. 2008). In brief, subjects were recruited for participation in the parent multisite protocol that was executed at the Bronx Children's Psychiatric Center (BCPC), Sagamore Children's Psychiatric Center (SCPC), and The Zucker Hillside Hospital. The study was designed to evaluate the effectiveness of antipsychotic treatments under real-world conditions and in representative patient samples. Thus, the majority of subjects were recruited at state-funded mental health hospitals (i.e., BCPC, SCPC) in the metropolitan New York area that serve as a tertiary-care referral center for children and adolescents with severe emotional disturbances in need of long-term treatment. The institutional review board at each site approved the study, and written informed consent was obtained from a parent or guardian of each subject prior to enrollment. Each child also gave informed consent or assent.
The subject eligibility criteria included boys and girls aged 10–18 years, inclusive, with a diagnosis of schizophrenia or schizoaffective disorder (determined by the Kiddie-Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version [K-SADS-PL], Kaufman et al. 1997) and failure of two prior antipsychotic treatments. Exclusion criteria were premorbid mental retardation, active alcohol or drug abuse, past serious adverse reactions to clozapine or olanzapine, pregnancy, or serious, unstable medical conditions. In addition, subjects who had failed an adequate trial of clozapine (12 weeks) at adequate doses (300 mg/day or higher) and/or who had failed an adequate trial of olanzapine (8 weeks) at high doses (20 mg/day or higher) were excluded from participation. Subjects with treatment refractory schizophrenia frequently have been observed to test poorly prior to stabilization on antipsychotic medication, and it is difficult to obtain the optimal level of their cognitive function (Kumra et al. 1996). For this study, subjects with low intelligence quotients (IQs) were included in the trial only if a diagnosis of mental retardation could be ruled out based on evaluation by a psychologist of current and previous available testing, school reports, and pediatric records.
Interventions
Thirty nine patients who completed the 12-week, double-blind portion of the study were initially randomly assigned to double-blind treatment with clozapine (n = 19) or olanzapine (n = 21). During the double-blind study, both the prescribing clinician and a blinded team, which performed clinical ratings, were blinded to treatment assignment. After the completion of the 12-week, double-blind phase of the study, the safety officer unblinded the patient and the patient was referred where possible to a physician outside of the study for ongoing care.
All 39 randomly assigned participants from the parent study were tracked for follow up. A total of 37 participants were located for follow-up interviews (2 patients were discharged and could not be located). However, only 33 (85%) of those subjects located completed follow-up interviews. The reasons for missing interviews included incarceration (n = 1) and parent/patient refusal (n = 3). There were no significant differences in baseline demographic or clinical characteristics between those participants who were (n = 33) versus those participants who were not (n = 6) available for follow up (all p values >0.1).
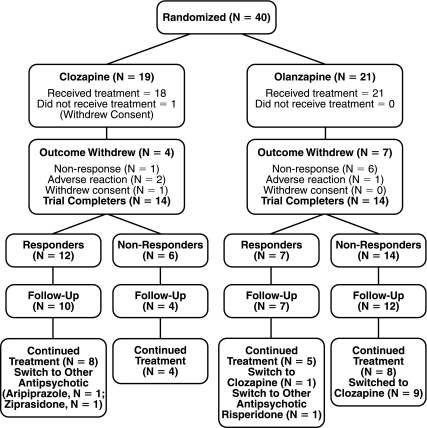
After completion of the double-blind protocol, patients were treated in a naturalistic fashion by a physician in consultation with the unblinded safety officer at each study site. Although some of the patients were discharged from hospital after the completion of the double-blind protocol, all of the patients treated with clozapine were seen once weekly to monitor their white blood cell (WBC) count, which most likely enhanced compliance. Patients who remained on olanzapine were not monitored at the same frequency. As shown in Fig. 1, 33 subjects (14 randomized to clozapine, 19 randomized to olanzapine) were available and agreed to participate in a 12-week, open-label follow-up evaluation.
FIG. 1.
Patient enrollment and outcomes in the double-blind and open-label extension phase of the study.
Symptom ratings by the rating team were made available to the treatment team along with a synopsis of the subject's progress through the clinical trial. At the completion of the double-blind trial, the treating clinician could adjust the dosage of the antipsychotic, change the antipsychotic and/or add adjunctive medications to improve symptoms, and limit adverse effects during the continuation phase. If clinically appropriate, patients who discontinued their initial study treatment either due to treatment nonresponse or intolerability were offered an opportunity to have a 12-week, open-label trial of the alternative study treatment.
During the open-label extension phase, clinicians were free to use adjunctive medications to target residual symptoms and augment clinical response. The decision to add concomitant medications was made by the primary clinician in consultation with nursing staff, the research team, parents/guardians, and the safety monitor at each site. In general, the decision to add concomitant medications was considered for patients who showed evidence of residual psychosis, mood instability, and aggressive or self-injurious behaviors that were considered sufficiently problematic in the hospital milieu and/or to diminish the likelihood of hospital discharge or time spent out of psychiatric hospital.
Indications for use of mood stabilizers was as follows: lithium was used in some cases to increase WBC counts as a significant proportion of our subjects were from ethnic backgrounds (i.e., African-American) that tend to have low WBC counts (Kranzler et al. 2005), topiramate was used to limit drug-induced weight-gain (Lévy et al. 2007), and lamotrigine was used to target residual psychotic symptoms (Dursun et al. 1999).
Using an intention-to-treat analysis, data for safety and effectiveness outcomes are presented for subjects based on their initial randomization to clozapine or olanzapine (Kumra et al. 2008) in Tables 2 and 3, below, respectively. However, 10 of the 19 subjects who were initially randomized to olanzapine were switched to clozapine due to insufficient therapeutic response. To enhance clarity, the clinical outcome data for these subjects are presented in a separate table (Table 4, below). After the patients were unblinded at the end of 12 weeks of olanzapine treatment, these 10 patients were immediately switched to clozapine. There was a crossover period of 2–4 weeks (which was considered as part of the 12-week open trial) where both medications were co-administered to allow patients to reach a therapeutic dosage of clozapine before olanzapine was discontinued. The schedule for dose titration and the maintenance doses was determined by the prescribing clinicians in accordance with clinical guidelines set forth by the Office of Mental Health (OMH) in New York (Kranzler et al. 2005). These guidelines recommend that clozapine therapy should be started at a dose of 25 mg/day and increased in 25-mg or 50-mg increments every 3 days until clinical response occurs or side effects limit the upward titration, to a maximum dosage of 900 mg/day of clozapine. In addition, a complete blood count (CBC) with differential was monitored weekly.
Table 2.
Intention-to-Treat Analysis of Outcome Measures of Safety Among Randomized Patientsa
| |
|
Week 12b |
Week 24 |
|
|
|
||
|---|---|---|---|---|---|---|---|---|
| Measure | Therapy | n | Mean (SD) | n | Mean (SD) | F | df | p |
| Cholesterol | Clozapine | 13 | 171.5 (27.2) | 13 | 141.9 (55.5) | 0.79 | 1, 28 | 0.38 |
| Olanzapine | 17 | 180.0 (33.9) | 17 | 170.8 (38.2) | ||||
| Triglycerides | Clozapine | 13 | 161.0 (82.3) | 14 | 154.9 (65.9) | 0.07 | 1, 28 | 0.80 |
| Olanzapine | 17 | 134.9 (68.9) | 18 | 140.5 (72.0) | ||||
| Glucose | Clozapine | 13 | 96.2 (17.1) | 14 | 94.2 (10.9) | 0.03 | 1, 28 | 0.87 |
| Olanzapine | 17 | 84.1 (7.9) | 18 | 93.3 (15.1) | ||||
| Weight (kg) | Clozapine | 14 | 76.9 (11.9) | 13 | 76.3 (13.1) | 4.06 | 1, 29 | 0.05 |
| Olanzapine | 19 | 84.0 (18.8) | 18 | 87.0 (16.9) | ||||
| Body mass index (BMI) | Clozapine | 14 | 28.7 (3.6) | 13 | 28.5 (3.9) | 3.35 | 1, 29 | 0.08 |
| Olanzapine | 19 | 29.5 (5.6) | 19 | 30.2 (5.5) | ||||
| BMI percentilec | Clozapine | 14 | 93.7% (5.3) | 13 | 93.3% (6.3) | |||
| Olanzapine | 19 | 91.4% (10.7) | 18 | 92.1% (9.9) | ||||
| At healthy weightd | Clozapine | 14 | 1 (7.1%) | 14 | 2 (14.3%) | |||
| Olanzapine | 19 | 4 (21.1%) | 19 | 4 (21.1%) | ||||
| Overweighte | Clozapine | 14 | 7 (50.0%) | 14 | 5 (35.7%) | |||
| Olanzapine | 19 | 5 (26.3%) | 19 | 4 (21.1%) | ||||
| Obesef | Clozapine | 14 | 6 (42.9%) | 14 | 6 (42.9%) | |||
| Olanzapine | 19 | 10 (52.6%) | 19 | 10 (52.6%) | ||||
| Prolacting | Clozapine | 14 | 13.9 (8.3) | 14 | 18.2 (14.1) | 6.57 | 1, 27 | 0.02 |
| Olanzapine | 15 | 41.3 (39.7) | 15 | 23.6 (16.8) | ||||
Laboratory data are missing for some patients due to non-compliance, patient refusal and/or administrative reasons.
Unless otherwise indicated, data are presented as mean (standard deviation). Cholesterol, triglycerides, and glucose were obtained fasting.
Or trial endpoint. Using an intention-to-treat analysis, data are presented for subjects based on their initial randomization assignment to the clozapine or olanzapine group in the double blind study (Kumra et al 2008) regardless of which drug they received during the current open-label 12-week extension study.
Age adjusted. From: http://www.kidsnutrition.org/bodycomp/bmiz2.html/.
BMI percentile ≥5 to <85.
BMI percentile ≥85 to <95.
BMI percentile ≥95 (Koplan et al. 2005).
The mean threshold for abnormal prolactin values varied by laboratory (males, 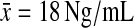 ; females,
; females, 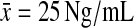 ).
).
SD = standard deviation.
Table 3.
Outcome Measures of Effectiveness Among Randomized Patients
| |
Week 12a |
Week 24 |
|
|
|
|
||
|---|---|---|---|---|---|---|---|---|
| Measure | n | Mean (SD) | n | Mean (SD) | F | df | p | Partial eta-square |
| BPRS totalb | ||||||||
| Clozapine | 13 | 31.1 (8.4) | 13 | 31.7 (12.1) | 1.36 | 1, 30 | .25 | .04 |
| Olanzapine | 19 | 34.7 (14.3) | 19 | 30.3 (9.8) | ||||
| BPRS Psychosis Clusterb | ||||||||
| Clozapine | 14 | 6.1 (2.1) | 14 | 6.6 (3.2) | .83 | 1, 30 | .37 | .03 |
| Olanzapine | 18 | 7.9 (4.7) | 18 | 7.3 (3.7) | ||||
| SANS total scorec | ||||||||
| Clozapine | 14 | 6.1 (3.6) | 14 | 6.6 (4.9) | 3.7 | 1, 31 | .06 | .11 |
| Olanzapine | 19 | 7.4 (3.9) | 19 | 5.4 (3.8) | ||||
| CGI-Improvementd | ||||||||
| Clozapine | 14 | 2.0 (.73) | 14 | 2.4 (1.3) | 8.34 | 1, 31 | .007 | .21 |
| Olanzapine | 19 | 2.9 (1.3) | 19 | 1.9 (.78) | ||||
| CGASe | ||||||||
| Clozapine | 14 | 49.5 (14.1) | 14 | 46.9 (16.8) | 1.98 | 1, 30 | .17 | .06 |
| Olanzapine | 18 | 48.5 (19.8) | 18 | 55.6 (17.5) | ||||
All data are presented as mean (standard deviation). Using an intention-to-treat analysis, data are presented for subjects based on their initial randomization assignment to the clozapine or olanzapine group in the double-blind study (Kumra et al. 2008), regardless of which drug they received during the current open-label 12-week extension study.
Or trial end point.
Brief Psychiatric Ratings Scale.
Scale for the Assessment of Negative Symptoms. Total Score = sum of global scores (affective flattening, alogia, avolition, asociality-an-hedonia).
Clinical Global Impressions—Improvement Scale.
Children's Global Assessment Scale.
Table 4.
Outcome Measures of Effectiveness in Ten Subjects Switched from Olanzapine to Clozapine During the 12-Week Open-Label Extension Study
| Measure | Week 12 | Week 24a | t | df | p | Effect size (d) |
|---|---|---|---|---|---|---|
| BPRSb | ||||||
| Total | 42.7 (13.81) | 30.4 (11.01) | 3.54 | 9 | 0.006 | 0.18 |
| Psychosis cluster | 10.7 (4.61) | 8.3 (4.21) | 2.09 | 9 | 0.07 | 0.58 |
| SANS total scorec | 9.2 (4.21) | 6.0 (3.61) | 3.53 | 9 | 0.006 | 0.97 |
| CGId | ||||||
| Improvement | 3.7 (1.31) | 2.1 (0.31) | 3.54 | 9 | 0.006 | 1.74 |
| Severity of Illness | 4.3 (1.1) | 3.0 (1.1) | 3.88 | 9 | 0.004 | 0.48 |
| CGASe | 36.2 (17.21) | 54.0 (17.61) | −2.59 | 8 | 0.03 | 0.36 |
All data are presented as mean (standard deviation). Effect sizes calculated as d = (Mean1 – Mean2)/pooled standard deviation.
Or trial end point.
Brief Psychiatric Ratings Scale.
Scale for the Assessment of Negative Symptoms. Total Score = sum of global scores (affective flattening, alogia, avolition, asociality-anhedonia).
Clinical Global Impressions—Improvement Scale.
Children's Global Assessment Scale.
During the open-label phase of the study, an unblinded clinician treated the patient and then a centralized team of raters performed clinical ratings. The raters were not aware of what the patient had been receiving as part of the double-blind protocol. The following evaluations were performed by trained members of the centralized team of raters who were not blinded to the treatment that patients were receiving during the open-label phase of the study at weeks 12 and 24: an anchored 18-item version of the BPRS (Overall and Gorham 1961; Woerner et al. 1988), the Clinical Global Impressions Scale–Severity of Illness (CGI-S) (Guy 1976), the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen 1982), the Children's Global Assessment Scale (CGAS) (Shaffer et al. 1983), the Subjective Treatment Emergent Symptoms Scale (STESS) (modified to include side effects associated with clozapine) (Campbell and Palig 1985), fasting laboratory measures (i.e., glucose, triglycerides and cholesterol), serum prolactin, weight, and body mass index (BMI). Laboratory data are missing for some patients due to noncompliance, patient refusal, and/or administrative reasons. At the week-24 follow-up visit, clinical response was defined as a decrease of 30% or more in the total Brief Psychiatric Rating Scale score from baseline and a CGI-I rating of 1 (very much improved) or 2 (much improved). Cognitive testing was administered at the baseline phase of the double-blind study as described elsewhere (Kumra et al. 2008).
Statistical analysis
Repeated-measures analyses of variance (RM ANOVA) was used to compare the two treatment groups on the pattern of change in clinical symptoms and other continuous study outcomes. A partial Eta-squared value (η2p) was calculated for each of the continuous outcome measures, representing the proportion of the total variability accounted for by the treatment condition.
For the subset of patients (10 of the 33 subjects) who were initially randomized to olanzapine, but who subsequently received a clozapine trial, a paired-samples t-test was used to compare clinical outcomes between baseline (week 12/end point) and end point (week 24) during open treatment with clozapine. We also calculated the magnitude of change (Cohen effect size d) in continuous measures of symptom ratings using the difference between changes in ratings from baseline to end point during the open trial of clozapine divided by the pooled standard deviation (SD): d = (mean change1 – mean change2)/pooled SD of change. The Cohen d values are considered to be a small effect size at 0.2, a moderate effect size at 0.5, and a large effect size at 0.8.
Results
Baseline characteristics and disposition
The mean (SD) age of the 33 participants in the open-label continuation phase was 15.5 (2.0) years with a mean (SD) age of onset of psychosis of 11.8 (2.9) years. Patients were diagnosed as having schizophrenia (n = 20) or schizoaffective disorder (n = 13). Adolescents had a long history of illness and were documented by the investigators as being treatment-resistant, which was defined as response failure to at least two antipsychotic agents. Prior to study entry, the median number of months of hospitalization was 10.5 and the median number of antipsychotic trials was 3. The mean (SD) full-scale IQ of the participants was 76.2 (13.4). There were no significant differences between randomly assigned patients to clozapine or olanzapine on any pretreatment demographic or clinical variables (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of Randomized Patients
| Clozapinea(n = 14) | Olanzapineb(n = 19) | Analysis | |
|---|---|---|---|
| Gender (male/female) | 5/9 | 11/8 | χ2 = 1.58, p = 0.21 |
| Age at trial entry (years) | 15.3 (2.3) | 15.6 (1.7) | t = 0.46, p = 0.65 |
| Ethnicity | |||
| Caucasian | 1 | 5 | χ2 = 5.51, p = 0.24 |
| African-American | 5 | 9 | |
| Hispanic | 4 | 4 | |
| Asian | 2 | 0 | |
| Other | 2 | 1 | |
| Parental SEs (high/low)c | 6/8 | 13/6 | χ2 = 2.2, p = 0.14 |
| Diagnosis | |||
| Schizophrenia | 8 | 12 | χ2 = 0.12, p = 0.73 |
| Schizoaffective disorder | 6 | 7 | |
| Total months of hospitalization at trial entry | 13.1 (15.2) | 12.6 (11) | t = 0.10, p = 0.92 |
| Median number of antipsychotic trials (range) | 3 (2–6) | 3 (2–5) | χ2 = 3.3, p = 0.50 |
| Full-scale IQd | 78.1 (16.3) | 76.6 (9.7) | t = 0.98, p = 0.34 |
All qualitative data are presented as mean (standard deviation) unless otherwise stated.
Two patients were switched to another antipsychotic from clozapine during the open-label extension phase (i.e., aripiprazole = 1, ziprasidone = 1) due to metabolic problems.
Ten patients were switched from clozapine to risperidone during the open-label extension phase due to lack of therapeutic efficacy or problematic side effects. An additional patient was switched from olanzapine to risperidone during the open-label extension phase due to lack of therapeutic efficacy.
Hollingshead-Redlich Scale (Hollingshead and Redlich, 1958) (High = Levels 1–3, Low = 4,5).
Wechsler Abbreviated Scale of Intelligence (The Psychological Corporation, 1999).
SES = socioeconomic status; IQ = intelligence quotient.
Fourteen of 33 patients who were initially randomized to clozapine treatment were observed for 24 weeks. Another subgroup of the 33 participants (n = 10), received open-label clozapine trials after having experienced inadequate therapeutic benefit from olanzapine as judged by the prescribing clinician, safety monitor, parent/guardian and/or patient. This subset of olanzapine patients (n = 10) who went on to receive clozapine treatment did not significantly differ from those who did not (n = 9) with respect to any pretreatment demographic or clinical characteristic (i.e., age, gender ratio, race, age of illness onset, schizophrenia subtype, and number of hospitalizations, baseline BPRS total scores, baseline CGI-S scores or mean final olanzapine dose during the 12-week double-blind trial) (all p values >0.10).
Patient disposition
As shown in Fig. 1, by week 24 of the study (observation period includes 12 week double-blind trial and 12-week open-label extension phase), patients were primarily being treated with the following antipsychotics: clozapine (n = 22), olanzapine (n = 8), risperidone (n = 1), aripiprazole (n = 1), and ziprasidone (n = 1). A significantly higher proportion of children and adolescents who were initially assigned to clozapine therapy (12 of 14, 86%) remained on their initial assigned treatment as compared to adolescents initially assigned to olanzapine therapy (8 of 19, 42%) at week 24 (Fisher exact test, p = 0.01). The reasons for treatment discontinuation were inadequate therapeutic effect (10 olanzapine) and unacceptable side effects (2 clozapine, 1 olanzapine). The two clozapine patients were switched at follow up to aripiprazole and ziprasidone because of concerns about weight gain and glucose intolerance, respectively. One olanzapine patient was switched to risperidone because of concerns about recurrent neutropenia and weight gain. Another olanzapine patient met “clinical response” criteria during the double-blind trial, but the clinician and family elected to conduct a 12-week, open-label clozapine trial because they were not fully satisfied with her clinical response due to persistent anxiety and social deficits and concern that she would destabilize after being discharged from hospital.
At week 24, 18 patients (10 olanzapine, 8 clozapine) were receiving co-medications in addition to their primary antipsychotic. The concomitant medications that were prescribed during the open-label continuation phase included: a secondary antipsychotic (1 clozapine, 5 olanzapine); antidepressant (1 clozapine, 4 olanzapine); clonidine (1 clozapine, 1 olanzapine); and/or a mood stabilizer (7 clozapine, 4 olanzapine). Mood stabilizers were used in patients with schizophrenia (n = 8) and schizoaffective disorder (n = 3).
Adverse side effects
As shown in Table 2, weight gain and associated metabolic problems emerged as the major side-effect burden associated with both olanzapine and clozapine treatments. The mean weight at the beginning of the open-label phase for all subjects was 79.5 kg (SD = 16.5), which corresponded to a mean BMI percentile of 91.3 (SD = 10.0). These data most likely reflect the considerable exposure of the study cohort to psychotropic medications, and, in particular, second-generation antipsychotic medications prior to study entry.
Changes in laboratory values were similar across groups with the exception of weight and prolactin values. For weight, the RM ANOVA indicated a significant treatment condition × time interaction [F(1, 29) = 4.06, p = 0.05], suggesting that patients initially randomized to olanzapine continued to gain weight. However, the condition × time interaction for glucose and triglyceride values was not significant for the olanzapine group. For prolactin, the RM ANOVA indicated a significant treatment condition × time interaction [F(1, 27) = 6.57, p = 0.02], suggesting that there was a reduction in prolactin values in patients initially randomized to olanzapine.
At week 24 of the study using criteria from Correll et al. (2006) to define clinically relevant abnormalities, 10 of 31 (32%) study participants (3 clozapine, 7 olanzapine) gained greater than 7% of their baseline body weight compared to baseline study prior to randomization to double-blind medications. Laboratory values for weeks 12 and 24 are shown in Table 2. At week 24, 6 (20%) of 30 children were noted to have elevated total serum cholesterol (>200 mg/dL) (2 clozapine, 4 olanzapine); 18 (56%) of 32 patients (10 clozapine, 8 olanzapine) were noted to have elevated fasting triglycerides >125 mg/dL; and 5 (16%) children (4 clozapine, 1 olanzapine) were observed to have a fasting blood glucose ≥100, which would meet current guidelines for “prediabetes” or impaired fasting glucose (American Diabetes Association 2004). These patients were provided nutritional counseling and diet modification.
At the completion of the study, four males treated with olanzapine and 8 females (4 olanzapine, 4 clozapine) were found to have elevated prolactin values (18 ng/mL for females, 25 ng/mL for males).
Maintenance of clozapine treatment effect
Analyses of longitudinal data
For patients initially randomized to clozapine and then continued on the agent during the open-label extension phase (n = 14), clinical response was measured from week 12. As shown in Table 3, in this subgroup examination of our data revealed that the majority of clozapine response was observed during the 12-week, double-blind trial. We observed only 1 patient who might be deemed a “late responder” to clozapine. The patient did not show substantial improvement during the 12-week, double-blind trial of clozapine (final dose of 500 mg/day), but did achieve what was described by the milieu staff as a “miraculous improvement” in the clarity of her thought processes and stability of mood by the end of 24 weeks on clozapine monotherapy.
Patients who were switched from olanzapine to clozapine
A subset of olanzapine patients (n = 10) received open-label clozapine trials after having experienced inadequate therapeutic benefit from olanzapine as judged by the prescribing clinician, safety monitor, parent/guardian, and/or patient. For these 10 subjects, the mean (SD) clozapine dose at the follow-up visit was 480 mg (153) (range, 200–750 mg/day). Four of these 10 patients were administered concomitant medications during the open-label clozapine trial that were prescribed primarily to target residual psychotic symptoms, mood symptoms and aggression (quetiapine [n = 1], olanzapine [n = 1], antidepressant [n = 1]) and to limit weight gain (topiramate [n = 1]).
On the basis of our clinical response criteria, 7 of 10 (70%) patients met responder criteria at the end of a 12-week clozapine trial. Clinical response in this subgroup of 10 patients both at baseline (after 12 weeks of double-blind olanzapine treatment) and end point (after 12 weeks of clozapine treatment) is shown in Table 4. A statistically significant difference between the week 12 and week 24 ratings was noted for most outcome measures, with the exception of the BPRS psychosis cluster scores which still showed evidence of a moderate effect size difference (Cohen effect size d = 0.58).
Discussion
This report describes an add-on study to a randomized controlled clinical trial investigating the risks and benefits of clozapine versus “high-dose” olanzapine in a relatively large group of well-characterized children and adolescents with treatment refractory ‘primary’ psychotic disorders. Subjects were recruited mainly from state-funded mental health hospitals in New York, and the retention rate of subjects from the double-blind portion of the study was 33/39 (85%). In this study, we report data from subjects who were followed from week 12 to week 24 of the study. All of the subjects were treated in a naturalistic fashion during this period of the study. This report documented that clozapine continued to have an apparent advantage in treatment-resistant patients, including youth and that the benefits of clozapine may occur well into treatment e.g., 12–24 weeks. Second, both clozapine and olanzapine were particularly problematic, and perhaps more so in youth as compared to adults, with regard to metabolic side effects.
Although weight gain reached a plateau, several clozapine-treated subjects developed significant metabolic disturbances, including glucose and lipid abnormalities, as seen in follow-up studies of adults with schizophrenia (Henderson 2001). Specifically, in patients initially randomized to the clozapine treatment arm the incidence of hypertriglyceridemia (10/14 = 71%) and the incidence of “prediabetes” (4/14 = 29%) during the 6-month follow up were high and required 2 patients to discontinue treatment prematurely. Because adverse weight/metabolic effects of clozapine treatment are likely to differ across patient populations and may depend upon age, baseline BMI percentile, past treatment exposure and co-medications, these data will need to be replicated in a larger sample of children and adolescents.
It remains unclear whether clozapine directly affects glucose metabolism or simply increases known risk factors for diabetes, such as obesity, lipid abnormalities, and sedentary lifestyle due to sedative effects in children and adolescents (Gothelf et al. 2002; Correll and Carlson 2006). On the basis of the adult literature, the incidence of these metabolic adverse effects likely would increase over longer-term follow-up with continued clozapine exposure (Henderson et al. 2005). These data are important for clinicians and consumers to know as they consider whether to initiate a clozapine trial. While such children are acutely psychotic, it would have been difficult to institute behavior and dietary modifications successfully on an individual basis. However, from a rehabilitation perspective, administrators in chronic-care facilities should consider how to implement dietary programs aimed at reducing food intake and boosting physical activity for all children and adolescents treated with SGAs such as olanzapine or clozapine during their hospital stay. Alternatively, there may be a role for metformin therapy in limiting the problems of decreased insulin sensitivity and abnormal glucose metabolism resulting from clozapine treatment (Klein et al. 2006).
Despite the potential risks of medical morbidities, clozapine continues to have a major role in the care of treatment-resistant children and adolescents with schizophrenia. We observed that subjects who responded to clozapine in the acute trial for the most part maintained their response at 24 weeks, suggesting sustained longer-term benefit. In spite of the superior efficacy for treatment-refractory schizophrenia, a substantial proportion of children and adolescents receiving clozapine continued to experience disabling symptoms such as persistent psychosis, mood instability, aggression, and/or self-injurious behavior as seen in the adult literature (Buckley et al. 2001).
Children and adolescents with treatment-refractory schizophrenia who receive an inadequate response to clozapine monotherapy pose a major clinical problem for which there are sparse data available to guide clinicians. One could consider the addition of other treatments as an end point, indicating that clozapine itself was not providing sufficient benefit as a monotherapy and therefore the subjects were in fact “out of protocol” when secondary treatments were added. In this scenario, our data supporting the efficacy of clozapine as a monotherapy are much less impressive. Eight (57%) of 14 subjects in the clozapine arm had treatments added during the open-label extension phase, including adjunctive antipsychotic medication, mood stabilizers, and/or an antidepressant to target aggression, self-injurious behaviors and residual psychosis.
Overall, these naturalistic data suggest that monotherapy with clozapine is suitable and appropriate for only a small percentage of treatment-refractory children and adolescents with schizophrenia and accurately portray the rather limited role of clozapine monotherapy in this population. The clinical value of these augmentation strategies is uncertain, as it is possible that there may be a higher metabolic side-effect burden associated with polypharmacy (Correll 2007), particularly in the scenario of combining two atypical antipsychotics (Correll et al. 2007). However, in our experience, maximal improvement with a clozapine trial may only be evident after the addition of an adjunctive medication. The relationship between adjunctive medications in clozapine-treated pediatric subjects and adverse metabolic side effects has not been adequately examined in pediatric subjects. There are some preliminary data in adults with schizophrenia that show the addition of aripiprazole to clozapine may be associated with a significant decrease in weight, BMI, fasting total serum cholesterol, and total triglycerides (Henderson et al. 2006). Thus, this strategy needs to be explored in pediatric cases as it may target both clozapine-associated medical morbidity and residual symptoms.
In the olanzapine-treated patients, we observed a significant condition × time interaction for weight and prolactin. This most likely reflected that approximately half of these subjects were switched to clozapine and continued to gain weight and resulted in a drop in prolactin due to the medication change. A high percentage of these patients showed a good treatment response to 12 weeks of open-label clozapine. The fact that all of the patients who were nonresponders were switched from olanzapine to clozapine most likely reflects the currently available evidence base that suggests that clozapine may be the only effective treatment option available in this scenario (Conley et al. 1999; Lieberman et al. 2005; McEvoy et al. 2006). However, because the raters and clinicians were not blind to treatment assignment, it is hard to ignore the strong expectancy bias for these results.
Limitations
It could be argued that the low number of subjects randomized in the original clinical trial (n = 39) limits the significance of the results generated from the open-label extension phase of our study presented herein. However, the sample of 39 children and adolescents recruited in our double-blind study (Kumra et al. 2008) was larger than any previous published controlled study in the literature (Kumra et al. 1996 [n = 21]; Shaw et al. 2006 [n = 25]) in children and adolescents with treatment-refractory schizophrenia. As discussed elsewhere, to recruit this sample of 39 subjects, we had to screen 248 subjects over a 5-year period (Kumra et al. 2008). Also, it was a difficult task to randomize subjects prospectively in the context of clinical urgency to initiate treatment. In general, children and adolescents with psychotic disorders represent a particularly difficult population to recruit into clinical trials despite the use of study designs that compare active treatments. Recruitment may be limited due to the relatively low incidence of schizophrenia in youth, a delay in diagnosis of schizophrenia in youth, a delay in acknowledgement of treatment-refractoriness on the part of clinicians, logistic obstacles related to enrolling subjects placed in child protective services, the increased burden on participants created by study assessments, the discomfort associated with administering blinded medications to children, and public skepticism regarding research participation (Sikich et al. 2004). Historically, these problems have resulted in the ascertainment of relatively small and heterogeneous samples of psychotic patients that have been treated with multiple medications rather than a single agent or placebo. In a controlled clinical trial with small groups, one could argue the rates of overall clinical response and the effect size difference on key symptom domains (i.e., positive and negative symptoms) would be of most interest. In this respect, the data from the acute phase trial were very clear and dramatic and supported the superiority of clozapine versus olanzapine (Kumra et al. 2008).
Another limitation of this study was its open-label nature and the issue of co-medications. We should emphasize, however, that our data differ from previous naturalistic observational data (e.g., Gerbino-Rosen et al. 2005). Specifically, we recruited a diagnostically homogeneous group of patients who were prospectively administered laboratory assessments and standardized clinical ratings by a centralized team of raters and by the use of random assignment in the original study. Although some of the patients were discharged from hospital prior to the completion of the study, the majority of the patients were seen at least once weekly throughout the first 6 months of treatment by the prescribing physician to monitor WBC counts.
Disclosures
Dr. Kumra has received honoraria and/or grant support from Janssen, Eli Lilly, Pfizer and Bristol-Myers Squibb. Dr. Kane has worked as a consultant for AstraZeneca, Aventis, Bristol-Meyers Squibb, Eli Lilly, Pfizer, Janssen, and Novartis. Drugs supplies were donated by the respective drug manufacturers. Drs. Kranzler, Gerbino-Rosen, Cullen, and Kane and Ms. Kester, Ms. DeThomas, and Mr. Regan have no conflicts of interest or financial ties to disclose.
Footnotes
This study was supported by National Institute of Mental Health grants P30 MH074543 to Dr. Kane and MH-60229 to Dr. Kumra.
Acknowledgments
We thank Carolyn Dombrowsky A.P.N.P, Laurie Nusser, M.Pharm., Guy Beauzile, M.D., Franz Moise, M.D., Christoph Correll, M.D., Nina Schooler Ph.D., Delbert Robinson, M.D., Margaret Woerner, Ph.D., the New York State Office of Mental Health, Sharon Carpinello R.N., Ph.D., and Mark D. Bienstock, M.P.A. for their support of and consultation to this project, as well as the children and families who participated. Additionally, we thank all of the physicians and nurses who provided care for the patients in this study.
References
- American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- Andreasen N. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1982. [Google Scholar]
- Bloch Y. Vardi O. Mendlovic S. Levkovitz Y. Gothelf D. Ratzoni G. Hyperglycemia from olanzapine treatment in adolescents. J Child Adolesc Psychopharmacol. 2003;13:97–102. doi: 10.1089/104454603321666234. [DOI] [PubMed] [Google Scholar]
- Buckley P. Miller A. Olsen J. Garver D. Miller DD. Csernansky J. When symptoms persist: Clozapine augmentation strategies. Schizophr Bull. 2001;27:615–628. doi: 10.1093/oxfordjournals.schbul.a006901. [DOI] [PubMed] [Google Scholar]
- Campbell M. Palig M. Subjective treatment emergent symptoms scale (STESS) Psychopharmacology Bull. 1985;21:1063–1082. [Google Scholar]
- Conley RR. Tamminga CA. Kelly DL. Richardson CM. Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry. 1999;46:73–77. doi: 10.1016/s0006-3223(99)00029-3. [DOI] [PubMed] [Google Scholar]
- Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: A systematic review and pooled analysis of short-term trials. J Am Acad Child Adolesc Psychiatry. 2007;46:687–700. doi: 10.1097/chi.0b013e318040b25f. [DOI] [PubMed] [Google Scholar]
- Correll CU. Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Correll CU. Frederickson AM. Kane JM. Manu P. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89:91–100. doi: 10.1016/j.schres.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM. Chen N. Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60:553–564. doi: 10.1001/archpsyc.60.6.553. [DOI] [PubMed] [Google Scholar]
- Dursun SM. McIntosh D. Milliken H. Clozapine plus lamotrigine in treatment-resistant schizophrenia. Arch Gen Psychiatry. 1999;56 doi: 10.1001/archpsyc.56.10.950. [DOI] [PubMed] [Google Scholar]
- Findling RL. Frazier JA. Gerbino-Rosen G. Kranzler HN. Kumra S. Kratochvil CJ. Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry. 2007;46:423–428. doi: 10.1097/chi.0b013e3180ed94e. [DOI] [PubMed] [Google Scholar]
- Freedman R. The choice of antipsychotic drugs for schizophrenia. N Engl J Med. 2005;353(12):1286–1288. doi: 10.1056/NEJMe058200. [DOI] [PubMed] [Google Scholar]
- Gerbino-Rosen G. Roofeh D. Tompkins DA. Feryo D. Nusser L. Kranzler H. Napolitano B. Frederickson A. Henderson I. Rhinwine J. Kumra S. Hematological adverse events in clozapine-treated children and adolescents. J Am Acad Child Adolescent Psychiatry. 2005;44:1024–1031. doi: 10.1097/01.chi.0000171904.23947.54. [DOI] [PubMed] [Google Scholar]
- Gothelf D. Bareket F. Singer P. Kairi M. Phillip M. Zigel L. Poraz I. Frishman S. Constantini N. Zalsman G. Abraham W. Apter A. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry. 2002;159:1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology revised edition. Rockville (Maryland): National Institute of Mental Health; 1976. [Google Scholar]
- Henderson DC. Clozapine: Diabetes mellitus, weight gain, lipid abnormalities. J Clin Psychiatry. 2001;62(Suppl 23):39–44. [PubMed] [Google Scholar]
- Henderson DC. Kunkel L. Nguyen DD. Borba CP. Daley TB. Louie PM. Freudenreich O. Cather C. Evins AE. Goff DC. An exploratory open-label trial of aripiprazole as an adjuvant to clozapine therapy in chronic schizophrenia. Acta Psychiatr Scand. 2006;114 doi: 10.1111/j.1600-0447.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- Henderson DC. Nguyen DD. Copeland PM. Hayden DL. Borba CP. Louie PM. Freudenreich O. Evins AE. Cather C. Goff DC. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: Results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66:1116–1121. doi: 10.4088/jcp.v66n0905. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klein DJ. Cottingham EM. Sorter M. Barton BA. Morrison JA. A randomized, double-blind, placebo-controlled trial of Metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072–2079. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- Kranzler HN. Roofeh D. Gerbino-Rosen G. Dombrowski C. McMeniman M. DeThomas C. Frederickson A. Nusser L. Beinstock MD. Fisch GS. Kumra S. Clozapine: its impact on aggressive behavior among children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2005;44:53–63. doi: 10.1097/01.chi.0000145371.23122.5a. [DOI] [PubMed] [Google Scholar]
- Kranzler HN. Kester HM. Gerbino-Rosen G. Kumra S. Treatment refractory schizophrenia in children and adolescents: An update on clozapine and other pharmacologic interventions. Child Adolesc Psychiatr Clin N Am. 2006;15:35–159. doi: 10.1016/j.chc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskaya L. Schulz C. McDougle CJ. Frazier JA. Dittmann R. Robertson-Plouch C. Bauer T. Xu W. Wang WV. Carlson J. Tohen M. A double-blind, placebo-controlled study of olanzapine in adolescents with schizophrenia. Schizophr Res. 2006;81:S44–S45. doi: 10.1097/CHI.0b013e3181900404. [DOI] [PubMed] [Google Scholar]
- Kumra S. Frazier JA. Jacobsen LK. McKenna K. Gordon CT. Lenane MC. Hamburger SD. Smith AK. Albus KE. Alaghband-Rad J. Rapoport JL. Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry. 1996;53:1090–1097. doi: 10.1001/archpsyc.1996.01830120020005. [DOI] [PubMed] [Google Scholar]
- Kumra S. Kranzler H. Gerbino-Rosen G. Kester HM. Dethomas C. Kafantaris V. Correll CU. Kane JM. Clozapine, “high-dose” olanzapine in refractory early-onset schizophrenia: A 12-week randomized and double-blind comparison. Biol Psychiatry. 2008;63:524–529. doi: 10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Lévy E. Agbokou C. Ferreri F. Chouinard G. Margolese HC. Topiramate-induced weight loss in schizophrenia: A retrospective case series study. Can J Clin Pharmacol. 2007;14(2):e234–239. [PubMed] [Google Scholar]
- Lieberman JA. Stroup TS. McEvoy JP. Swartz MS. Rosenheck RA. Perkins DO. Keefe RS. Davis SM. Davis CE. Lebowitz BD. Severe J. Hsiao JK Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- McClellan J. Sikich L. Findling RL. Frazier JA. Vitiello B. Hlastala SA. Williams E. Ambler D. Hunt-Harrison T. Maloney AE. Ritz L. Anderson R. Hamer RM. Lieberman JA. Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS): Rationale, design, methods. J Am Acad Child Adolesc Psychiatry. 2007;46 doi: 10.1097/CHI.0b013e3180691779. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. Lieberman JA. Stroup TS. Davis SM. Meltzer HY. Rosenheck RA. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry. 2006;16:600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Overall J. Gorham D. The brief psychiatric rating scale. Psychiatry Res. 1961;10:799–812. [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) 1999.
- Ratzoni G. Gothelf D. Brand-Gothelf A. Reidman J. Kikinzon L. Gal G. Phillip M. Apter A. Weizman R. Weight gain associated with olanzapine and risperidone in adolescent patients: A comparative prospective study. J Am Acad Child Adolesc Psychiatry. 2002;41:337–43. doi: 10.1097/00004583-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Shaffer D. Gould MS. Brasic J. Ambrosini P. Fisher P. Bird H. Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shaw P. Sporn A. Gogtay N. Overman GP. Greenstein D. Gochman P. Tossell JW. Lenane M. Rapoport JL. Child-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63:721–730. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]
- Sikich L. Hamer RM. Bashford RA. Sheitman BB. Lieberman J. A pilot study of Risperidone, Olanzapine, and Haloperidol in psychotic youth: A double-blind, randomized, 8-week trial. Neuropsychopharmacology. 2004;29:133–145. doi: 10.1038/sj.npp.1300327. [DOI] [PubMed] [Google Scholar]
- Simpson GM. Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Woerner MG. Mannuzza S. Kane JM. Anchoring the BPRS: An aid to improved reliability. Psychopharmacol Bull. 1988;24:112–117. [PubMed] [Google Scholar]