Abstract
Objective
The aim of this study was to assess the effectiveness and tolerability of a long-acting methylphenidate (MPH) formulation, beaded MPH (B-MPH), for treatment of attention-deficit/hyperactivity disorder (ADHD) in 4- and 5-year-old children.
Method
Eleven children (9 boys and 2 girls) with ADHD received 4 weeks of B-MPH treatment in a single-site, open-label pilot study. Medication dosing was flexible, with titration to a maximum of 30 mg/day. A brief education session on behavior management was offered to parents at each treatment visit.
Results
Subjects experienced a mean decrease of 1.09 (standard deviation [SD] = 0.73, p < 0.01) on the Swanson, Nolan, and Pelham Questionnaire (SNAP-IV) ADHD composite score to an end point of 1.18 (SD = 0.64). Subjects demonstrated mean decreases in scores of inattention of 1.01 (SD = 0.85, p < 0.01) and in hyperactivity/impulsivity of 1.17 (SD = 0.74, p < 0.01), with end point scores of 1.10 (SD = 0.61) and 1.26 (SD = 0.77), respectively. The Clinical Global Impressions-Severity (CGI-S) scale showed a statistically significant improvement from a baseline mean of 5 to the final visit mean of 3.36 (p < 0.01). At the final visit, the mean daily B-MPH dose was 17.73 mg. Subjects did not experience any statistically significant changes in weight, blood pressure, or pulse during the study. The most common adverse event was decreased appetite.
Conclusion
B-MPH was safe and effective for the treatment of ADHD in the 4- and 5-year-olds participating in this study.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most commonly diagnosed childhood psychiatric disorders. Although it has been thoroughly researched in school-aged children, only limited data are available about its manifestation and treatment in preschoolers. Recent research suggests that ADHD has a prevalence rate ranging from 2 to 5.7% and can be accurately diagnosed in this age group (Lavigne et al. 1996; DuPaul et al. 2001; Egger and Angold 2006). In a study of over 500 children, preschoolers were described as having similar co-morbidities to those present in school-aged children with ADHD. Furthermore, comparable difficulties were observed in school and social settings, as well as in overall functioning (Wilens et al. 2002). Studies that examined the clinical presentation of ADHD reported that preschoolers tend to exhibit more disruptive behaviors and are less socially skilled than their unaffected counterparts. They also have more negative social behavior and score significantly lower on tests of preacademic skills. These factors place preschoolers with ADHD at risk for falling behind both psychologically and educationally (Du-Paul et al. 2001). A review by Sonuga-Barke and colleagues (Sonuga-Barke et al. 2004) described preschool ADHD as a threat to normal development and called for studies to investigate its treatment.
The pharmacological treatment of ADHD in preschoolers has not been extensively studied, with only 10 out of 160 pediatric controlled clinical trials conducted in this age group (Kratochvil et al. 2007). Furthermore, study samples have often combined preschoolers with school-aged children, thus yielding underpowered results for analyses of treatment efficacy and safety. Early in the decade, several small studies investigated methylphenidate (MPH) in preschoolers, and a review published in 2000 (Conners 2000) concluded that it was an effective treatment modality in this population.
To address the lack of randomized controlled clinical trials, in 2001, the National Institute of Mental Health (NIMH) launched the multisite Preschool ADHD Treatment Study (PATS) (Kollins et al. 2006). The study sample included a total of 303 children, ages 3–5. All subjects were first treated with behavioral therapy. Subsequently, nonresponders were randomized to medication treatment with immediate-release MPH (IR-MPH) or placebo. The mean optimal IR-MPH daily dose was 0.75 mg/kg per day (14 mg), which is lower than the mean 1.0 mg/kg per day optimal dose reported for school-aged subjects in the Multimodal Treatment of ADHD Study (MTA) (Greenhill et al. 2006). In both PATS and MTA, children treated with active MPH reported a significant reduction in ADHD symptoms compared to those assigned to placebo treatment. However, the adverse event prevalence rate was significantly higher in preschoolers than that in older children participating in the MTA and other clinical trials.
While PATS helped establish MPH as a safe and effective treatment in younger children, there were no comparable studies investigating long-acting MPH formulations in this age group (Gleason et al. 2007). A long-acting formulation would have a clear utility for preschoolers, because its once-daily administration obviates the necessity of noontime dosing. However, because long-acting formulations offer less flexible dosing strategies than those available for short-acting stimulants, one concern would be their tolerability in preschoolers who tend to be more vulnerable to side effects (Wigal et al. 2006).
This pilot study evaluated a long-acting MPH formulation, otherwise known as beaded MPH (B-MPH) or Ritalin LA™, for ADHD treatment in 4 to 5 year olds. The Food and Drug Administration (FDA) approved B-MPH in 2002 for the treatment of ADHD in children 6 years and older. This medication requires a once-daily administration, because MPH is distributed throughout the day, with the first 50% immediately after absorption and the remaining 50% approximately 4 hours later, mimicking the effects of a twice-daily dosing regimen (Lopez et al. 2003). The capsules may be opened and the contents sprinkled on food without any diminution in the duration of action of the medication. This administration method offers an advantage for preschoolers, because many patients in this age group are not yet able to swallow pills.
Methods
Subjects
Eleven subjects, 9 boys and 2 girls aged 4–5 inclusive, were enrolled in the study. The mean age at the time of consent was 5.1 years. Eight subjects were Hispanic. All children received an ADHD diagnosis based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000), with symptoms present for at least 9 months. The diagnosis was confirmed by a psychiatric evaluation, semistructured diagnostic interview, and history completed by a child psychiatrist. Ten children met criteria for ADHD combined subtype and 1 child was diagnosed with ADHD hyperactive/impulsive subtype. One subject was co-morbid for oppositional defiant disorder (ODD). Parents and children had to speak English or Spanish. Only children who were enrolled in an educational setting with at least 8 same-age peers for at least 2 half-days per week were eligible for the study. Five subjects were attending preschool, 4 were enrolled in kindergarten, and 2 were in first grade. Exclusion criteria included history of intolerance or nonresponse to stimulants, current adjustment disorders, autism, psychosis, bipolar disorder, or suicidality. Children with a history of significant physical, sexual, or emotional abuse and medical abnormalities that would make use of B-MPH clinically inappropriate were also excluded. Concomitant treatments with antihypertensives, medication affecting blood pressure or heart rate, sedating antihistamines, antiseizure medications, diphenhydramine, and/or other psychotropic agents were not allowed during the study.
Prior to initiating the study procedures, the child psychiatrist met with the families to review the consent document, which included risk and benefits of treatment and alternatives to study treatment. There was an oral description and discussion of the study, and written informed consent was obtained from a parent or guardian for each subject. The study was reviewed and approved by the New York State Psychiatric Institute/Columbia University Institutional Review Board (IRB) and was conducted in accordance with the ethical standards of the 1975 Declarations of Helsinki as revised in 2000 (World Medical Association).
Study design
The study was conducted at the New York State Psychiatric Institute (NYSPI), Columbia University in New York, New York. Subjects were recruited through the NYSPI Child and Adolescent Psychiatric Evaluation Service (CAPES), which provides comprehensive evaluations at no cost to families referred by community pediatricians. Children who were diagnosed with ADHD were referred to the study for further evaluation. The study included a screening visit, a 1-week washout (if needed), a baseline visit, and 4 weeks of B-MPH treatment.
Measures
The initial CAPES assessment included the Conners' Parent Rating Scale-Revised (CPRS-R) (Conners et al. 1998a) and Conners' Teacher Rating Scale-Revised (CTRS-R) (Conners et al. 1998b), which were used to inform the ADHD diagnosis. Diagnosis and inclusion in the study were dependent on-subjects meeting DSM-IV-TR criteria for ADHD inattentive, hyperactive-impulsive, or combined type. At the screening visit, the child psychiatrist reviewed the CAPES diagnostic evaluation, inquired about concomitant medications and adverse events, and administered the Clinical Global Impressions-Severity Scale (CGI-S) (Guy 1976) and Children's Global Assessment Scale (C-GAS) (Shaffer et al. 1983). Parents completed the Swanson, Nolan and Pelham Questionnaire (SNAP-IV), which includes 18 items of ADHD symptoms indicated in the DSM-IV. Each symptom was scored on a 4-point scale, from 0 = Not at all to 3 = Very much (Swanson 1992). Subsequently, these scales were completed at each visit. Following baseline, the Clinical Global Impressions-Improvement (CGI-I) was also administered at every visit.
Study visits
At the screening visit, the child psychiatrist assessed the ADHD symptom severity and ascertained the presence or absence of other co-morbid disorders using a semistructured interview that was developed in PATS. This interview includes questions based on the DSM-IV definition of ADHD. The CGI-S and C-GAS were also completed. To be eligible for the study, subjects had to have a CGI-S ≥4 (Moderately Mentally Ill) and a C-GAS ≤55. The Kaufman Brief Intelligence Test (K-BIT) was administered to confirm an intelligence quotient (IQ) ≥70.
After the screening visit, eligible subjects returned to the clinic for a baseline visit, during which B-MPH was initiated at 10mg/day. Dose–response was defined by the subject having a CGI-S of 2 or 1. Subjects who did not respond to this dose were titrated to 20 mg/day at visit 4 if they were not experiencing adverse events and the parents agreed to the increase. For those not responding at 20 mg, the dose could be increased again to 30 mg at visit 5.
Parents were instructed to administer the medication once daily in the morning. For subjects who could not swallow the capsule, it could be opened and the contents sprinkled on food (e.g., apple sauce, ice cream). Novartis Pharmaceuticals Corporation, the manufacturer of B-MPH, supplied the study medication.
Following IRB-recommended guidelines suggesting that treatment of ADHD in this age group include a nonpharmacological intervention, the study used a parent-training component. During the 4-week treatment period, the parents participated in 15-minute individual parent-training sessions. These sessions were designed according to an abbreviated protocol that was based on the Helping the Noncompliant Child manual (McMahon and Forehand 2003). The parent training aimed to identify problem behaviors and causes of disruptions at home, as well as with family and friends. It included behavioral reinforcement and practical suggestions on structuring time-outs. A Spanish version of the protocol was implemented for Spanish-speaking parents.
Safety analyses
The study pediatrician conducted a physical examination and obtained a medical history at the screening visit. A physical examination was repeated at visit 6 or at the last study visit for subjects who terminated the study early. Vital signs (i.e., weight, blood pressure, pulse rate) were measured at each visit. Height was obtained at the screening visit only. No laboratory testing was performed during the study. Using open-ended questions, the child psychiatrist elicited information about concomitant medications and adverse events at baseline and at each subsequent visit.
Data analysis
Statistical analyses were conducted using SPSS 15.0 (SPSS Inc, Chicago, IL). The SNAP scores were calculated for the inattentive and hyperactive/impulsive ADHD subscales and for the composite score as well. All of these were primary-outcome measures. The calculations were performed by taking the mean of the respective items in each subscale. Paired-sample t-tests were used to evaluate mean difference scores on each of the measures at baseline and visit 6 (early termination). The frequency of adverse events was also calculated using SPSS.
Results
Sample retention
Of the 11 subjects who signed consent, 3 subjects (27%) discontinued the study due to adverse events. Their individual reasons for early termination were, respectively, intolerable stomach ache and emesis, increased irritability, and sedation. Eight subjects completed the entire study (Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics (Prior to Treatment)
| Entered treatment | Completed treatment | Discontinued treatment | |
|---|---|---|---|
| n | 11 | 8 | 3 |
| Age, years, mean (SD) | 5.1 (.71) | 5.4 (.66) | 4.6 (.50) |
| 4-Year-olds, n (%) | 5 (45%) | 2 (18%) | 3 (27%) |
| 5-Year-olds | 6 (55%) | 6 (55%) | 0 |
| Gender, n (%) | |||
| Male | 9 (82%) | 7 (64%) | 2 (18%) |
| Female | 2 (18%) | 1 (9%) | 1 (9%) |
| Race/ethnicity, n (%) | |||
| White | 3 (27%) | 2 (18%) | 1 (9%) |
| African American | |||
| Hispanic | 8 (73%) | 6 (55%) | 2 (18%) |
| Asian | |||
| American Indian | |||
| ADHD type, n (%) | |||
| Hyperactive-impulsive | 1 (9%) | 1 (9%) | 0 (0%) |
| Combined | 10 (91%) | 7 (64%) | 3 (27%) |
| Co-morbidity, n (%) | |||
| ODD | 1 (9%) | 0 (0%) | 1 (9%) |
| Grade in school, n (%) | |||
| Preschool | 5 (45.5%) | 3 (27%) | 2 (18%) |
| Kindergarten | 4 (36.4 %) | 3 (27%) | 1 (9%) |
| First grade | 2 (18.2%) | 2 (18%) | |
| Prior treatment with psychotropic medication, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| C-GAS, mean (SD) | 49.45 (5.34) | 49.25 (5.78) | 50.00 (5.00) |
Abbreviations: ADHD = attention-deficit/hyperactivity disorder; ODD = oppositional defiant disorder; SD = standard deviation; C-GAS = Children's Global Assessment Scale.
Adverse events
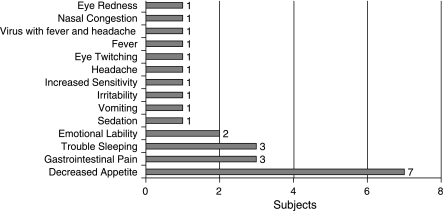
The most commonly reported adverse event was decreased appetite, which was experienced by 7 subjects (64%) following treatment initiation. Five out of these 7 subjects continued to report decreased appetite for the duration of the study. Difficulty sleeping occurred in 3 subjects (27%). Emotional lability and gastrointestinal pain were reported by 2 subjects (18%). These adverse events were resolved by the end of the study (Fig. 1). There were no adverse events judged as severe. One subject who terminated the study early experienced moderate levels of insomnia, vomiting, decreased appetite, and stomach pain, and another who also terminated early experienced moderate irritability. All other adverse events were coded as mild.
FIG. 1.
Adverse events.
Overall severity of illness and level of impairment
At baseline, all subjects were rated as markedly mentally ill with the CGI-S score of 5. The mean end point CGI-S score was 3.36 (standard deviation [SD] = 1.29), with a mean decrease of 1.64 (SD = 1.29, p < 0.01), indicating a significant reduction in the severity of illness. At their last study visit, 6 subjects (55%) were rated as much improved on the CGI-I scale, 1 subject (9%) was minimally improved, and 4 (36%) were rated as no change.
ADHD symptoms
The mean baseline SNAP-IV ADHD composite score was 2.27 (SD = 0.40), with a baseline inattention score of 2.11 (SD = 0.44) and hyperactivity/impulsivity score of 2.43 (SD = 0.54). The mean end point ADHD composite score was 1.18 (SD = 0.64), with a mean decrease of 1.09 (SD = 0.73, p < 0.01); the inattention score was 1.10 (SD = 0.61), with a mean decrease of 1.01 (SD = 0.85, p < 0.01); hyperactivity/impulsivity score was 1.26 (SD = 0.77), with a mean decrease of 1.17 (SD = 0.74, p < 0.01). These results show a significant reduction in the ADHD composite score, as well as in the inattention and hyperactivity/impulsivity scales (see Table 2).
Table 2.
Symptom and Functioning Scores
| Rating Scale | Measure of | Rater | Baseline mean (SD) | Endpoint mean (SD) | Baseline vs. end point mean change (SD) | Baseline vs. end point Paired t-test: t(p) |
|---|---|---|---|---|---|---|
| SNAP-IV | ADHD symptoms | Parent | ||||
| Inattention | 2.11 (0.44) | 1.10 (0.61) | −1.01 (0.85) | 3.942 (<0.01) | ||
| Hyperactivity/Impulsivity | 2.43 (0.54) | 1.26 (0.77) | −1.17 (0.74) | 5.268 (<0.01) | ||
| ADHD symptoms composite | 2.27 (0.40) | 1.18 (0.64) | −1.09 (0.73) | 4.988 (<0.01) | ||
| CGI-S | Severity of illness | Clinician | 5.00 (0.00) | 3.36 (1.29) | −1.64 (1.29) | 4.219 (<0.01) |
| C-GAS | Functioning | Clinician | 49.45 (5.34) | 60.00 (7.17) | 10.55 (7.70) | 4.543 (<0.01) |
Abbreviations: SD = standard deviation; SNAP–IV = Swanson, Nolan and Pelham questionnaire; ADHD = attention-deficit/hyperactivity disorder; CGI-I = Clinical Global Impressions–Improvement; C-GAS = Children's Global Assessment Scale.
Level of functioning
The mean baseline C-GAS score was 49.45 (SD = 5.34). The score increased to 60.00 (7.17) by the last study visit, thus indicating significant improvement in the level of functioning (p < 0.01).
Medication dose
The most frequently administered B-MPH dose was 10 mg/day, the lowest dose allowed in the study. At the end of the study, the mean B-MPH dose was 17.73 mg/day (SD = 6.07). Six of the subjects received B-MPH doses of 20 mg/day, and only 1 of these subjects was titrated up to 30 mg/day, the highest dose permitted in the study. All of the 5 subjects who stayed at the lowest dose did so because of side effects. None of these subjects achieved a CGI-S of 2 or 1 by the end of the study.
Cardiovascular and weight parameters
Subjects did not experience any statistically significant changes in weight, blood pressure, or pulse during the study. The mean baseline weight was 21.44 kg (SD = 3.75). At visit 6 (early termination), the mean weight was 21.30 kg (SD = 3.53), which indicated a decrease of 0.15 kg (SD = 0.92, p = 0.61). The mean baseline systolic blood pressure was 96.18 mmHg (SD = 14.02), and the mean diastolic blood pressure was 61.36 mmHg (SD = 13.74). The mean end point systolic blood pressure was 96.09 mmHg (SD = 10.05), with a mean decrease of 0.09 (SD = 13.07, p = 0.98); and the mean end point diastolic blood pressure was 60.91 mmHg (SD = 14.49), with a mean decrease of 0.46 (SD = 21.66, p = 0.95). The mean baseline pulse was 95.45 beats/minute (bpm) (SD = 16.00), and the mean end point pulse was 91.82bpm (SD = 12.24), with a mean decrease of 3.64 (SD = 22.00, p = 0.60) (Table 3).
Table 3.
Weight, Blood Pressure, and Pulse at Baseline and End Point
| Measures | Baseline mean (SD) | End point mean (SD) | Baseline vs. end point mean change (SD) | Baseline vs. end point paired t-test: t(p) |
|---|---|---|---|---|
| Weight (kg) | 21.44 (3.75) | 21.30 (3.53) | −0.15 (0.92) | 0.525 (0.61) |
| Systolic BP (mmHg) | 96.18 (14.02) | 96.09 (10.05) | −0.91 (13.07) | 0.023 (0.98) |
| Diastolic BP (mmHg) | 61.36 (13.74) | 60.91 (14.49) | −0.46 (21.66) | 0.070 (0.95) |
| Pulse (bpm) | 95.45 (16.00) | 91.82 (12.24) | −3.64 (22.00) | 0.548 (0.60) |
Abbreviations: SD = standard deviation; BP = blood pressure; bpm = beats per minute.
Discussion
This was a pilot study using a common treatment, MPH, in a slightly less common long-acting preparation to treat ADHD in a relatively unstudied population of 4–5 year olds. B-MPH was selected because it does not require repeated dosing throughout the day and because the capsule can be opened and sprinkled on food without detracting from its efficacy or duration of action.
Demographically, our sample was close to being evenly divided between 4- and 5-year-old children. The preponderance of boys over girls (9:2) may reflect a bias toward males in ADHD identification at this early age. The ethnic concentration of Hispanic families is characteristic of the population in the community located in the vicinity of our medical center. It is worth noting that all subjects were medication naive, likely suggesting not only the young age of our subjects, but also the limited availability of child psychiatric services for this age group. All subjects met the DSM-IV criteria for ADHD diagnosis, combined or hyperactive-impulsive subtype.
While the young children in our study showed clinically meaningful improvement, their ratings indicated that they did not achieve the remission level that might have been anticipated based on the MTA findings. Although the SNAP scores improved convincingly throughout the study, with a drop in mean ADHD composite score from 2.27 to 1.18 (p < 0.01) and significant declines in both hyperactive (p < 0.01) and inattentive scores (p < 0.01), they did not reach the benchmark of the SNAP ADHD score of less than 1, which had been characterized as an “excellent response” in the MTA study (Swanson et al. 2001). The percentage of children attaining a composite score of 1 was 45% in our sample compared with 56% in the MTA sample and 22% in the PATS study (Greenhill et al. 2006). If one is to ignore the different scales of the respective studies and compare the current study to the PATS (3–5.5 year olds) and MTA (6–12 year olds) studies, it appears that the younger cohorts of children are less likely to have an excellent response than older school-aged children. This significant though incomplete improvement in symptomatology was also reflected in the CGI-S, which dropped from 5 (“markedly ill”) at the beginning of the study to 3.36 (SD = 1.26; slightly higher than the anchor for “mildly ill”) at the final visit. None of the children achieved a score of 1 (“not at all ill”). A similar decrease in the severity of illness was reported in PATS, in which the CGI-S score was 3.07 (SD = 1.11) (corresponding to “mildly ill”) at the end of study (Vitiello et al. 2007).
Similarly, the C-GAS had a meaningful mean increase from 49.45 (SD = 5.34) at baseline to 60 (SD = 7.17) at the end of treatment, but no individual score reached 70, which is the benchmark for normal clinical functioning. These results are also consistent with the PATS findings that reported the C-GAS score mean increase from 47.33 (SD = 4.07) at study entry to 65.76 (SD = 2.82) at the end of treatment maintenance (Vitiello et al. 2007).
A possible factor limiting the efficacy of B-MPH in bringing about an even more robust improvement in symptoms is the vulnerability of younger children to adverse events and the collateral effect this had on slowing and restricting the titration of medication doses. B-MPH was most frequently administered at its lowest available dose (10mg/day), with only 1 subject reaching the highest dose (30 mg/day) by the end of treatment. Furthermore, all subjects who ended the study at the lowest dose had CGI-S ratings of 3 or higher, indicating that side effects may indeed have limited the ability to treat their symptoms more thoroughly. It is possible that if patients were able to tolerate side effects, then higher doses would have resulted in increased efficacy, as demonstrated in the MTA study, where the school-aged subjects had optimal responses at higher MPH doses (Greenhill et al. 2001). In our preschool group, similarly to the larger placebo-controlled PATS sample, subjects had a vigorous response but did not achieve remission.
At the other end of the dosing spectrum, the 10-mg denominations of B-MPH tablets did not allow for a finer titration. Following study completion, several subjects continued treatment with B-MPH at intermediate doses but, finding that the titration done in 10-mg increments offered either too-high dosing leading to adverse events or too-low dosing without significant therapeutic effects, parents were instructed to open the capsules and administer half of the contents, roughly 5 mg, each day, leading to positive outcomes. This lack of dose tolerability in young children is consistent with the PATS data, showing that preschoolers have significantly lower MPH clearance compared to that of the school-aged children, even when the dosing is corrected for weight (Wigal et al. 2006). Therefore, future research may benefit from 5-mg and 2.5-mg B-MPH tablet formulations, which will allow enhancing the therapeutic effect while avoiding the occurrence of adverse events.
With our dosing regimen, the most commonly reported adverse event was decreased appetite. It was reported by 7 subjects (64%) shortly following the treatment initiation and continued in 5 subjects through the end of treatment (45%). Trouble sleeping was experienced by 3 subjects (27%), while mood lability and gastrointestinal pain were reported by 2 subjects (18%). The prevalence of these adverse events was along the lines of that seen in the PATS study (Wigal et al. 2006) and greater than that seen in the MTA study, thus underscoring the relative vulnerability of younger children to adverse events.
Despite the reported adverse events, there were no significant changes in vital signs noted. Furthermore, even weight showed no significant change, which suggested one of two possibilities: Either losses in the weight percentile were obscured by looking at weight alone—although this is unlikely based on the short study duration—or children were able to compensate for decreased appetite before and after the medication took effect. Indeed, we often counseled families to serve larger breakfasts and evening snacks to make up for decreased appetite at lunchtime.
The conclusions of this study are limited by its small size, the open-label design, and the possible confound of the parent intervention. To follow up on the findings and to characterize better both similarities and differences between preschool-and school-aged populations, a placebo-controlled trial using a long-acting MPH formulation would be useful, perhaps employing finer dosing gradations to maximize the medication benefits while minimizing adverse events.
A commentary on the PATS results (Kuehn 2007) notes that, although MPH use in preschoolers has been controversial due to the paucity of data, PATS provides evidence about its efficacy and safety for ADHD treatment in this population. Our study provides preliminary open-label data that support long-acting MPH treatment in preschoolers. These data can be added to the existing research on mixed amphetamine salts and preliminary data on atomoxetine treatment (Kratochvil et al. 2007), thus serving to expand the pharmacopoeia for the treatment and amelioration of ADHD symptoms in preschoolers and to guide the way for larger placebo-controlled clinical trials.
Conclusion
This open-label study found B-MPH to be an effective and well-tolerated option for the ADHD treatment in preschoolers. However, subjects reported higher rates of adverse events than would have been expected from the available school-age data. Close monitoring and careful medication titration are clearly indicated in treating preschoolers with stimulants. Future double-blind, placebo-controlled trials are recommended in this age group, possibly with lower-dose preparations to allow for a more finely tuned medication titration.
Disclosures
Dr. Maayan receives grant support from Eli Lilly and Pfizer. Dr. Greenhill received support from Novartis in the form of medication supplies to conduct this study. For the past 2 years, he has a consultant arrangement with Pfizer Pharmaceuticals to serve as chair of their Ziprasidone Pediatric Clinical Trials Data and Safety Monitoring Board. He has been awarded a research contract to study risperidone by Johnson and Johnson pharmaceuticals. Finally, he has been awarded an investigator-initiated grant to study aripiprazole by Otsuka. No financial support was received from Novartis Pharmaceuticals. Ms. Paykina, Dr. Fried, Ms. Strauss, and Ms. Gugga have no conflicts of interest or financial ties to disclose.
References
- American Psychiatric Association. Diagnostic, statistical manual of mental disorders. 4th. Washington (DC): American Psychiatric Association; 2000. (DSM-IV-TR) [Google Scholar]
- Conners CK. Attention-deficit/hyperactivity disorder—historical development and overview. J Attention Disord. 2000;3:173–191. [Google Scholar]
- Conners CK. Sitarenios G. Parker JD. Epstein JN. Revision and restandardization of the Conners' Teacher Rating Scale (CTRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998a;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Conners CK. Sitarenios G. Parker JD. Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998b;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ. McGoey KE. Eckert TL. VanBrakle J. Preschool children with attention-deficit/hyperactivity disorder: Impairments in behavioral, social, and school functioning. J Am Acad Child Adolesc Psychiatry. 2001;40:508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Egger HL. Angold A. Common emotional and behavioral disorders in preschool children: Presentation, nosology, and epidemiology. J Child Psychol Psychiatry. 2006;47:313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Gleason MM. Egger HL. Emslie GJ. Greenhill LL. Kowatch RA. Lieberman AF. Luby JL. Owens J. Scahill LD. Scheeringa MS. Stafford B. Wise B. Zeanah CH. Psychopharmacological treatment for very young children: Contexts and guidelines. J Am Acad Child Adolesc Psychiatry. 2007;46:1532–1572. doi: 10.1097/chi.0b013e3181570d9e. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Swanson JM. Vitiello B. Davies M. Clevenger W. Wu M. Arnold LE. Abikoff HB. Bukstein OG. Conners CK. Elliott GR. Hechtman L. Hinsha SP. Hoza B. Jensen PS. Kraemer HC. March JS. Newcorn JH. Severe JB. Wells K. Wigal T. Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40:180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Kollins S. Abikoff H. McCracken JT. Riddle M. Swanson J. McGough J. Wigal S. Wigal T. Vitiello B. Skrobala A. Posner K. Ghuman J. Cunningham C. Davies M. Chuang S. Cooper T. Efficacy and safety of immediate-release methylphenidate for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284–1293. doi: 10.1097/01.chi.0000235077.32661.61. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. 2nd. Washington (DC): U.S. Government Printing Office; 1976. DHEW Publication 76–388. [Google Scholar]
- Kollins S. Greenhill L. Swanson J. Wigal S. Abikoff H. McCracken J. Riddle M. McGough J. Vitiello B. Wigal T. Skrobala A. Posner K. Ghuman J. Davies M. Cunningham C. Bauzo A. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS) J Am Acad Child Adolesc Psychiatry. 2006;45:1275–1283. doi: 10.1097/01.chi.0000235074.86919.dc. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ. Vaughan BS. Mayfield-Jorgensen ML. March JS. Kollins SH. Murray DW. Ravi H. Greenhill LL. Kotler LA. Paykina N. Biggins P. Stoner J. A pilot study of atomoxetine in young children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:175–185. doi: 10.1089/cap.2006.0143. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Scientists examine benefits, risks of treating preschoolers with ADHD drugs. JAMA. 2007;298:1747–1749. doi: 10.1001/jama.298.15.1747. [DOI] [PubMed] [Google Scholar]
- Lavigne JV. Gibbons RD. Christoffel KK. Arend R. Rosenbaum D. Binns H. Dawson N. Sobel H. Isaacs C. Prevalence rates and correlates of psychiatric disorders among preschool children. J Am Acad Child Adolesc Psychiatry. 1996;35:204–214. doi: 10.1097/00004583-199602000-00014. [DOI] [PubMed] [Google Scholar]
- Lopez F. Silva R. Pestreich L. Muniz R. Comparative efficacy of two once daily methylphenidate formulations (Ritalin LA and Concerta) and placebo in children with attention deficit hyperactivity disorder across the school day. Paediatr Drugs. 2003;5:545–555. doi: 10.2165/00148581-200305080-00005. [DOI] [PubMed] [Google Scholar]
- McMahon RJ. Forehand RL. Family-Based Treatment for Oppositional Behavior. New York: The Guilford Press; 2003. Helping the Noncompliant Child. [Google Scholar]
- Shaffer D. Gould MS. Brasic J. Ambrosini P. Fisher P. Bird H. Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. Swanson JM. Coghill D. DeCory HH. Hatch SJ. Efficacy of two once-daily methylphenidate formulations compared across dose levels at different times of the day: Preliminary indications from a secondary analysis of the COMACS study data. BMC Psychiatry. 2004;4:28. doi: 10.1186/1471-244X-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM. School-Based Assessments Interventions for ADD Students. Irvine (California): K.C. Publications; 1992. [Google Scholar]
- Swanson JM. Kraemer HC. Hinshaw SP. Arnold LE. Conners CK. Abikoff HB. Clevenger W. Davies M. Elliott GR. Greenhill LL. Hechtman L. Hoza B. Jensen PS. March JS. Newcorn JH. Owens EB. Pelham WE. Schiller E. Severe JB. Simpson S. Vitiello B. Wells K. Wigal T. Wu M. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Abikoff HB. Chuang SZ. Kollins SH. McCracken JT. Riddle MA. Swanson JM. Wigal T. McGough JJ. Ghuman JK. Wigal SB. Skrobala AM. Davies M. Posner K. Cunningham C. Greenhill LL. Effectiveness of methylphenidate in the 10-month continuation phase of the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) J Child Adolesc Psychopharmacol. 2007;17:593–604. doi: 10.1089/cap.2007.0058. [DOI] [PubMed] [Google Scholar]
- Wigal T. Greenhill L. Chuang S. McGough J. Vitiello B. Skrobala A. Swanson J. Wigal S. Abikoff H. Kollins S. McCracken J. Riddle M. Posner K. Ghuman J. Davies M. Thorp B. Stehli A. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1294–1303. doi: 10.1097/01.chi.0000235082.63156.27. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Biederman J. Brown S. Tanguay S. Monuteaux MC. Blake C. Spencer TJ. Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41:262–268. doi: 10.1097/00004583-200203000-00005. [DOI] [PubMed] [Google Scholar]