Abstract
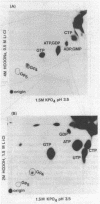
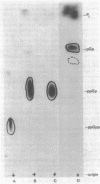
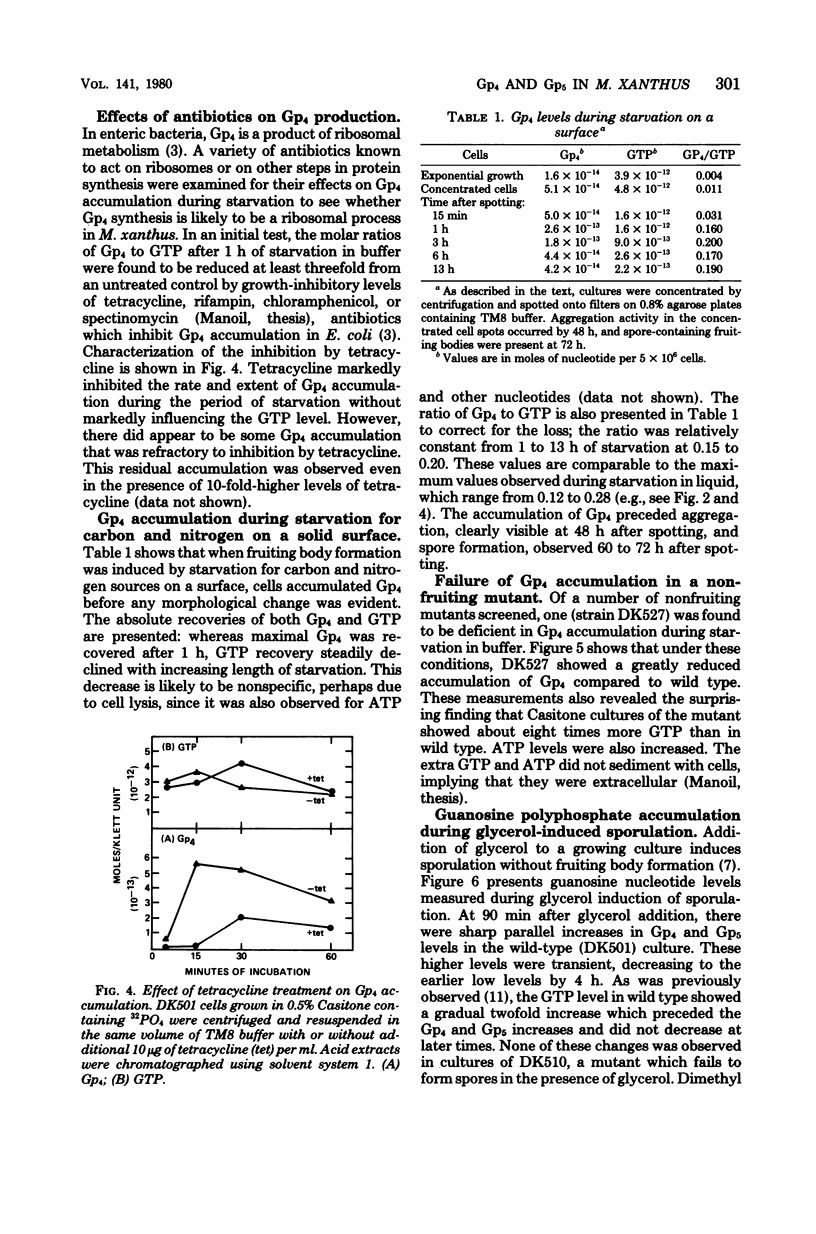
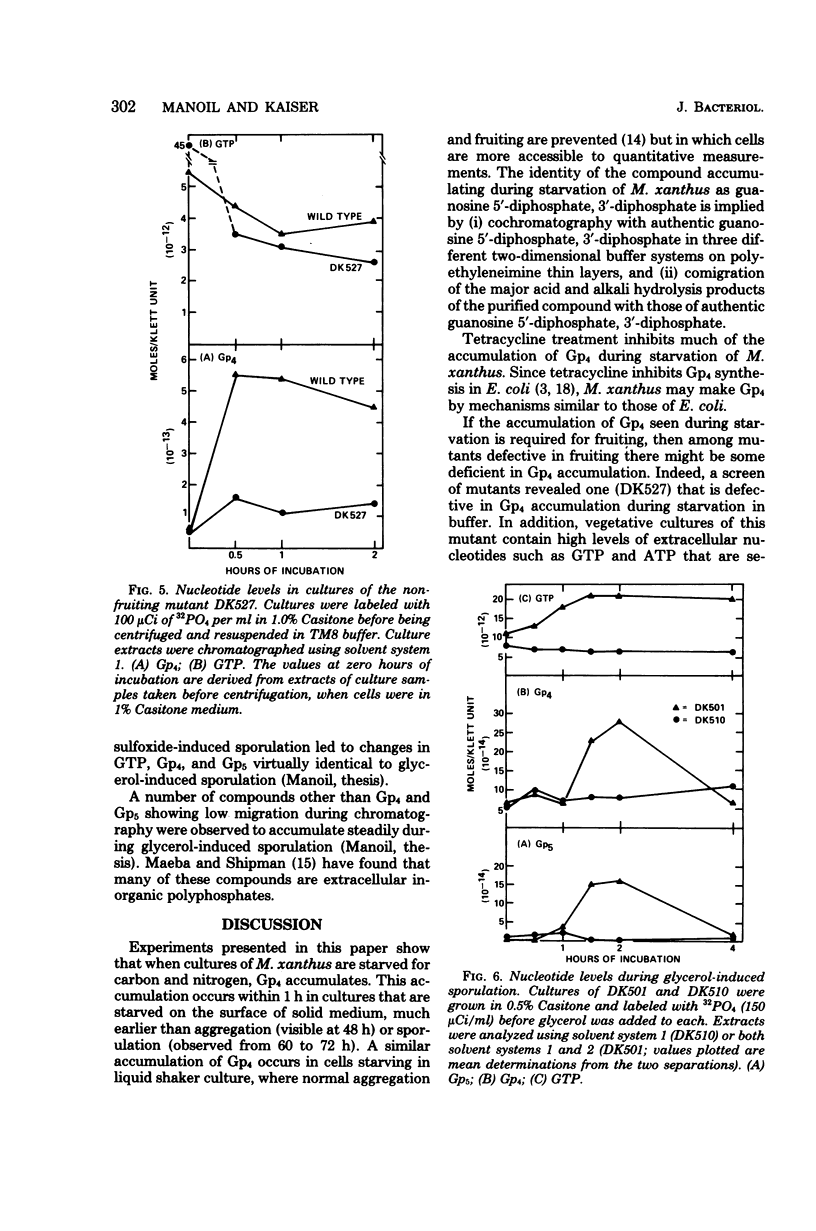
Cultures of Myxococcus xanthus develop multicellular fruiting bodies when starved for carbon and nitrogen sources on an agar surface. Under these conditions of severe starvation, cultures rapidly accumulated a compound identified as guanosine tetraphosphate by chromatographic migration of the compound and of its major acid and alkali breakdown products. The accumulation of guanosine tetraphosphate was reduced in the presence of tetracycline, indicating that it may be synthesized by mechanisms similar to those of Escherichia coli. The guanosine tetraphosphate level was also reduced in starved cultures of a mutant unable to fruit normally, although it has been determined whether the defect in guanosine tetraphosphate accumulation is responsible for the inability to fruit. Induction of spores by glycerol addition led to transient increases in both guanosine tetraphosphate and guanosine pentaphosphate at a stage following most cell shortening, but before spores had acquired full refractility.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- Gallant J., Shell L., Bittner R. A novel nucleotide implicated in the response of E. coli to energy source downshift. Cell. 1976 Jan;7(1):75–84. doi: 10.1016/0092-8674(76)90257-9. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hanson C. W., Dworkin M. Intracellular and extracellular nucleotides and related compounds during the development of Myxococcus xanthus. J Bacteriol. 1974 May;118(2):486–496. doi: 10.1128/jb.118.2.486-496.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Involvement of the relA gene product and feedback inhibition in the regulation of DUP-N-acetylmuramyl-peptide synthesis in Escherichia coli. J Bacteriol. 1978 Sep;135(3):766–774. doi: 10.1128/jb.135.3.766-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- MCVITTIE A., MESSIK F., ZAHLER S. A. Developmental biology of Myxococcus. J Bacteriol. 1962 Sep;84:546–551. doi: 10.1128/jb.84.3.546-551.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba P. Y., Shipman R. Incorporation of 32Pi into nucleotides, polyphosphates, and other acid-soluble compounds by Myxococcus xanthus during myxospore formation. J Bacteriol. 1978 Dec;136(3):1058–1069. doi: 10.1128/jb.136.3.1058-1069.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Gallant J. A gene involved in the metabolic control of ppGpp synthesis. Mol Gen Genet. 1978 Jan 17;158(3):271–277. doi: 10.1007/BF00267198. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Paietta J., Gallant J. A. Synthesis of guanosine tetraphosphate (magic spot I) in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1977 Jan 10;74(1):314–322. doi: 10.1016/0006-291x(77)91410-3. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey W. S., Dworkin M. Stable messenger ribonucleic acid and germination of Myxococcus xanthus microcysts. J Bacteriol. 1970 Feb;101(2):531–540. doi: 10.1128/jb.101.2.531-540.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M., Mitchell A. R. Chromatography of nucleic acid digests on thin layers of cellulose impregnated with polyethyleneimine. Biochem J. 1971 Jul;123(4):613–617. doi: 10.1042/bj1230613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]