Abstract
Mutations in the gene encoding the Krebs cycle enzyme fumarate hydratase (FH) predispose to hereditary leiomyomatosis and renal cell cancer in affected individuals. FH-associated neoplasia is characterized by defective mitochondrial function and by upregulation of transcriptional pathways mediated by hypoxia-inducible factor (HIF), although whether and by what means these processes are linked has been disputed. We analysed the HIF pathway in Fh1−/− mouse embryonic fibroblasts (MEFs), in FH-defective neoplastic tissues and in Fh1−/− MEFs re-expressing either wild-type or an extra-mitochondrial restricted form of FH. These experiments demonstrated that upregulation of HIF-1α occurs as a direct consequence of FH inactivation. Fh1−/− cells accumulated intracellular fumarate and manifested severe impairment of HIF prolyl but not asparaginyl hydroxylation which was corrected by provision of exogenous 2-oxoglutarate (2-OG). Re-expression of the extra-mitochondrial form of FH in Fh1−/− cells was sufficient to reduce intracellular fumarate and to correct dysregulation of the HIF pathway completely, even in cells that remained profoundly defective in mitochondrial energy metabolism. The findings indicate that upregulation of HIF-1α arises from competitive inhibition of the 2-OG-dependent HIF hydroxylases by fumarate and not from disruption of mitochondrial energy metabolism.
INTRODUCTION
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is an inherited human cancer syndrome characterized by benign smooth muscle tumours and malignant renal papillary carcinoma (1). Genetic studies revealed that the syndrome is caused by inactivating mutations of the fumarate hydratase (FH) gene (2). FH is not a ‘traditional’ suppressor gene with a recognized function in cell proliferation or survival, but encodes an enzyme that is part of the mitochondrial Krebs cycle, suggesting the operation of novel oncogenic mechanisms.
It has been suggested that activation of hypoxia-inducible factor (HIF) contributes to FH-associated oncogenesis by activation of hypoxia pathways that promote tumour growth or associated pro-tumourigenic processes such as angiogenesis (3). FH-associated human tumours display upregulation of HIF (4–6). However, the mechanisms contributing to HIF activation have been disputed. As FH inactivation disrupts oxidative mitochondrial metabolism, it has been postulated that dysregulation of energy metabolism may itself contribute directly or indirectly to activation of HIF (7). In succinate dehydrogenase-deficient cells, activation of HIF has been associated with enhanced generation of reactive oxygen species (ROS) arising from defective mitochondrial function (8), and similar mechanisms have been proposed in FH deficiency (9). Enhanced ROS production arising from increased glucose metabolism has also been proposed to contribute to activation of HIF in FH deficiency (10). An alternative, but not mutually exclusive, hypothesis proposes that the accumulation of fumarate itself activates HIF by competitive inhibition of the 2-oxoglutarate (2-OG) oxygenases that control the levels and activity of HIF-α sub-units (4,11,12).
In oxygenated cells, prolyl hydroxylation at two sites within an internal HIF-α degradation domain promotes binding to the von Hippel–Lindau E3 ligase complex and proteolysis by the ubiquitin–proteasome pathway, whereas asparaginyl hydroxylation at a C-terminal site reduces transcriptional activity by blocking co-activator recruitment. HIF prolyl hydroxylation is catalysed by three closely related enzymes [prolyl hydroxylase domain (PHD) 1, 2 and 3, also termed EGLN, 2, 1 and 3]. HIF asparaginyl hydroxylation is catalysed by a less closely related 2-OG oxygenase [factor inhibiting HIF (FIH)] (reviewed in 13,14). It has been proposed that inhibition by fumarate mimics hypoxia, inhibiting these enzymes and allowing HIF to escape destruction and to activate transcription. This hypothesis is of particular interest because it implies that fumarate accumulation itself is a key step in the oncogenic pathway and might be targeted for therapeutic modulation (15).
FH is expressed not only in the mitochondrion, but also in the cytosol, where it has been proposed to participate in nucleotide, urea cycle and amino acid metabolic pathways (16,17). As the HIF hydroxylases are extra-mitochondrial enzymes, this raises a key question as to whether cytosolic expression of FH would be sufficient to correct the dysregulation of HIF, irrespective of the mitochondrial defect, thus distinguishing between the proposed mechanisms for activation of HIF.
Both mitochondrial and cytosolic forms of FH are encoded by the same transcript. The resulting propeptide is targeted to the mitochondrial membrane via its N-terminal mitochondrial targeting sequence (MTS), where it is then cleaved into two smaller peptides: the peptide encoding the MTS sequence is retained within the mitochondrion and the remaining peptide is released into the cytosol (18,19). In this work, we have taken advantage of this property to create stable transfectants re-expressing either wild-type or an extra-mitochondrial, but otherwise identical, form of human FH in Fh1-deficient cells. We demonstrate striking upregulation of the HIF pathway in Fh1-deficient cells and that cytoplasmic re-expression of FH is sufficient to completely correct the observed dysregulation of HIF-1α, irrespective of persisting mitochondrial dysfunction.
RESULTS
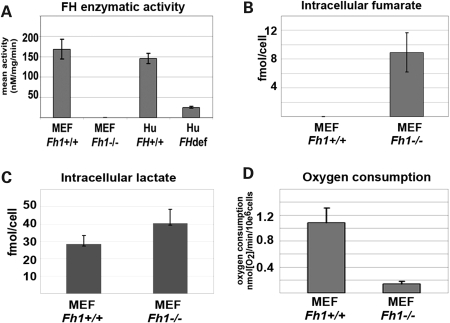
To establish a system for the study of Fh1 inactivation, we first isolated mouse embryonic fibroblasts (MEFs) from animals bearing a conditionally inactivated Fh1 allele (20). Adenoviral expression of Cre recombinase in these cells resulted in complete absence of Fh1 expression (Supplementary Material, Fig. S1). Fh1 activity in wild-type, Fh1+/+ MEFs was comparable with that in human skin fibroblasts, but totally absent in Fh1−/− MEFs (Fig. 1A). To define the metabolic consequences of Fh1 inactivation in these cells, we performed 1H magnetic resonance spectroscopy (MRS) metabolite analysis and measured oxygen consumption in Fh1+/+ and Fh1−/− MEFs. Striking intracellular fumarate accumulation was observed in Fh1−/− cells (Fig. 1B); in keeping with the absence of a specific mechanism for fumarate transport across the plasma membrane, fumarate did not appear in medium extracts (data not shown). Fh1−/− MEFs also manifest increased lactate production resulting in increased cellular levels (Fig. 1C) consistent with a shift towards glycolysis-driven metabolism. In line with the decreased Fh1 activity, cellular respiration in Fh1−/− MEFs was suppressed. Using a Clark-type oxygen electrode, basal unstimulated mitochondrial respiration was measured in intact cells and was found to be decreased by ∼80% in the Fh1−/− MEFs compared with Fh1+/+ MEFs (Fig. 1D).
Figure 1.
Analysis of Fh1-deficient (Fh1−/−) MEFs. (A) FH activity was compared between Fh1+/+ and Fh1−/− MEFs. Skin fibroblast cells from FH-deficient patients (28) and FH+/+ patients were used as negative and positive controls, respectively. Fh1−/− MEFs show no detectable FH activity, whereas the Fh1+/+ MEFs and normal human skin fibroblasts show equivalent levels of activity. (B) Analysis of intracellular fumarate in cell extracts of Fh1−/− and Fh1+/+ MEFs. (C) Analysis of lactate levels in cellular extracts of Fh1−/− and Fh1+/+ MEFs. Fh1−/− MEFs show increased levels of intracellular lactate compared with Fh1+/+ MEFs. (D) Oxygen consumption was significantly reduced in Fh1−/− MEFs compared with Fh1+/+ MEFs. All data are shown as mean ± SD of triplicate assays.
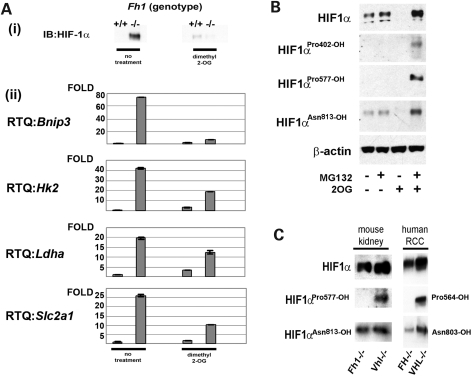
To determine whether Hif-1α was directly upregulated by Fh1 inactivation, heterogeneous pools of Fh1-deficient MEFs, and individual Fh1−/− clones derived from these pools, were analysed by immunoblotting. These experiments revealed consistent and striking upregulation of Hif-1α protein levels in all Fh1-defective MEFs [Fig. 2A(i) and data not shown]. As a first test of the hypothesis that upregulation of Hif-1α might be mediated by competitive inhibition of the 2-OG oxygenases that catalyse HIF hydroxylation, we examined the effects of a cell-permeable esterified derivative of 2-OG (dimethyl-2-OG) that increases 2-OG levels within cells. Exposure of Fh1−/− cells to this treatment resulted in the loss of the accumulated Hif-1α. We next determined the effects on a panel of HIF- target genes: BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3), hexokinase 2 (Hk2), hactate dehydrogenase A (Ldha) and Glut1 (Slc2a1) [Fig. 2A(ii)]. All were strongly upregulated in Fh1−/− cells and downregulated by the addition of dimethyl-2-OG to these cells.
Figure 2.
Fh1-deficient cells exhibit severe impairment of HIF prolyl hydroxylation. (A) (i) Immunoblot and (ii) qRT–PCR analyses of Fh1−/− and Fh1+/+ MEFs. Hif-1α protein stabilization and upregulation of HIF- target genes is observed in Fh1−/− MEFs under normoxic conditions. The addition of exogenous dimethyl-2-OG reduces the expression of HIF- target genes to levels comparable with wild-type cells. Results are shown as mean ± SD of triplicate assays. (B) Immunoblot analysis using hydroxy-residue-specific antibodies. Hif-1α Pro402 and Pro577 hydroxylation is absent in Fh1−/− MEFs, but is restored following addition of dimethyl-2-OG (2 mm) for 8 h. Hydroxylation of Asn813 is relatively unaffected. (C) Immunoblot analyses of kidney tissue from Fh1−/− or Vhl−/− mice and human type II papillary RCC (FH−/−) or human clear cell RCC (VHL−/−) tissues. HIF-1α prolyl hydroxylation is suppressed in FH-defective but not (VHL)-defective tissues.
In normal cells, Hif prolyl hydroxylation occurs at two proline sites (Pro402 and Pro577 in mouse Hif-1α), whereas HIF asparaginyl hydroxylation occurs at a single site (Asn813 in mouse Hif-1α) To assess directly whether the effects of Fh1 inactivation were due to actions on the hydroxylation of Hif-1α at any or all of these sites, we determined the hydroxylation status of Hif-1α using hydroxy-residue-specific antibodies (Fig. 2B). Under normoxic conditions, hydroxylated Hif-1α is targeted by the ubiquitin–proteasome pathway for proteasomal degradation. We demonstrated that Hif-1α in Fh1−/− MEFs treated with the proteasomal inhibitor N-[(phenylmethoxy)carbonyl]-l-leucyl-N-[(1S)-1-formyl-3-methylbutyl]-l-leucinamide (MG132) remained unhydroxylated at both Pro402 and Pro577, indicating essentially a complete inhibition of the PHDs. The addition of dimethyl-2-OG to Fh1−/− cells under the same conditions strongly enhanced hydroxylation at these sites, consistent with the reversal of that inhibition. In contrast, detectable asparaginyl hydroxylation was observed even in untreated Fh1−/− cells, although this was also increased by the addition of dimethyl-2-OG, suggesting that it was also partially restricted in Fh1−/− cells. Taken together, these experiments indicate that Hif-1α hydroxylation is inhibited in Fh1−/− cells and suggest that prolyl hydroxylation is more sensitive than asparaginyl hydroxylation. The findings also indicate that despite persistent Hif-1α asparaginyl hydroxylation, the HIF transcriptional system is activated. To test whether dysregulation of Hif-1α hydroxylation also occurs in a similar way in FH-associated neoplasia, we examined hyperplastic renal cystic tissue from Fh1−/− mouse kidneys and human FH-deficient papillary renal carcinoma. Results shown in Figure 2C confirm that, in FH-associated neoplasia, HIF-1α prolyl, but not asparaginyl hydroxylation, is strongly inhibited (for comparison, Von Hippel-Lindau (VHL)-defective material is shown, in which HIF-1α accumulates in a form that is hydroxylated on both prolyl and asparaginyl residues). Taken together, these experiments indicate that profound dysregulation of HIF occurs in FH-associated neoplasia as a direct consequence of FH inactivation.
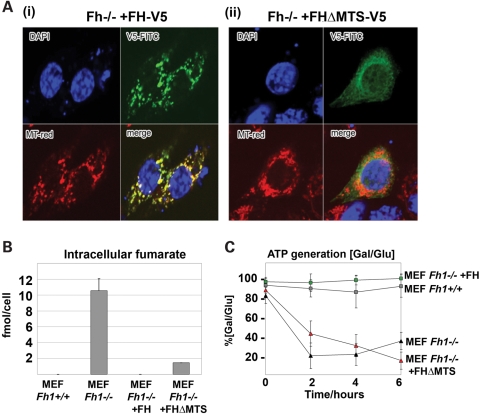
We next sought to define the contribution or otherwise of the mitochondrial defect to this process by comparing the effects of re-expression of either a full-length C-terminal V5-tagged human FH gene (FH-V5) or an identical gene lacking the mitochondrial targeting sequence (FH-V5ΔMTS) in Fh1−/− cells (Supplementary Material, Fig. S2). Following transfection of Fh1−/− MEFs with each gene, multiple independent clones were generated. Immunofluorescence microscopy confirmed the predominant intramitochondrial localization of the transfected FH-V5 gene and the cytoplasmic localization of FH-V5ΔMTS in all clones (Fig. 3A and data not shown).
Figure 3.
Complementation of Fh1-deficient MEFS. (A) Immunofluorescence studies confirming localization, by merging of MitoTracker (red) and V5-FITC-tagged FH protein (green), of (i) the FH-V5 construct exclusively to the mitochondria and (ii) the FHΔMTS-V5 construct to the cytoplasm, excluding the mitochondria. (B) Intracellular levels of fumarate in Fh1+/+, Fh1−/−, Fh1−/− +FH and Fh1−/− +FHΔMTS MEFs. (C) ATP levels in Fh1−/−, Fh1+/+, Fh1−/− +FHΔMTS and Fh1−/− +FH MEFs were measured at 0, 2, 4 and 6 h after supplementation with either glucose (permissive for both oxidative phosphorylation and glycolysis) or galactose (permissive for oxidative phosphorylation only). Maintenance of [ATP] in galactose medium is expressed as a percentage of that maintained in a parallel culture in glucose medium calculated using the formula [ATP]Gal/[ATP]Glux100. Both Fh1−/− and Fh1−/− +FHΔMTS MEFs showed a reduced capacity to maintain ATP levels (80 and 60%, respectively), whereas Fh1+/+ and Fh1−/− +FH MEFs showed comparable levels of ATP. Results are shown as mean ± SD of triplicate assays.
Both FH-V5ΔMTS and FH-V5 transfections substantially corrected the fumarate accumulation that was observed in Fh1−/− cells, although a low level of fumarate persisted in the FH-V5ΔMTS transfectants (Fig. 3B). In contrast, measurements of both substrate-stimulated (glutamate) and uncoupled [carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP)] mitochondrial respiration revealed marked differences between the transfectants. Although the introduction of full-length FH (FH-V5) into Fh1−/− cells restored respiration to wild-type levels, FH-V5ΔMTS transfectants remained defective in respiration, manifesting oxygen consumption similar to the non-mitochondrial oxygen consumption rate observed after inhibition of mitochondrial ATP synthase using oligomycin (Table 1). In keeping with defective mitochondrial respiration, Fh1−/− +FHΔMTS transfectants, and the parent Fh1−/− MEFs, were unable to maintain cellular ATP levels when cultured in galactose- as opposed to glucose-containing medium. In contrast, this capacity was restored in full-length FH (FH-V5) transfectants. Taken together, these findings demonstrate that although full restoration of respiration via oxidative phosphorylation in Fh1−/− MEFs requires re-expression of mitochondrial FH, cytoplasmic FH is sufficient to largely ablate fumarate accumulation. Thus we argue that comparative studies of the HIF system in these cells should distinguish the effects of the mitochondrial defect (and consequent glucose addiction) from those of fumarate accumulation.
Table 1.
Oxygen consumption measurements in MEFs
| Oxygen consumption (nmol O2/min/106 cells) | ||||
|---|---|---|---|---|
| Fh1+/+ | Fh1−/− | Fh1+/+ +FH | Fh1−/− +FHΔMTS | |
| Glutamate | 1.08 ± 0.22 | 0.14 ± 0.04* | 0.72 ± 0.05# | 0.29 ± 0.06* |
| Oligomycin | 0.24 ± 0.01 | 0.18 ± 0.03 | 0.34 ± 0.13 | 0.25 ± 0.05 |
| FCCP | 1.50 ± 0.13 | 0.58 ± 0.08* | 1.33 ± 0.24# | 0.76 ± 0.2 |
Data are average ± SEM. A Clark-type oxygen electrode was used to determine the respiratory rates of Fh1+/+, Fh1−/−, Fh1−/− +FH and Fh1−/− +FHΔMTS MEFs in the presence of glutamate, oligomycin and FCCP.
*P < 0.05 versus Fh1+/+.
#P < 0.05 versus Fh1−/−.
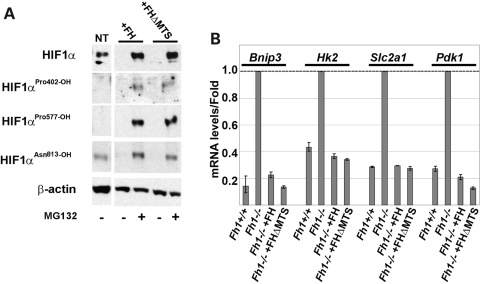
Strikingly, both sets of transfectants manifest complete loss of the normoxic Hif-1α accumulation that was observed in untransfected Fh1−/− cells. To confirm the effects on the hydroxylation of Hif-1α directly, we again used hydroxy-residue-specific antibodies and studied cells under conditions of MG132-mediated proteasomal blockade to stabilize all forms of Hif-1α irrespective of hydroxylation (Fig. 4A). These studies revealed that both sets of transfectants completely restored HIF-1α prolyl hydroxylation. Finally, to determine the overall effects on the HIF transcriptional activity, we measured expression of the HIF- target gene transcripts, Bnip3, HK2, Slc2a1 and pyruvate dehydrogenase kinase isoenzyme 1 (Pdk1), by RT–PCR (Fig. 4B). In each case, both FH-V5 and FH-V5ΔMTS transfections corrected the upregulation of these transcript levels in normoxic Fh1−/− MEFs. These findings indicate that re-expression of extra-mitochondrial FH is sufficient for complete correction of the normoxic upregulation of the HIF pathway in Fh1−/− cells, irrespective of persistent deficiency in mitochondrial respiration.
Figure 4.
Cytoplasmic FH is sufficient to restore Hif-1α hydroxylation and degradation. Immunoblot (A) and qRT–PCR (B) analyses of Fh−/− transfectants. Immunoblotting using hydroxy-residue-specific antibodies confirmed that Fh1−/− MEFs transfected with either FH-V5 or FH-V5ΔMTS restored Hif-1α hydroxylation at the Pro402 and Pro577 residues. Neither FH-V5 nor FH-V5ΔMTS transfection affected hydroxylation at Asn813. qRT–PCR analysis of Hif-1α target genes shows that mRNA levels in both Fh1−/− +FH and Fh1−/− +FHΔMTS MEFs are reduced to a similar range to levels observed in Fh1+/+ MEFs. Results are shown relative to the mRNA levels in Fh1−/− cells; mean ± SD of triplicate assays.
DISCUSSION
Taken together, our findings provide clear evidence that upregulation of the HIF pathway in Fh1-deficient cells occurs as a result of inhibition of HIF-1α prolyl hydroxylation by accumulated intracellular fumarate and is independent of the defect in mitochondrial oxidative metabolism (Fig. 5). Total correction of Hif-1α pathway activation in Fh1−/− MEFs by extra-mitochondrial expression of FH indicates that, at least in this context, neither impaired mitochondrial function itself nor the consequent dependence of energy metabolism on glycolysis contribute importantly to this process. Experiments using hydroxy-residue-specific antibodies revealed that Hif-1α prolyl and asparaginyl hydroxylation were differentially impaired in both Fh1-deficient cells and tumours. These results are in agreement with analyses on recombinant PHDs and FIH, which demonstrate that fumarate is a better inhibitor of the PHDs than FIH in kinetic assays conducted in vitro (11,12).
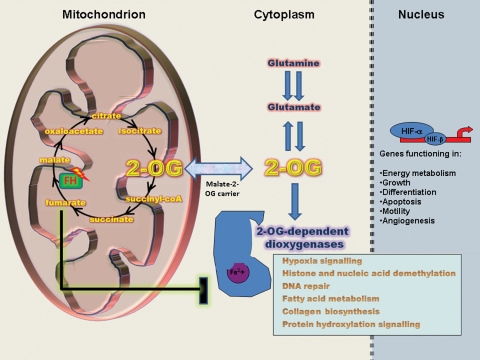
Figure 5.
Fumarate as an inhibitor of 2-OG-dependent enzymes. Mutations in FH result in intracellular fumarate accumulation that may inhibit 2-OG-dependent enzymes. The increased levels of Hif-1α and HIF- target genes observed in Fh1-deficient cells can be explained by fumarate-mediated inhibition of HIF–PHDs. This allows Hif-1α to escape hydroxylation and subsequent degradation and to accumulate in the nucleus. Other 2-OG-dependent oxygenases that function in different cellular processes may also be inhibited by high levels of fumarate.
Although both the current and previous studies have consistently demonstrated upregulation of HIF in FH-associated neoplasia (6,20,21), this does not prove that HIF activation is the causative oncogenic pathway in this setting. Recent work has indicated that the human genome encodes as many as 60–70 known or predicted 2-OG-dependent oxygenases (22). Described functions include prolyl and lysyl hydroxylation of collagen, histone lysyl and arginyl demethylation, lysyl hydroxylation of proteins associated with RNA splicing, different steps in carnitine metabolism, the repair of alkylated bases in DNA, single- and double-stranded nucleic acid demethylation and the hydroxylation of 5-methyl-cytosine (reviewed in 22). Our finding that Hif-1α prolyl and asparaginyl hydroxylations are differentially inhibited in Fh1−/− cells, in a manner that is consistent with in vitro efficacy of fumarate as a catalytic inhibitor, suggests that studies of the action of fumarate across a range of 2-OG oxygenases should be of interest in defining possible consequences of FH inactivation (23).
To date, no HLRCC mutations that occur in the MTS have been described consistent with the ‘tumour suppressor’ function of FH being extra-mitochondrial. In this context, it is of interest that recent studies of Saccharomyces cerevisiae have revealed that yeast cells, which have been engineered to be defective in cytosolic, but not mitochondrial fumarase (equivalent to FH), show defective DNA repair responses (24). Based on these findings, and on the action of exogenous fumarate, it has been proposed that fumarate itself may have a role in DNA repair. Whether these effects are due to an action on a 2-OG oxygenase function is not yet clear. However, the derivation of a mammalian cellular model that enables the effects of fumarate accumulation to be distinguished from those of defective mitochondrial function should now be of considerable use in dissecting the potentially complex effects of FH inactivation on 2-OG oxygenase functions and in defining the oncogenic pathways operating in FH-associated disease. It will also provide a platform for the definition and monitoring of potential therapeutic interventions designed to restrict fumarate accumulation in this setting.
MATERIALS AND METHODS
Generation and maintenance of immortalized Fh1-deficient MEFs
Fh1 conditional knockout mice (20) were maintained in accordance with Home Office guidelines and licensing regulations. Fh1 mice were inter-crossed, and MEFs were isolated from littermate embryos and dissected at 14.5 days of gestation using standard protocols. Cells (at passage number 3) were transfected with pBabe-puro SV40 Large T antigen (25) (108 PFU) and immortalized cells were selected with puromycin. Excision of the Fh1 loxP-flanked exon was effected by transfection using an adenovirus- type 5 (dE1/E3) vector, with a cytomegalovirus (CMV) promoter-driven Cre recombinase transgene and green fluorescent protein (GFP) tag (Ad-Cre-GFP) (Vector Biolabs). A similar vector lacking the Cre transgene but also driven by the CMV promoter (Ad-CMV-GFP) was used as a control. Transfection efficiency was ∼80%, as assessed by GFP expression. Clones were established and the genotype was confirmed by PCR of genomic DNA (20). All MEFs were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma) containing 4500 g/l glucose supplemented with 10% (v/v) fetal bovine serum (Sigma), 2 mm glutamine (Sigma) and maintained in a humidified atmosphere of 5% CO2 and 21% O2.
Generation of MEFs with stable FH expression
Wild-type human FH with and without the MTS was inserted into pEF1/V5-His (Invitrogen) using PCR (details on request). Fh1−/− MEFs were transfected using FuGene®6 Transfection Reagent (Roche), according to the manufacturer's instructions. Post-transfection (72 h), cells were placed under G418 selection. Surviving cells were then analysed by immunofluorescence to confirm appropriate sub-cellular localization. Independent clones were isolated from these heterogeneous populations of transfected cells, and expression and localization were re-confirmed.
Human and murine tissues and cell lines
The frozen FH-mutant type II papillary renal cancer, VHL-mutant clear cell renal cancer and FH-deficient cells were collected with full ethical approval in collaboration with Professor Ian Tomlinson (Oxfordshire REC B 05/Q1605/66 ‘Molecular analyses of archival tumours’). Fh1- and Vhl-deficient mice have been previously described (20,26).
Immunoblotting and antibodies
Whole-cell extracts and immunoblot analysis were carried out as described previously (6). The following primary antibodies were used: HIF-1α (Novus), Hydroxy-HIF-1α (Pro564; Cell Signalling Technology, no. 3434), Hydroxy-HIF-1α (Pro402; Millipore, no. 07-1585), Hydroxy-HIF-1α (Asn803) (27), β-actin-HRP (Abcam) and FH (Autogen Bioclear).
Immunofluorescence and confocal analysis
Prior to fixation, cells were labelled with 250 nm Mitotracker® Red CMXRos (Invitrogen). Cells were fixed in 10% neutral buffered formalin (Sigma), with an additional fixation in methanol (Sigma) at −20°C, and permeabilized with 0.025% Triton X-100 (Sigma). V5-tagged FH protein was visualized with anti-V5-FITC-conjugated antibody (Invitrogen). Cells were mounted in Vectashield® with DAPI 4',6-diamidino-2-phenylindolel (Vector Labs). Pictures were acquired using the Zeiss LSM510 MetaHead confocal microscope and using LSM 510 software v4.2 (Carl Zeiss GmbH).
Measurement of FH activity
Determination of enzymatic activity of FH was performed as described previously (28).
MRS metabolite analysis
MRS was performed as described previously (6). In brief, metabolites were extracted from cells, using perchloric acid, and freeze-dried. The samples were resuspended in D2O and analysed using 1H MRS. Cell culture media were also collected and analysed by 1H MRS. Sodium 3-trimethylsilyl-2,2,3,3-tetradeuterpropionate (TSP) was added to the samples for chemical shift calibration and quantification.
Quantitative reverse transcription–PCR
qRT–PCR for quantification of mRNA employed Taqman gene expression assays on a StepOne thermocycler (Applied Biosystems). Total RNA was extracted using Tri-reagent (Sigma) according to the manufacturer's protocol. Single-strand cDNA synthesis was generated from 2 µg of total RNA (Applied Biosystems). Normalization was to β-actin mRNA and relative gene expression was calculated using the ΔΔCT method. Fifty nanograms of cDNA template per reaction were used and three biological replicates, each in triplicate, were performed for each experiment.
Measurement of mitochondrial respiration
MEFs (2 × 106 cells) were harvested and washed in 100 mm KCl, 50 mm MOPS and 0.5 mm EGTA to remove any cell culture media. Cells were resuspended in respiratory media (100 mm KCl, 50 mm MOPS, 1 mm EGTA, 5 mm KH2PO4 and 1 mg/ml BSA, pH 7.4) and transferred to a Clark-type oxygen electrode (Strathkelvin, UK) at 30°C for the measurement of mitochondrial respiration (29). Respiration was measured under basal unstimulated conditions, following the addition of glutamate (20 mm), the ATP synthase inhibitor oligomycin (2 µm) and the metabolic uncoupler (FCCP; 10 µm).
ATP measurements
Levels of ATP were determined using a CellTiter-Glo® Assay (Promega, Madison) on white 96-well plates (Greiner Bio One, Frickenhausen, Germany) according to the manufacturer's instructions. Briefly, cells were plated at a density of 5 × 104 cells per well in 96-well plates and grown in DMEM supplemented with either 25 mm glucose or 25 mm galactose. Cells grown in each condition were lysed simultaneously at 0, 2, 4 and 6 h and luminescent signals measured using a plate reader (Victor 2; PerkinElmer Life Sciences). Maintenance of [ATP] in galactose medium is expressed as a percentage of that in glucose medium at each time point, i.e., ([ATP]Gal/[ATP]Glu)×100.
Statistical analysis
Statistical comparisons for qRT–PCR were performed using the Student's unpaired t-test. Data on mitochondrial respiration were analysed using one-way ANOVA and Tukey's post hoc analysis.
SUPPLEMENTARY MATERIAL
FUNDING
The work was supported by the Medical Research Council; Cancer Research UK (C6079/A9485 to P.J.R. and P.J.P., C10843/A12027 to P.J.P.); The Wellcome Trust (WT091112MA to P.J.P.); and The Science Foundation of Ireland (07/IN.1/B1804). P.J.P. is a Beit Memorial Fellow.
Supplementary Material
ACKNOWLEDGEMENTS
We thank S.H. Lee for providing the anti-HIF-1α Asn803-OH antibody, P. Jat for the pBabe-puro construct and I. Tomlinson for provision of tumour samples. We would also like to thank C. Schofield, N. Masson, Y.M. Tian and members of the Ratcliffe Laboratory and Chemistry Research Laboratory for technical advice and assistance, reagents and critical discussions.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Launonen V., Vierimaa O., Kiuru M., Isola J., Roth S., Pukkala E., Sistonen P., Herva R., Aaltonen L.A. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc. Natl Acad. Sci. USA. 2001;98:3387–3392. doi: 10.1073/pnas.051633798. doi:10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomlinson I.P., Alam N.A., Rowan A.J., Barclay E., Jaeger E.E., Kelsell D., Leigh I., Gorman P., Lamlum H., Rahman S., et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002;30:406–410. doi: 10.1038/ng849. doi:10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 3.Harris A. Hypoxia–a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. doi:10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs J.S., Jung Y.J., Mole D.R., Lee S., Torres-Cabala C., Chung Y.L., Merino M., Trepel J., Zbar B., Toro J., et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. doi:10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Pollard P., Wortham N., Barclay E., Alam A., Elia G., Manek S., Poulsom R., Tomlinson I. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J. Pathol. 2005;205:41–49. doi: 10.1002/path.1686. doi:10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- 6.Pollard P.J., Briere J.J., Alam N.A., Barwell J., Barclay E., Wortham N.C., Hunt T., Mitchell M., Olpin S., Moat S.J., et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. doi:10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb E., Tomlinson I.P. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. doi:10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 8.Guzy R.D., Sharma B., Bell E., Chandel N.S., Schumacker P.T. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell. Biol. 2008;28:718–731. doi: 10.1128/MCB.01338-07. doi:10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudarshan S., Linehan W.M., Neckers L. HIF and fumarate hydratase in renal cancer. Br. J. Cancer. 2007;96:403–407. doi: 10.1038/sj.bjc.6603547. doi:10.1038/sj.bjc.6603547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudarshan S., Sourbier C., Kong H.S., Block K., Valera Romero V.A., Yang Y., Galindo C., Mollapour M., Scroggins B., Goode N., et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol. 2009;29:4080–4090. doi: 10.1128/MCB.00483-09. doi:10.1128/MCB.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewitson K.S., Lienard B.M., McDonough M.A., Clifton I.J., Butler D., Soares A.S., Oldham N.J., McNeill L.A., Schofield C.J. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. doi:10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 12.Koivunen P., Hirsila M., Remes A.M., Hassinen I.E., Kivirikko K.I., Myllyharju J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem. 2007;282:4524–4532. doi: 10.1074/jbc.M610415200. doi:10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- 13.Kaelin W.G., Jr, Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Pouyssegur J., Dayan F., Mazure N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. doi:10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie E.D., Selak M.A., Tennant D.A., Payne L.J., Crosby S., Frederiksen C.M., Watson D.G., Gottlieb E. Cell-permeating {alpha}-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol. Cell. Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. doi:10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson D., Cox M. Lehninger Principles of Biochemistry. 4th edn. W. H. Freeman; 2004. [Google Scholar]
- 17.Salway J.G. Metabolism at a Glance. Blackwell Publishers; 1999. [Google Scholar]
- 18.Stein I., Peleg Y., Even-Ram S., Pines O. The single translation product of the FUM1 gene (fumarase) is processed in mitochondria before being distributed between the cytosol and mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 1994;14:4770–4778. doi: 10.1128/mcb.14.7.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sass E., Blachinsky E., Karniely S., Pines O. Mitochondrial and cytosolic isoforms of yeast fumarase are derivatives of a single translation product and have identical amino termini. J. Biol. Chem. 2001;276:46111–46117. doi: 10.1074/jbc.M106061200. doi:10.1074/jbc.M106061200. [DOI] [PubMed] [Google Scholar]
- 20.Pollard P.J., Spencer-Dene B., Shukla D., Howarth K., Nye E., El-Bahrawy M., Deheragoda M., Joannou M., McDonald S., Martin A., et al. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell. 2007;11:311–319. doi: 10.1016/j.ccr.2007.02.005. doi:10.1016/j.ccr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs J.S., Jung Y.J., Mole D.R., Lee S., Torres-Cabala C., Merino M., Trepel J., Zbar B., Toro J., Ratcliffe P.J., et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. doi:10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Loenarz C., Schofield C.J. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat. Chem. Biol. 2008;4:152–156. doi: 10.1038/nchembio0308-152. doi:10.1038/nchembio0308-152. [DOI] [PubMed] [Google Scholar]
- 23.Pollard P.J., Ratcliffe P.J. Cancer. Puzzling patterns of predisposition. Science. 2009;324:192–194. doi: 10.1126/science.1173362. doi:10.1126/science.1173362. [DOI] [PubMed] [Google Scholar]
- 24.Yogev O., Singer E., Shaulian E., Goldberg M., Fox T.D., Pines O. Fumarase: a mitochondrial metabolic enzyme a cytosolic nuclear component of the DNA damage response. PLoS Biol. 8:e1000328. doi: 10.1371/journal.pbio.1000328. doi:10.1371/journal.pbio.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gjoerup O.V., Wu J., Chandler-Militello D., Williams G.L., Zhao J., Schaffhausen B., Jat P.S., Roberts T.M. Surveillance mechanism linking Bub1 loss to the p53 pathway. Proc. Natl Acad. Sci. USA. 2007;104:8334–8339. doi: 10.1073/pnas.0703164104. doi:10.1073/pnas.0703164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haase V.H., Glickman J.N., Socolovsky M., Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc. Natl Acad. Sci. USA. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. doi:10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S.H., Jeong Hee M., Eun Ah C., Ryu S.E., Myung Kyu L. Monoclonal antibody-based screening assay for factor inhibiting hypoxia-inducible factor inhibitors. J. Biomol. Screen. 2008;13:494–503. doi: 10.1177/1087057108318800. doi:10.1177/1087057108318800. [DOI] [PubMed] [Google Scholar]
- 28.Alam N.A., Rowan A.J., Wortham N.C., Pollard P.J., Mitchell M., Tyrer J.P., Barclay E., Calonje E., Manek S., Adams S.J., et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum. Mol. Genet. 2003;12:1241–1252. doi: 10.1093/hmg/ddg148. doi:10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 29.Heather L.C., Carr C.A., Stuckey D.J., Pope S., Morten K.J., Carter E.E., Edwards L.M., Clarke K. Critical role of complex III in the early metabolic changes following myocardial infarction. Cardiovasc. Res. 85:127–136. doi: 10.1093/cvr/cvp276. doi:10.1093/cvr/cvp276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.