Abstract
The permeabilities of amino acids for isolated cuticular membranes of ivy (Hedera helix L.) were measured at different pH. Cuticular permeances were lowest for the zwitterionic form at pH 6, followed by the cationic form at pH 1. Highest permeances were obtained for the anionic form at pH 11. Permeances were not correlated with octanol/water partition coefficients and decreased at a given pH with increasing molar volume of the solute. This finding suggests that permeation takes place in the polar cuticular pathways. The effect of pH on the cuticular transport properties was analysed according to the porous membrane model considering the polyelectrolytic character of the cuticle in terms of porosity, tortuosity, and size selectivity of the aqueous cuticular pathway which is altered by pH. An increase of water content and permeability of the cuticular membrane was caused by the dissociation of weak acidic groups with increasing pH leading to a swelling of the cuticle induced by fixed negative charges. In addition, the pH-dependent size of the hydration shell of the amino acids was identified as a secondary factor explaining the variability of cuticular permeances.
Keywords: Amino acids, aqueous pathways, hydration, permeability, pH, plant cuticle
Introduction
Plant leaf surfaces are covered by a cuticle, which serves as a lipophilic transport barrier in order to reduce uncontrolled water loss to the atmosphere (Riederer and Schreiber, 2001; Burghardt and Riederer, 2006). In addition, the cuticle counteracts the leaching of nutrients and metabolites from the inner leaf tissues (Tukey, 1970). Leaf surfaces represent a habitat for consortia of epiphyllic micro-organisms (Krimm et al., 2005; Leveau, 2006) and the availability of nutrients is a prerequisite for colonization. Carbohydrates and amino acids are frequently found on leaf surfaces (Derridj, 1996). These compounds are assumed to originate from the leaf tissue reaching the surface by permeation through the cuticle (Singh et al., 2004).
Cuticles are mainly composed of the lipid components cutin and cuticular waxes. The transport of lipophilic solutes across cuticular membranes can be described by applying the principles of a solution-diffusion membrane (Schönherr and Riederer, 1989; Riederer and Friedmann, 2006; Schreiber and Schönherr, 2009).
However, the recent description of polar pathways has become a main focus of research in cuticular transport physiology (Schreiber, 2005, 2006; Schönherr, 2006). It was shown that inorganic salts and ionic organic solutes permeate through cuticular membranes with higher rates than expected from the sorption–diffusion model of the lipophilic pathway (Schönherr, 2000, 2002; Schönherr and Schreiber, 2004). Aqueous polar pores were proposed as an alternative route. They are assumed to be formed by polar functional groups of the cutin polymer or strands of polysaccharides expanding from the epidermal cell wall across the cuticle. Sorption of water to these structures leads to the formation of an aqueous phase traversing the cuticular membrane.
For cuticular water permeability it was demonstrated that pH may modify the transport properties of the polar pathway (for dewaxed isolated cuticles: Schönherr, 1976; for native isolated cuticles: Luque et al., 1995). Physico-chemical properties like octanol/water partition coefficients and hydration shells of ionizable solutes are also affected by pH (Yunger and Cramer, 1981; Gulyaeva et al., 2003; Burakowski and Gliński, 2008). In particular, this is the case for amphoteric amino acids which exist in cationic, anionic, and zwitterionic forms, depending on the pH of the solvent (Fig. 1). Although the permeation of amino acids through cuticular membranes was demonstrated for pear fruit cuticular membranes, interpretation of data was limited due to the extreme variability of the permeabilities (Ersöz et al., 1995) potentially caused by the omission of the impact of pH. Since there is only scarce information on cuticular permeation of amino acids, the aim of the present work is to evaluate the multiple effects of pH on cuticular permeability in order to explore fundamental mechanisms of amino acid transport. This will contribute to a better understanding of the main parameters affecting the permeation of hydrophilic solutes.
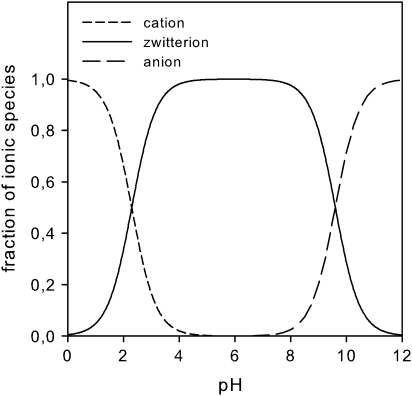
Fig. 1.
Fraction of ionic species of glycine in aqueous solution as a function of pH. The fractions of cationic, zwitterionic, and anionic species were calculated from the Henderson–Hasselbalch equation using the acid and base dissociation constants (Table 1).
Materials and methods
Cuticular membranes
Cuticular membranes (CM) were isolated enzymatically (Schönherr and Riederer, 1986) from the upper, astomatous leaf surfaces of Hedera helix L.. Leaf discs were obtained from fully emerged, trichome-less leaves harvested from generative stems of ivy plants growing outside in the Botanical Garden of the University of Würzburg. Only the 4th to 8th leaves below the inflorescence were chosen and leaf discs were punched out very carefully from the marginal regions of the leaves with major veins being omitted as completely as possible.
Extraction of cuticular waxes with chloroform (Roth, Karlsruhe, Germany) for 12 h at room temperature yielded polymer matrix membranes (MX). The composition of the cuticular membranes (cuticular waxes, cutin, hydrolysable components) was determined gravimetrically using an electronic microbalance (S3D, Sartorius, Göttingen, Germany). Acidic hydrolysis by treatment of matrix membranes with 6 N HCl at 110 °C for 12 h removed polar hydrolysable components such as polysaccharides and phenolics. The solid residue is referred to as cutin (Riederer and Schönherr, 1984).
Amino acids
Amino acids (Table 1) with purity always better than 99% were purchased from Fluka (Neu-Ulm, Germany). Aqueous solutions of the amino acids (0.01 mol l−1) were prepared for pH 1 with hydrochloric acid (HCl, 0.1 mol l−1), for pH 6 with 2-(N-morpholino)ethanesulphonic acid buffer (MES, 0.1 mol l−1 adjusted with NaOH), and for pH 11 with sodium hydroxide (NaOH, 0.1 mol l−1). These pH values were selected in order to study the permeation of the cationic (pH 1), zwitterionic (pH 6), and anionic (pH 11) forms of the amino acids, respectively (Fig. 1).
Table 1.
Physico-chemical properties of the amino acids studied
| Compound | MW (g mol−1) | MV (cm3 mol−1) | pKa | pKb | pl | log Ko/w (pl) |
| Glycine | 75.1 | 56.5 | 2.34 | 9.60 | 5.97 | −3.41 |
| Alanine | 89.1 | 70.6 | 2.34 | 9.69 | 6.00 | −2.99 |
| Proline | 115.1 | 87.9 | 1.99 | 10.60 | 6.30 | −2.54 |
| Valine | 117.2 | 98.7 | 2.32 | 9.62 | 5.96 | −2.08 |
| Leucine | 131.2 | 112.8 | 2.36 | 9.60 | 5.98 | −1.59 |
| Phenylalanine | 165.2 | 131.3 | 1.83 | 9.13 | 5.48 | −1.28 |
Molecular weights (MW), molar volumes (MV) (calculated according to Abraham and McGowan, 1987), acid dissociation constants (pKa), base dissociation constants (pKb), pH at the isoelectronic points (pl) (Lide, 1992) and octanol/water partition coefficients at the isoelectronic point (Ko/w) (estimated using the structure/property prediction and calculation programme ChemAxon, http://www.chemaxon.com) are given.
After derivatization with a commercially available amino acid analysing kit (EZ:faast, Phenomenex Ltd., Aschaffenburg, Germany), the amino acids were quantified by gas chromatography (GC-FID 6850 Series II, Agilent Technologies, Böblingen, Germany). The column used was included in the kit (Zebron ZB-AAA, 10 m×0.25 mm) and the gas chromatographic parameters were applied as recommended in the user manual. The amounts of amino acids were determined in relation to norvaline (included in the kit) as the internal standard. According to Cicchetti et al. (2008) individual response factors were measured for each amino acid. The sensitive gas chromatographic method which is able to detect amino acids in the range of pmoles is best suited for the measurement of the very small cuticular flux rates of hydrophilic solutes (octanol/water partition coefficient KO/W <0). Radiolabelled solutes, which are commonly used in permeation studies, rarely have radiochemical purities better than 99% and the results may be compromised by the contribution of lipophilic impurities (Bukovac, 1976).
Cuticular permeance
Cuticular membranes, which were previously incubated under corresponding pH conditions, were mounted between a donor and receiver compartment of transport chambers made out of stainless steel (Schreiber et al., 1995). The exposed area of the cuticle was 1.14 cm2. Solutions of amino acids (1 ml) at the respective pH were added to the donor side of the camber and cuticles were incubated overnight at 25 °C. Subsequently, experiments were initiated by adding 1 ml HCl (0.1 mol l−1), MES buffer (0.1 mol l−1) or NaOH (0.1 mol l−1 adjusted to pH 11 with HCl) to the receiver compartment.
When studying the trans-membrane diffusion of ions, the contribution of the electrical potential to the total driving force has to be considered, in addition to the concentration gradient. According to Tyree et al. (1990) absolute values of electrical potentials across isolated cuticles decrease with increasing ionic strength and decreasing concentration ratios of the adjacent solutions. Since the ionic strength of the solutions on both sides of the cuticle is high (100 mmol l−1) and the concentrations of amino acids applied to the donor side are lower by one order of magnitude (10 mmol l−1) the overall concentration ratio of total ions is close to unity (1.1). Therefore, the resulting electrical potential is very small (Tyree et al., 1990) and thus can be neglected. Hence, the concentration gradient of the amino acids is the sole driving force for permeation.
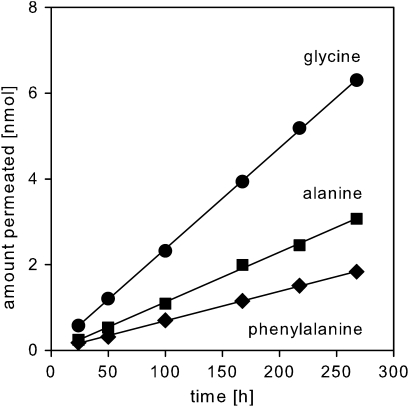
In time intervals (in the range of 1–3 d) the receiver solution was completely exchanged with fresh solution and the amount of amino acids permeated was quantified as described above. Therefore, the amino acid concentration in the receiver solution was practically zero at any time and steady-state conditions were obtained. The permeation chambers were continuously rotated at 25 °C between the sampling points. In all cases, linear transport kinetics were obtained (Fig. 2) and flux (F) was calculated from the slope of the linear plot of the amount permeated (M) versus time (t):
| (1) |
Fig. 2.
Representative kinetics for the permeation of amino acids across isolated ivy cuticular (Hedera helix) membranes at pH 6.
The cuticular permeance (P) was calculated from the flux divided by the exposed area (A) and the concentration gradient between the donor and receiver compartment (Δc):
| (2) |
Water permeability was measured with modified transport chambers without a receiver compartment (Schönherr and Lendzian, 1981). Cuticular membranes were mounted in transpiration chambers made from stainless steel with the upper side orientated towards the atmosphere. The donor compartment was filled with water of adjusted pH. The transpiration chambers were placed upside-down into plastic boxes containing dry silica gel and stored between the sampling points at 25 °C. The water flux was determined by measuring the weight loss of the chambers as a function of time. In all cases linear plots were obtained. The cuticular permeance (P) for water was obtained according to equation 2 from the transpiration rate (F) divided by the exposed area (A) and the concentration difference of water across the cuticular membrane (Δc) amounting to 0.998 g cm−3 at maximum driving force. Permeances were calculated on the basis of the water density in the liquid state. These values can easily be converted to vapour-based permeances by multiplication with a factor of 43270, i.e. the ratio of the densities of water in the liquid and the vapour state (Burghardt and Riederer, 2006).
Cuticular water sorption
The gravimetric method used by Chamel et al. (1991) for the investigation of the sorption of water vapour from the atmosphere is excellent, but is not suited for measuring the effect of pH and cations or anions on swelling. Therefore, the method of Lippold et al. (1999) was adapted to determine the pH-dependent water sorption of the cuticle. Isolated cuticular membranes of known dry weight were incubated in aqueous solutions of different pH (0.1 mol l−1 HCl pH 1; 0.1 mol l−1 MES buffer pH 6, 0.1 mol l−1 NaOH adjusted to pH 11 with HCl) for at least 3 d at 25 °C. Subsequently, the cuticles were taken from the liquid and the excess water adhering to the surfaces of the cuticular membranes was quickly removed with paper tissue. Afterwards the cuticles were weighed again. The amount of sorbed water within the cuticle was given by the difference between the dry and wet masses.
Titration of cuticular membranes
Dry cuticular membranes (200 mg) were added to 100 ml deionized water. Titration was carried out with 1.0 N HCl in order to adjust to an acidic pH in the external solution. Re-basification was achieved by the addition of small amounts of 1.0 N NaOH (5–100 μl). After each titration step, the sample was stirred until the pH reached a constant value. A blank sample of deionized water was treated in the same way as the control. Identical titration curves were obtained after re-acidification. For the potentiometric determination of pH, a glass electrode (inLab micro, Mettler-Toledo GmbH, Germany) linked to a pH meter (inoLab pH Level1, WTW, Germany) was used.
Statistics
All measurements were based on at least 10 replications. Results are given as means with 95 percentage confidence intervals (95% CI).
Results
Cuticle composition
Cuticular membranes of ivy consist of two lipophilic fractions: cuticular waxes (17.8±0.9%), which can be extracted by chloroform, and the insoluble polymer cutin (63.6±1.4%). In addition, cuticular membranes contain hydrolysable components (18.1±0.9%). This polar fraction is at least partially made up of polysaccharides (Lendzian and Kerstiens, 1991). The mass per unit area of the cuticular membrane amounted to 0.5±0.05 mg cm−2 yielding a thickness of about 4.6 μm, when a density of 1090 mg cm−3 (Schreiber and Schönherr, 1990) was assumed.
Cuticular permeances of amino acids
The permeances of glycine, alanine, proline, valine, leucine, and phenylalanine for native isolated cuticular membranes varied over one order of magnitude and ranged from 1.6×10−12 m s−1 (phenylalanine, pH 6) to 1.3×10−11 m s−1 (glycine, pH 11). Permeances were different for each ionic species. Permeances were lowest at pH 6 for the zwitterionic forms, followed by the cationic forms at pH 1. Highest permeances were obtained at pH 11 for the anionic forms (Table 2).
Table 2.
Cuticular permeances (PCM) of amino acids determined with isolated ivy (Hedera helix) cuticular membranes (CM) at different pH; means ±95% CIs
| Compound | PCM×1012(m s−1) pH 1 | PCM×1012 (m s−1) pH 6 | PCM×1012 (m s−1) pH 11 |
| Glycine | 9.6±1.4 | 7.8±1.9 | 13.4±2.3 |
| Alanine | 7.4±1.1 | 5.3±1.4 | 10.3±1.6 |
| Proline | 6.7±1.1 | 2.6±0.7 | 11.0±2.3 |
| Valine | 5.5±0.8 | 2.2±0.6 | 8.3±1.4 |
| Leucine | 5.0±0.8 | 1.9±0.5 | 8.4±1.4 |
| Phenylalanine | 5.0±0.91 | 1.6±0.4 | 7.8±1.4 |
Permeances of amino acids for matrix membranes
Permeances of the matrix membranes for the amino acids investigated at pH 6 ranged from 3.0×10−11 m s−1 (phenylalanine) to 9.8×10−11 m s−1 (glycine). Removal of cuticular waxes enhanced amino acid permeability by factors between 12.5 (glycine) and 19.1 (phenylalanine) (Table 3).
Table 3.
Permeances (PMX) of amino acids determined with isolated ivy (Hedera helix) dewaxed matrix membranes (MX) at pH 6 and the effect of wax extraction on the permeance (PMX×PCM−1); means ±95% CIs.
| Compound | PMX×1011 (m s−1) | PMX×PCM−1 |
| Glycine | 9.8±1.5 | 12.5 |
| Alanine | 7.0±1.2 | 13.2 |
| Proline | 4.6±0.8 | 17.5 |
| Valine | 4.0±0.7 | 18.5 |
| Leucine | 3.4±0.6 | 18.1 |
| Phenylalanine | 3.0±0.6 | 19.1 |
Cuticular bulk water permeability and water sorption
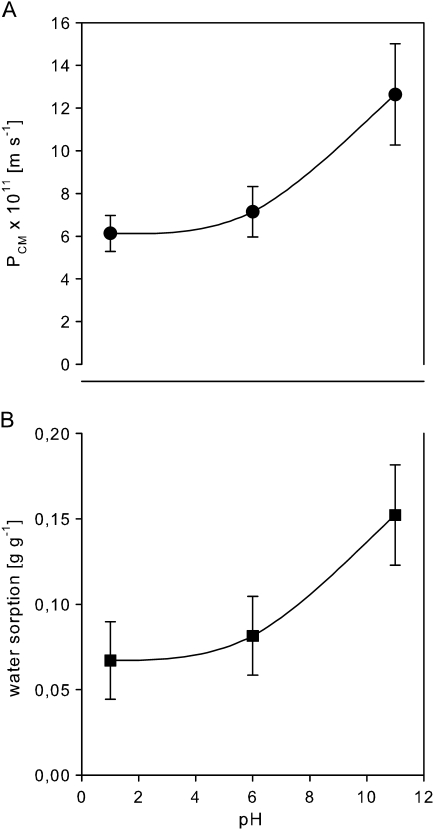
Cuticular permeances for water increased from 6.1×10−11 m s−1 at pH 1 to 1.3×10−10 m s−1 at pH 11 by a factor of 2.1 (Fig. 3A). Water sorption to isolated cuticular membranes amounted to 0.11 g g−1 at pH 1 and increased to 0.25 g g−1 at pH 11 varying by a factor of 2.2 (Fig. 3B).
Fig. 3.
Cuticular permeance (PCM) for water (A) and water sorption by isolated cuticular membranes (B) as a function of pH (means ±95% CIs).
Titration of cuticular membranes
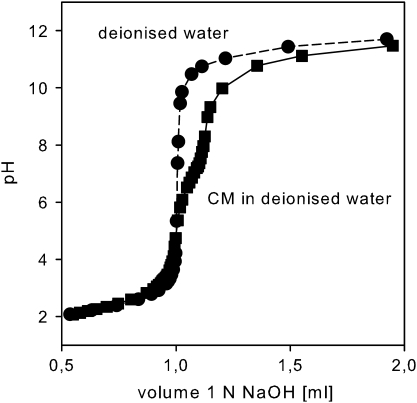
The comparison of the titration curves of isolated cuticular membranes with the blank sample of deionized water revealed the existence of charged sites within the cuticle (Schönherr and Bukovac, 1973; McFarlane and Berry, 1974). The shapes of the titration curves were identical in the acidic pH range. The buffering action of the cuticular membrane became obvious starting from pH 6 and proceeded towards the basic pH range (Fig. 4).
Fig. 4.
Titration of isolated cuticular membranes in comparison to a blank sample of deionized water.
Discussion
The solution–diffusion model (Riederer and Friedmann, 2006) of the lipophilic pathway implies that cuticular permeances increase with increasing octanol/water partition coefficients (Schönherr and Riederer, 1989). Estimating the cuticular permeances of the amino acids included in this study by using a predictive equation established for lipophilic compounds (Popp et al., 2005) yields values between 7.4×10−14 m s−1 (glycine) and 1.1×10−12 m s−1 (phenylalanine). These values are 1.5-fold (phenylalanine) to 105-fold (glycine) lower than the experimentally determined values for the zwitterionic species at pH 6 (Table 2). Further studies have confirmed that permeation rates of polar solutes are generally too high to be explained by the sorption–diffusion model (Schönherr and Schreiber, 2004; Popp et al., 2005).
The porous membrane model
As an alternative path of diffusion, the porous membrane model was proposed which states that, within the cuticular lipid matrix, water-filled strands cross the membrane forming a tortuous pathway (Schönherr and Schmidt, 1979). Hydration studies using infrared spectrometry revealed that water molecules in isolated cuticular membranes are mainly associated with the hydroxyl groups of polysaccharides (Maréchal and Chamel, 1996), which can be found in substantial amounts in cuticular membranes of several plant species (Riederer and Schönherr, 1984; Schreiber and Schönherr, 1990; Dominguez and Herédia, 1999) including ivy cuticular membranes. Carbonyl groups of the cutin polymer can also attract water via hydrogen bonds (Maréchal and Chamel, 1997). The sorption of liquid water to ivy cuticular membranes (Fig. 3B) is within the range of values reported previously for the sorption of water vapour to cuticles of different plant species (1% to 18% of the cuticle dry weight; Kerstiens and Lendzian, 1989; Chamel et al., 1991).
Fixed negative charges contribute to the porous nature of the CM.
The titration curve of ivy cuticular membranes reveals the presence of fixed weak acidic groups, which start to dissociate with rising pH (Fig. 4). According to Schönherr and Bukovac (1973) these dissociable groups are most likely carboxyl groups of pectin (dissociating between pH 3 to pH 6), non-esterified carboxyl groups of hydroxy fatty acids of the cutin polymer (dissociating between pH 6 to pH 9) and phenolic hydroxyl groups (dissociating above pH 9). Dissociation of the weak acidic groups induces a swelling of the cuticle by enhanced water sorption. This effect originates from the electrostatic repulsion of fixed charges of equal sign and the osmotically driven tendency of the interstitial fluid to dilute itself (Schönherr, 1976). Accordingly, the increase of water permeability of ivy cuticular membranes with rising pH (Fig. 3A) can be attributed to an increase of the water content of the cuticular membrane (Fig. 3B) due to dissociation of weak acidic groups with increasing pH (Fig. 4).
As negative charges are present in the cuticle, one would expect that the anionic amino acids at pH 11 are repelled by the action of the Donnan potential (Helfferich, 1962). From the titration experiment, a total exchange capacity of about 260 meq kg−1 can be estimated, which is in accordance with values reported for matrix membranes of Citrus aurantium (250 meq kg−1) and Prunus armeniaca (290 meq kg−1) (Schreiber and Schönherr, 2009). By contrast, the ion exchange capacity of tomato fruit cuticles is as twice as much (580 meq kg−1) because they contain high amounts of dissociable flavonoids (Schreiber and Schönherr, 2009).
Approximately 1 mg of cuticle was used in each permeation experiment and, consequently, about 1×1017 charges where exposed in the cuticle at ph 11. The total amount of amino acids which were applied as donor solution was 20-fold higher (about 2×1018 molecules in total). Therefore, the impact of the Donnan exclusion on the permeation of negative charged amino acids can be neglected because the efficiency of electrolyte exclusion decreases with increasing solution concentration of the co-ion (Tyree et al., 1990) and increasing swelling of the sorption domain (Helfferich, 1962). Hence, the effect of swelling was overriding the effect of repelling in the presence of a relatively high ionic strength.
Transport properties of the polar pathway
The permeation of amino acids within a water continuum across the cuticular membrane from an aqueous donor to an aqueous receiver compartment implies that no phase change occurs during the permeation process. The partition coefficient between the aqueous cuticular phase and the external aqueous solution is similar for all solutes and equal to unity. Unfortunately, sorption of highly water-soluble compounds to cuticular membranes is very low and difficult to measure. Therefore it cannot be determined experimentally with sufficient accuracy (Schreiber and Schönherr, 2008).
In the absence of the impact of partitioning processes, the cuticular permeance is proportional to the diffusion coefficient which depends on the size of the diffusing species. Consequently, permeances of hydrophilic solutes are a function of the molar volume (Potts and Guy, 1992):
| (3) |
P (m s−1) is the measured permeance, P0 (m s−1) is the permeance of a hypothetical molecule having zero molar volume, MV (cm3 mol−1) is the molar volume according to Abraham and McGowan (1987) and β (mol cm−3) is the size selectivity describing the dependence of the permeance on the molar volume (Potts and Guy, 1992).
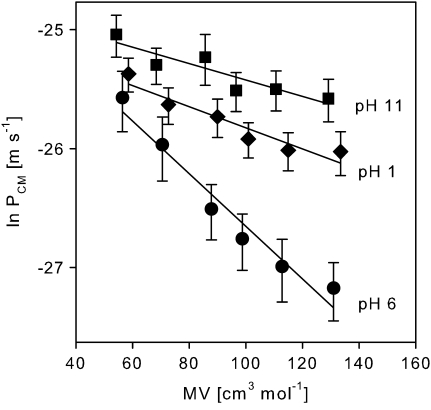
Cuticular permeances of the amino acids were dependent on pH and decreased within the respective pH with increasing molar volume. A plot of ln P versus MV results in a linear correlation (Fig. 5) with the slope of the regression line corresponds to the size selectivity (β) and the y-intercept is equal to P0 (Table 4). Both parameters can be related to a general expression describing the permeance (P) of a hydrophilic solute diffusing through a porous membrane (Peck et al., 1994; Mitragotri, 2003):
| (4) |
Fig. 5.
Correlation between cuticular permeance (PCM) (means ±95% CIs) and molar volume (MV) of the amino acids.
Table 4.
Parameters (±95% CI) of the regression lines obtained by plotting the natural logarithm of the permeances (P) versus the molar volumes (MV) (Figs 5, 6) according to equation 3
| Correlation: lnP versus MV | β (mol cm−3) | lnP0 (m s−1) | r2 |
| CM, pH 1 | 0.0089±0.004 | –24.9±0.6 | 0.91 |
| CM, pH 6 | 0.0221±0.006 | –24.4±0.6 | 0.96 |
| CM, pH 11 | 0.0069±0.004 | –24.7±0.4 | 0.83 |
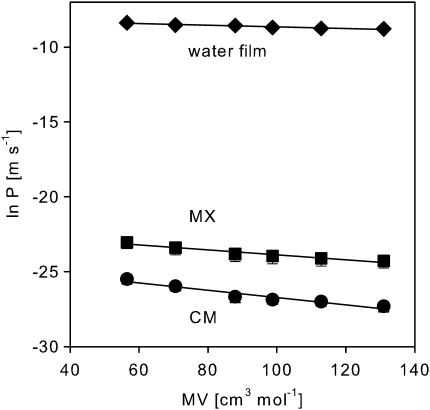
| MX, pH 6 | 0.0159±0.005 | –22.3±0.5 | 0.94 |
| Water film (thickness 4.6 μm) | 0.0055±0.002 | –8.1±0.2 | 0.94 |
The size selectivity (β) is given by the slope and the intercept is equal to the permeance (P0) of a hypothetical molecule of zero molar volume. CM, native isolated cuticle; MX, dewaxed isolated cuticle.
where ε is the porosity (relative pore surface), τ the tortuosity of the diffusional path which is many times longer than the bulk membrane thickness (l) and Dp the diffusion coefficient of the solute in the pore, which is a function of the pore radius (rp). Diffusion in narrow pores with small radii is hindered by steric restriction at the pore entrance (Ford and Gland, 1995) and friction at the pore wall (Knudsen diffusion). These two parameters decrease with decreasing molar volume of the solute, and turn out to be zero for a hypothetical molecule having zero molar volume (MV0). For such a molecule the diffusion coefficient in the pore is reduced to the diffusion coefficient in water and the permeance (P0) can be considered as a measure for the ratio of porosity to tortuosity (ετ−1).
In the present study the estimates of P0 remain unchanged over a wide range of pH (Table 4) which implies that porosity and tortuosity do not seem to be affected by pH. On the other hand it does not seem very likely that the pH-dependent swelling of the cuticle (Fig. 3) does not affect one or both of the parameters. It can be speculated that the changes of the two parameters compensate each other.
The size selectivity β reflects the transport properties of the polar pores for solute diffusion which are mainly determined by pore size (or more precisely: by the size distribution of passable polar pores within the cutin or the polysaccharide strands). The size selectivity is highest for the zwitterionic amino acids at pH 6 (0.0221 mol cm−3) and lowest for the anionic species at pH 11 (0.0069 mol cm−3) (Table 4). This finding may be linked to the polyelectrolytic character of the cuticle. It can be hypothesized that the pH-dependent increase in water sorption may increase the pore radius or broaden the pore size distribution reducing in this way the size selectivity for the permeation of the anionic species of the amino acids at pH 11. This effect was also shown for ethyl cellulose membranes whereas permeation at pH >6 takes place via polar pathways which become available due to the dissociation of the carboxyl groups in the ethyl cellulose (Lippold et al., 1999).
Dimensions of the polar pathway
It is instructive to compare the permeation of amino acids through cuticular membranes to the free diffusion of amino acids in an aqueous solution. Based on measured values taken from Longsworth (1953) permeances were recalculated for a water film of 4.6 μm which is equal to the average thickness of ivy cuticles. In relation, the permeance of a molecule of zero molar volume in cuticular membranes of Hedera helix is reduced by a factor of 1.2×107 (Fig. 6; Table 4). Also the size selectivity (β) for cuticular permeation at pH 6 is 4-fold higher than for unhindered diffusion in an aqueous solution (Fig. 6).
Fig. 6.
Permeance (P) of isolated cuticular membranes (CM) and matrix membranes (MX) measured at pH 6 (means ±95% CIs) in comparison to the permeance of a water film of equal thickness (4.6 μm) calculated from diffusion measurements in aqueous solution (Longsworth, 1953) as a function of the molar volume (MV) of the amino acids.
Eichert and Goldbach (2008) calculated mean pore radii of cuticular membranes considering an equation which was developed for technical membranes with defined pore size (Beck and Schulz, 1972).
According to
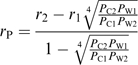 |
(5) |
(verified after Eichert and Goldbach, 2008) a mean pore radius (rP) of 0.6 nm at pH 6 can be calculated from the proportion of cuticular permeances (PC2×PC1−1) of two amino acids (1,2) of differing molecular radius (r2 >r1) and the reciprocal proportion of the corresponding permeances of a water film (PW1×PW2−1). This value lies in the range of pore radii reported previously for different plant cuticles (0.3–2.4 nm, Fernández and Eichert, 2009).
The polar pathway present seems to be restricted to a very small and limited fraction of the plant cuticle while cuticular waxes are regarded as the main constituent of the transport-limiting barrier. Permeances of water and solutes increase by several orders of magnitude after the removal of waxes (Schreiber and Schönherr, 2009). Wax extraction also affected the permeation of amino acids across ivy cuticular membranes at pH 6 (Table 3). While the size selectivity (β) is slightly reduced by a factor of 1.4 (not significant at the 95% CI level) the permeance of a molecule of zero molar volume (P0) increases by a factor of 8.2 (Fig. 6; Table 4). Under the assumption that cuticular waxes are embedded in the cutin polymer and, in addition, a superficial wax layer blocks a fraction of the aqueous pores (Schreiber and Schönherr, 2009), wax removal may enhance amino acid permeation by increasing porosity, decreasing tortuosity, and exposing additional polar pathways which were blocked by waxes before.
Impact of the hydration shell
All studies published so far on the permeation of ionic solutes across plant cuticles (Schönherr and Schreiber, 2004; Popp et al., 2005; Schlegel et al., 2005; Eichert and Goldbach, 2008) used either the molar volumes or molecular masses of the permeating solutes for describing the size selectivity of the corresponding pathway. These size parameters, however, do not reflect the real situation because ionic compounds bind hydration water very strongly by ion–dipole interactions.
Promising results on the hydration of non-electrolytes and electrolytes which were based on the measurement of the speed of sound in a sample solution of known density have been published recently (Burakowski and Gliński, 2007, 2008; Gliński and Burakowski, 2008). So data on the hydration of different ionic species of amino acids are available (Table 5).
Table 5.
Number of water molecules associated with amino acids (hydration number) at different pH, recalculated from Burakowski and Gliński (2008)
| Compound | pH 1 | pH 6 | pH 11 |
| Glycine | 4.9 | 5.5 | 0.2 |
| Alanine | 5.3 | 6.2 | 1.6 |
| Proline | 6.1 | 8.7 | 3.1 |
| Valine | 6.7 | 8.5 | 3.9 |
| Leucine | 7.9 | 9.7 | 4.7 |
Hydration numbers for phenylalanine could not be calculated, since no data are available on the hydration of the phenyl group.
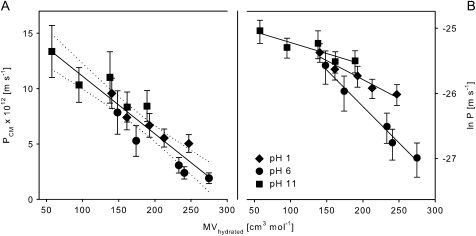
The effective molar volumes (MVhydrated) of the hydrated molecules can be calculated by adding up the molar volumes of the solute and the total volume of the surrounding water molecules (MV=16.7 cm3 mol−1). Excellent correlations are obtained when overall cuticular permeances of the amino acids (Table 2) are plotted versus the hydrated molar volume (Fig. 7A, r2=0.93). This implies that the hydration shell of a solute plays a decisive role in the cuticular permeation of ionic solutes.
Fig. 7.
Correlation between cuticular permeances (PCM) (A) or the natural logarithm of cuticular permeances (ln PCM) (B) and the molar volumes of amino acids taking the hydration shell into account (MVhydrated). Hydrated molar volumes were calculated, using hydration numbers from Burakowski and Gliński (2008) (Table 5). Phenylalanine was omitted from the correlation, since no data for the hydration number were available. Dotted lines (A) represent 95% confidence intervals of the regression line.
Most water molecules are associated with the zwitterionic amino acids and therefore the effective molar volume is biggest at pH 6. Since the measured permeances were lowest for the zwitterions, the impact of hydration shell outweighs the swelling effect of the cuticle and the proportion between the pore size and the size of the molecule is supposed to be less than at pH 1 or pH 11. This should favour a higher friction at the pore wall which is reflected by the highest size selectivity at pH 6 when lnP is plotted against the hydrated molar volume (Fig. 7B).
Consequently, for further in-depth analysis of the polar cuticular pathway it is fundamental to consider both the cuticular swelling due to the dissociation of fixed charges as well as the changeable hydration shell size of the permeating solutes.
Acknowledgments
This work was supported in part by a grant from Syngenta, Jealott's Hill International Research Centre, UK. Drs Jacek Glińsky and Andrzej Burakowski, University of Wrocław, contributed valuable advice on the hydration of organic molecules in water. We are also indebted to the Botanical Garden of the University of Würzburg for supplying plant material.
References
- Abraham MH, McGowan JC. The use of characteristic volumes to measure cavity terms in reversed phase liquid chromatography. Chromatographia. 1987;23:243–246. [Google Scholar]
- Beck RE, Schultz JS. Hindrance of solute diffusion within membranes as measured with microporous membranes of known pore geometry. Biochimica et Biophysica Acta. 1972;255:273–303. doi: 10.1016/0005-2736(72)90028-4. [DOI] [PubMed] [Google Scholar]
- Bukovac MJ. Herbicide entry into plants. In: Audus LJ, editor. Herbicides: physiology, biochemistry, ecology. New York, USA: Academic Press; 1976. pp. 335–364. [Google Scholar]
- Burakowski A, Gliński J. Hydration numbers of non-electrolytes. Application of the acoustic method of Pasynski. Chemical Physics. 2007;332:336–340. [Google Scholar]
- Burakowski A, Gliński J. Hydration of the zwitterionic forms of amino acids from the acoustic Pasynski method. Acta Physica Polonica A. 2008;114:39–44. [Google Scholar]
- Burghardt M, Riederer M. Cuticular transpiration. In: Riederer M, Müller C, editors. Biology of the plant cuticle. Oxford, UK: Blackwell Publishing; 2006. pp. 292–311. [Google Scholar]
- Chamel A, Pineri M, Escoubes M. Quantitative determination of water sorption by plant cuticles. Plant, Cell and Environment. 1991;14:87–95. [Google Scholar]
- Cicchetti E, Merle P, Chaintreau A. Quantitation in gas chromatography: usual practices and performances of a response factor database. Flavour and Fragrance Journal. 2008;23:450–459. [Google Scholar]
- Derridj S. Nutrients on the leaf surface. In: Morris CE, Nicot PC, Nguyen-The C, editors. Aerial plant surface microbiology. New York, USA: Plenum Press; 1996. pp. 25–42. [Google Scholar]
- Dominguez E, Herédia A. Water hydration in cutinised cell walls: a physico-chemical analysis. Biochimica et Biophysica Acta. 1999;1426:168–176. doi: 10.1016/s0304-4165(98)00152-4. [DOI] [PubMed] [Google Scholar]
- Eichert T, Goldbach HE. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces: further evidence for a stomatal pathway. Physiologia Plantarum. 2008;132:491–502. doi: 10.1111/j.1399-3054.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- Ersöz M, Vural US, Duncan HJ. Transport and selectivities of amino acids on periderm and cutciular membranes. Separation Science and Technology. 1995;30:2173–2187. [Google Scholar]
- Fernández V, Eichert T. Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Critical Reviews in Plant Sciences. 2009;28:36–68. [Google Scholar]
- Ford DM, Glandt ED. Steric hindrance at the entrances to small pores. Journal of Membrane Science. 1995;107:47–57. [Google Scholar]
- Gliński J, Burakowski A. Is the hydration number of a non-electrolyte additive with length and constituents of the solute molecule? The European Physical Journal Special Topics. 2008;154:275–273. [Google Scholar]
- Gulyaeva N, Zaslavsky A, Lechner P, Chait A, Zaslavsky B. pH dependence of the relative hydrophobicity and lipophilicity of amino acids and peptides measured by aqueous two-phase and octanol-buffer partitioning. Journal of Peptide Research. 2003;61:71–79. doi: 10.1034/j.1399-3011.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Helfferich F. Ion exchange. New York, San Francisco, Toronto, London: McGraw-Hill Book Company; 1962. [Google Scholar]
- Kerstiens G, Lendzian KJ. Interactions between ozone and plant cuticles. ll. Water permeability. New Phytologist. 1989;112:21–27. [Google Scholar]
- Krimm U, Abanda-Nkpwatt D, Schwab W, Schreiber L. Epiphytic microorganisms on strawberry plants (Fragaria ananassa cv. Elsanta): identification of bacterial isolates and analysis of their interaction with leaf surfaces. FEMS Microbiology Ecology. 2005;53:483–492. doi: 10.1016/j.femsec.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lendzian K, Kerstiens G. Sorption and transport of gases and vapors in plant cuticles. Reviews of Environmental Contamination and Toxicology. 1991;121:65–128. [Google Scholar]
- Leveau JHJ. Microbial communities in the phyllosphere. In: Riederer M, Müller C, editors. Biology of the plant cuticle. Oxford, UK: Blackwell Publishing; 2006. pp. 334–367. [Google Scholar]
- Lide DR. Handbook of chemistry and physics. Boca Raton, USA: CRC Press; 1992. [Google Scholar]
- Lippold BC, Gunder W, Lippold BH. Drug release from diffusion pellets coated with the aqueous ethyl cellulose dispersion aquacoat (R) ECD-30 and 20% dibutyl sebacate as plasticizer: partition mechanism and pore diffusion. European Journal of Pharmaceutics and Biopharmaceutics. 1999;47:27–32. doi: 10.1016/s0939-6411(98)00084-8. [DOI] [PubMed] [Google Scholar]
- Longsworth LG. Diffusion measurements, at 25°, of aqueous solutions of amino acids, peptides and sugars. Journal of the American Chemical Society. 1953;75:5705–5709. [Google Scholar]
- Lüttge U. Stofftransport der Pflanzen. Berlin, Heidelberg, New York: Springer-Verlag; 1973. [Google Scholar]
- Luque P, Bruque S, Herédia A. Water permeability of isolated cuticular membranes: a structural analysis. Archives of Biochemistry and Biophysics. 1995;317:417–422. doi: 10.1006/abbi.1995.1183. [DOI] [PubMed] [Google Scholar]
- Maréchal Y, Chamel A. Water in a biomembrane by infrared spectrometry. Journal of Physical Chemistry. 1996;100:8551–8555. [Google Scholar]
- Maréchal Y, Chamel A. Interaction configurations of H2O molecules absorbed in isolated plant cuticles by infrared spectrometry. Biospectroscopy. 1997;3:143–153. [Google Scholar]
- McFarlane JC, Berry WL. Cation penetration through isolated leaf cuticles. Plant Physiology. 1974;53:723–727. doi: 10.1104/pp.53.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. Journal of Controlled Release. 2003;86:69–92. doi: 10.1016/s0168-3659(02)00321-8. [DOI] [PubMed] [Google Scholar]
- Peck KD, Ghanem AH, Higuchi WI. Hindered diffusion of polar molecules through and effective pore radii estimates of intact and ethanol treated human epidermal membrane. Pharmaceutical Research. 1994;11:1306–1314. doi: 10.1023/a:1018998529283. [DOI] [PubMed] [Google Scholar]
- Popp C, Burghardt M, Friedmann A, Riederer M. Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: permeation of water and uncharged organic compounds. Journal of Experimental Botany. 2005;56:2797–2806. doi: 10.1093/jxb/eri272. [DOI] [PubMed] [Google Scholar]
- Potts RO, Guy RH. Predicting skin permeability. Pharmaceutical Research. 1992;9:663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- Riederer M, Friedmann A. Transport of lipophilic non-electrolytes across the cuticle. In: Riederer M, Müller C, editors. Biology of the plant cuticle. Oxford, UK: Blackwell Publishing; 2006. pp. 249–278. [Google Scholar]
- Riederer M, Schönherr J. Accumulation and transport of (2,4-dichlorophenoxy)acetic acid in plant cuticles. l. Sorption in the cuticular membrane and its components. Ecotoxicology and Environmental Safety. 1984;8:236–247. doi: 10.1016/0147-6513(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Riederer M, Schreiber L. Protecting against water loss: analysis of the barrier properties of plant cuticles. Journal of Experimental Botany. 2001;52:2023–2032. doi: 10.1093/jexbot/52.363.2023. [DOI] [PubMed] [Google Scholar]
- Schlegel TK, Schönherr J, Schreiber L. Size selectivity of aqueous pores in stomatous cuticles of Vicia faba leaves. Planta. 2005;221:648–655. doi: 10.1007/s00425-005-1480-1. [DOI] [PubMed] [Google Scholar]
- Schönherr J. Water permeability of isolated cuticular membranes: the effect of pH and cations on diffusion, hydrodynamic permeability and size of polar pores. Planta. 1976;128:113–126. doi: 10.1007/BF00390312. [DOI] [PubMed] [Google Scholar]
- Schönherr J. Calcium chloride penetrates pear leaf cuticles via aqueous pores. Planta. 2000;212:112–118. doi: 10.1007/s004250000373. [DOI] [PubMed] [Google Scholar]
- Schönherr J. A mechanistic analysis of penetration of glyphosate salts across astomatous cuticular membranes. Pest Management Science. 2002;58:343–351. doi: 10.1002/ps.462. [DOI] [PubMed] [Google Scholar]
- Schönherr J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. Journal of Experimental Botany. 2006;57:2471–2491. doi: 10.1093/jxb/erj217. [DOI] [PubMed] [Google Scholar]
- Schönherr J, Bukovac MJ. Ion exchange properties of isolated tomato fruit cuticular membrane: exchange capacity, nature of fixed charges and cation selectivity. Planta. 1973;109:73–93. doi: 10.1007/BF00385454. [DOI] [PubMed] [Google Scholar]
- Schönherr J, Lendzian K. A simple and inexpensive method for measuring water permeability of isolated plant cuticular membranes. Zeitschrift für Pflanzenphysiologie. 1981;102:321–327. [Google Scholar]
- Schönherr J, Riederer M. Plant cuticles sorb lipophilic compounds during enzymatic isolation. Plant, Cell and Environment. 1986;9:459–466. [Google Scholar]
- Schönherr J, Riederer M. Foliar penetration and accumulation of organic chemicals in plant cuticles. Reviews of Environmental Contamination and Toxicology. 1989;108:1–70. [Google Scholar]
- Schönherr J, Schreiber L. Size selectivity of aqueous pores in astomatous cuticular membranes isolated from Populus canescens (Aiton) Sm. leaves. Planta. 2004;219:405–411. doi: 10.1007/s00425-004-1239-0. [DOI] [PubMed] [Google Scholar]
- Schreiber L. Polar paths of diffusion across plant cuticles: new evidence for an old hypothesis. Annals of Botany. 2005;95:1069–1073. doi: 10.1093/aob/mci122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L. Characterization of polar paths of transport in plant cuticles. In: Riederer M, Müller C, editors. Biology of the plant cuticle. Oxford, UK: Blackwell Publishing; 2006. pp. 280–291. [Google Scholar]
- Schreiber L, Bach S, Kirsch T, Knoll D, Schalz K, Riederer M. A simple photometric device for analysing cuticular transport physiology: surfactant effect on permeability of isolated cuticular membranes of Prunus laurocerasus L. Journal of Experimental Botany. 1995;46:1915–1921. [Google Scholar]
- Schreiber L, Schönherr J. Phase transitions and thermal expansion coefficients of plant cuticles. The effects of temperature on structure and function. Planta. 1990;182:186–193. doi: 10.1007/BF00197109. [DOI] [PubMed] [Google Scholar]
- Schreiber L, Schönherr J. Water and solute permeability of plant cuticles. Measurement and data analysis. Berlin, Germany: Springer-Verlag; 2009. [Google Scholar]
- Singh P, Piotrowski M, Kloppstech K, Gau AE. Investigations on epiphytic living Pseudomonas species from Malus domestica with an antagonistic effect to Venturia inaequalis on isolated plant cuticle membranes. Environmental Microbiology. 2004;6:1149–1158. doi: 10.1111/j.1462-2920.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- Tukey HB. The leaching of substances from plants. Annual Review of Plant Physiology. 1970;21:305–324. [Google Scholar]
- Tyree MT, Scherbatskoy TD, Tabor CA. Leaf cuticles behave as asymmetric membranes. Plant Physiology. 1990;92:103–109. doi: 10.1104/pp.92.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunger LM, Cramer RD. Measurement and correlation of partition coefficients of polar amino acids. Molecular Pharmacology. 1981;20:602–608. [PubMed] [Google Scholar]