Abstract
Mature pollen is very sensitive to cold stress in chilling-sensitive plants. Plant WRKY DNA-binding transcription factors are key regulators in plant responses to abiotic and biotic stresses. Previous studies have suggested that WRKY34 (At4g26440) gene might be involved in pollen viability, although the mechanism involved is unclear. In this study, it is shown that cold treatment increased WRKY34 expression in the wild type, and promoter-GUS analysis revealed that WRKY34 expression is pollen-specific. Enhanced green fluorescent protein-tagged WRKY34 was localized in the nuclei. Pollen harbouring the wrky34 allele showed higher viability than pollen with the WRKY34 allele after cold treatment. Further functional analysis indicated that the WRKY34 transcription factor was involved in pollen development regulated by the pollen-specific MIKC* class of MADS-domain transcription factors under cold stress, and cold-insensitivity of mature wrky34 pollen might be partly attributable to the enhanced expression of transcriptional activator CBFs in the mutants. Thus, the WRKY34 transcription factor negatively mediated cold sensitivity of mature Arabidopsis pollen and might be involved in the CBF signal cascade in mature pollen.
Keywords: Arabidopsis, cold stress, pollen, transcription factor, WRKY34
Introduction
Temperature is one of the most important environmental factors affecting plant development and crop productivity. Certain stages of the plant life cycle are more sensitive to chilling than others. Seedlings appear to be more susceptible than plants at advanced stages of development (Lyons, 1973), and pollen maturation is the most sensitive process in the entire life cycle of cold-sensitive plants (Sataka and Koike, 1983; Patterson et al., 1987). Cold stress significantly reduces the pollen germination rate and seed production of Arabidopsis (Lee and Lee, 2003).
Because of its crucial function in the plant reproductive cycle, pollen has been the focus of considerable cytological, biochemical, and molecular research. In recent years, investigation of processes underlying pollen development and function has been extended by studying pollen-related gene expression. For example, the pollen-specific MIKC* (MADS DNA-binding domain, intervening domain, keratin-like domain, and c-terminal domain) class of MADS-domain transcription factors are required for pollen maturation and tube growth, and could affect the expression of many genes specific to mature pollen grains in Arabidopsis (Verelst et al., 2007a, b; Adamczyk and Fernandez, 2009). Arabidopsis phosphatidylinositol 3-kinase is essential for vacuole reorganization and nuclear division during pollen development (Lee et al., 2008). Arabidopsis MS1 is involved in the development of pollen and the tapetum (Ito et al., 2007). RPG1 encodes a plasma membrane protein and is required for exine pattern formation of microspores in Arabidopsis (Guan et al., 2008). The FLP1 protein is likely to play a role in the synthesis of the components of tryphine and sporopollenin of the exine (Ariizumi et al., 2003).
Most plants, such as Arabidopsis, develop tolerance to freezing after exposure to low, non-freezing temperatures. This adaptive process, known as cold acclimation, involves profound changes in biochemistry, physiology, and plant transcriptome. In Arabidopsis, cold-regulated genes have been estimated to constitute about 4–20% of the genome (Lee et al., 2005; Hannah et al., 2005). Considerable progress has been made in the past decade in elucidating the transcriptional networks regulating cold acclimation. The ICE1–CBF transcriptional cascade plays a central role in the cold-response pathway in Arabidopsis (Thomashow, 1999; Chinnusamy et al., 2007). CBF transcription factors regulate COR (COLD RESPONSIVE) genes by binding C-repeat/dehydration response elements (CRT/DRE) to their promoters (Stockinger et al., 1997; Fowler and Thomashow, 2002). CBFs regulate the expression of genes involved in phosphoinositide metabolism, transcription, osmolyte biosynthesis, ROS detoxification, membrane transport, hormone metabolism, and signalling, and many other genes with known or presumed cellular protective functions (Fowler and Thomashow, 2002; Maruyama et al., 2004; Lee et al., 2005). Over-expression of CBF1, CBF2, and CBF3 causes phenotypes associated with freezing tolerance, such as increased proline and sugar concentrations and transcriptional activation of COR genes (Jaglo-Ottosen et al., 1998; Gilmour et al., 2004). Many important genes in the cold-response pathway have been studied in detail, such as ICE1, ZAT12, HOS9, and MYB15 (Chinnusamy et al., 2003; Lee et al., 2005; Agarwal et al., 2006; Benedict et al., 2006). Although cold stress responses in whole plants have been extensively studied, the effect of cold stress on the development and function of plant organs, especially that of pollen, has received less attention.
The family of plant-specific WRKY transcription factors comprises over 70 members in Arabidopsis (Eulgem et al., 2000; Dong et al., 2003; Eulgem and Somssich, 2007). WRKY proteins typically contain one or two domains composed of about 60 amino acids with the conserved amino acid sequence WRKYGQK, together with a novel zinc-finger motif. The WRKY domain shows a high binding affinity to the TTGACC/T W-box sequence (Ulker and Somssich, 2004).
Accumulating evidence has demonstrated that WRKY genes are involved in regulating plant responses to biotic stresses. A majority of studies on WRKY genes address their involvement in disease responses and salicylic acid (SA)-mediated defence (Dellagi et al., 2000; Eulgem et al., 2000; Asai et al., 2002; Zheng et al., 2007; Lai et al., 2008). In addition, WRKY genes are involved in plant responses to wounding (Hara et al., 2000) and low Pi stress (Chen et al., 2009). Although most WRKY proteins studied thus far have been implicated in regulating biotic stress responses, some WRKY genes regulate plant responses to freezing, oxidative stress, drought, salinity, cold, and heat (Huang and Duman, 2002; Seki et al., 2002; Rizhsky et al., 2004; Li et al., 2009; Qiu and Yu, 2009). In addition, increasing evidence indicates that WRKY proteins are key regulators in certain developmental processes. Some WRKY genes regulate biosynthesis of anthocyanins (Johnson et al., 2002), starch (Sun et al., 2003), and sesquiterpenes (Xu et al., 2004). Other WRKY genes may regulate embryogenesis (Lagace and Matton, 2004), seed size (Luo et al., 2005), seed coat and trichome development (Johnson et al., 2002; Ishida et al., 2007), senescence (Robatzek and Somssich, 2001; Miao and Zentgraf, 2007; Jing et al., 2009), and seed germination and post-germination arrest of development by abscisic acid (Jiang and Yu, 2009).
Based on the number of WRKY domains and the pattern of the zinc-finger motif, WRKY proteins can be divided into three different groups in Arabidopsis (Eulgem et al., 2000). WRKY34 is a group I WRKY family transcription factor (Chinnusamy et al., 2007). The promoter of WRKY34 is male gametophyte-specific (Honys et al., 2006). In the present study, expression and subcellular localization of WRKY34 in response to cold stress was investigated in the wild type (WT) and wrky34 mutants using promoter–GUS analysis and quantitative RT-PCR. The objective was to elucidate the roles of the WRKY34 transcription factor in mediating the cold sensitivity of mature Arabidopsis pollen grains.
Materials and methods
Plant growth conditions and pollen collection
Seeds of wrky34-2 (SAIL_1284_C01), agl65 (SALK_009651C), agl66 (SALK_072108), and agl104 (SALK_066443C) were obtained from the Arabidopsis Biological Resource Center (ABRC). Wild-type A. thaliana Col-0 plants and the mutants were grown in a greenhouse with temperature controlled at 22 °C and a 16 h photoperiod (photosynthetically active radiation about 120 μmol m−2 s−1). For practical reasons the different genotypes were grown, harvested, and processed in separate batches, each concurrently with WT (control) plants.
Flowers at developmental stage 13 (Smyth et al., 1990) were marked at a fixed time in the morning. For the cold treatment, the plants were transferred to a growth chamber, maintained at 4 °C under the same light conditions as in the greenhouse, for 24 h or 48 h. After cold treatment, mature pollen grains were harvested from the marked flowers by shaking in 0.3 M mannitol, as described by Honys and Twell (2003). The freshly harvested pollen was ground with quartz sand and total RNAs were extracted using the RNAeasy Plant Mini Kit (Qiagen, Valencia, CA). DNA was removed via an on-column DNAse treatment, and the RNA extracts were stored at –80 °C prior to use.
Competition experiments and plant genotyping
To determine whether cold stress affects the segregation ratio of progeny from self-fertilized WRKY34/wrky34 heterozygotes produced by crosses between wrky34 mutants and the WT, and because WRKY34 was expressed only in pollen, plants with flowers at stage 13 were treated with cold stress; flowers from untreated greenhouse-grown plants were used as the control. After cold treatment, the WRKY34/wrky34 plants were transferred to the greenhouse and grown until the seeds were harvested. The seeds were germinated on Murashige and Skoog medium, and genomic DNA was isolated from the seedlings and used as a template for PCR amplification of DNA fragments corresponding to the WT and the T-DNA insertion loci. The number of plants with the WRKY34/wrky34 and wrky34/wrky34 genotype (w34), and the number of plants with the WRKY34/wrky34 and WRKY34/WRKY34 genotype (W34), were counted. To quantify the fertility of flowers exposed to cold stress further, the number of seeds in siliques from flowers that were treated at 4 °C for 24 h or 48 h was scored.
To determine which gametophyte was responsible for the abnormal segregation ratio, reciprocal crosses between cold-treated heterozygous and unstressed WT plants were performed. The F1 progeny was analysed by PCR-based genotyping.
All experiments were configured such that the expected transmission was always 50% and differences in the frequency of recombination between loci would have no impact on the outcome. In PCR-based genotyping, the presence of the T-DNA insertion allele was detected using the LB primer derived from the T-DNA sequence (5′-AAACGTCCGCAATGTGTTAT-3′) and a locus-specific primer. Presence of the WT allele was detected using the following gene-specific primers: LP1 (5′-GCGAGATCCGGGTTTAATGCACCG-3′) and RP1 (5′-GCATGTCTTGGCCAGTACCGGATG-3′) for wrky34-1, and LP2 (5′-GTCATTGGGCACAGCACTGTCT-3′) and RP2 (5′-ACTTGGGCTCAAGCTGAAACGTG-3′) for wrky34-2.
Pollen phenotyping
Light and epifluorescence microscopic examination of DAPI-stained pollen, including image capture and processing, were performed as described previously (Park et al., 1998). To characterize phenotypically mature pollen of the wrky34 homozygous mutants under cold stress, the pollen viability of homozygous plants was analysed. Evaluation of mature pollen viability by staining with fluorescein diacetate (FDA) in vitro (Verelst et al., 2007b) and pollen germination assays in vivo were performed as described previously with some modifications (Mori et al., 2006; Chhun et al., 2007).
To determine the pollen germination frequency in vivo, flowers at the beginning of stage 12 (Smyth et al., 1990) were emasculated. After waiting 12–24 h to ensure that the stigma was receptive, a saturating amount of pollen from a donor plant was placed on the stigma. After 30 min, the pistils were removed and directly stained with aniline blue on a glass slide for 30 min before observation with an Olympus BX51 ultraviolet (UV) epifluorescent microscope (Chhun et al., 2007). Pollen grains attached to the stigma and in which a pollen tube was detected with UV fluorescence were classified as having germinated.
To detect pollen tube elongation in vivo, after cold treatment the plants were moved to the greenhouse for 5 h, after which pistils were excised and the stigma fixed in acetic acid:ethanol (1:3, v/v) for 24 h, then softened with 8 M NaOH for 24 h. The stigma was washed in distilled water three times, and then stained with aniline blue on a glass slide for 30 min prior to observation by UV microscopy. Pollen grains attached to the stigma and in which pollen tube growth in the style was detected by UV fluorescence were classified as possessing elongated pollen tubes (Mayer and Gottsberger, 2000; Chhun et al., 2007).
Generation of 35S::WRKY34 and plant transformation
To produce transgenic plants, the WRKY34 full-length cDNA was cloned into the pOCA30 vector, which contains the modified CaMV 35S promoter. The recombinant plasmid was introduced into Agrobacterium tumefaciens GV3101 and used to transform A. thaliana using the floral dip method (Clough and Bent, 1998). The transformed lines were selected for resistance to kanamycin (50 μg ml−1). Northern blot analysis was used for further confirmation of their transgenic identity. High levels of homozygous lines (T3 generation) were obtained from independent transformants.
Construction of promoter::GUS reporters and GUS staining
The 1.5 kb promoter fragments of WRKY34 were amplified using ExTaq (TaKaRa) and the gene-specific primers. The resulting fragments were subcloned upstream of the GUS reporter gene in the pCAMBIA1300 Ti-derived binary vector (CAMBIA) and introduced into WT Arabidopsis plants. Transformation and GUS staining was performed as described by Yang et al. (2003).
Subcellular localization of WRKY34
The WRKY34 full-length cDNA was linked to the pBluscript KS II vector, and the enhanced green fluorescent reporter gene (eGFP) was subcloned behind the WRKY34 cDNA. The WRKY34–eGFP fragment was cloned by ligation into the sites behind the CaMV 35S promoter of pOCA30. The plasmid was isolated using Qiagen kits (Valencia, CA, USA), and concentrated to about 1 μg μl−1. The recombinant plasmid was introduced into A. tumefaciens GV3101 and used to transform Nicotiana benthamiana epidermal cells. A plasmid containing eGFP alone was concentrated as above and bombarded in parallel as a control. Transformation of N. benthamiana epidermal cells and localization of the protein were performed essentially as described previously (Asai et al., 2008).
Quantitative real-time PCR
cDNA was obtained from 1 μg total RNA in a 20 μl reaction volume using the First Strand cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany). Each cDNA sample was diluted 1:20 with water, and 2 μl of this dilution was used as template for quantitative RT-PCR. Half-reactions (10 μl each) were performed with the Lightcycler FastStart DNA Master SYBR Green I Kit (Roche, Mannheim, Germany) on a Roche LightCycler 480 real-time PCR machine, according to the manufacturer's instructions. ACT2 (AT3G18780) was used as a control. Gene-specific primers used to detect transcripts are listed in Supplementary Table S1 at JXB online.
MIKC*-type transcription factors are known to be important regulators during pollen maturation and tube growth in Arabidopsis, and are negative regulators of the WRKY34 gene (Verelst et al., 2007b). The effect of these genes (AGL66, AGL104, and AGL65) on WRKY34 expression under cold stress, and whether the expression of MIKC* genes is affected by WRKY34 and cold stress, was investigated in the present study. Quantitative RT-PCR was used to measure mRNA transcript levels for the five pollen-expressed MIKC* genes in mature pollen of both the WT and wrky34 mutants.
The effect of WRKY34 knockdown on cold regulation of CBF transcription factors was also examined, as WRKY34 is a cold-responsive transcription factor and the transcriptional activator CBF cold-response pathway plays a prominent role in cold acclimation (Stockinger et al., 1997; Thomashow, 1999; Fowler and Thomashow, 2002; Chinnusamy et al., 2007).
Results
Expression and subcellular localization of WRKY34
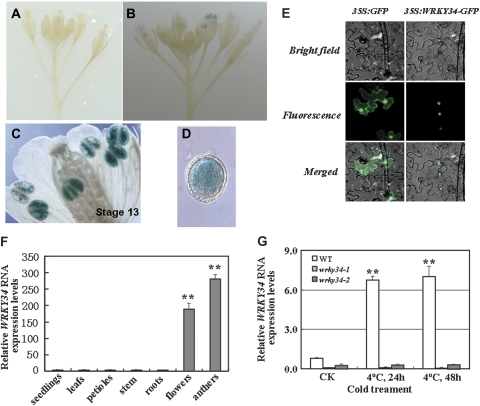
In total, 37 T2 plants were analysed for GUS activity in inflorescences, bud clusters, and flowers. In 35 plants, GUS activity was only detected in the male gametophyte (Fig. 1A, B), and was observed in mature pollen grains from flowers at stage 13 (Fig. 1C, D). In seedlings of ∼20 lines of T2 plants for each line, no GUS expression was observed in seedlings, leaves, roots, and stamen vascular tissue (data not shown). Measurement of WRKY34 mRNAs in WT plant organs by quantitative RT-PCR showed that WRKY34 mRNAs were strongly detected in male gametophytic tissues, and barely detected in seedling, leaves, petioles, stems, and roots (Fig. 1F).
Fig. 1.
Expression patterns and subcellular localization of WRKY34. (A) A negative control non-transgenic wild-type (WT) inflorescence, showing no GUS activity. (B) A transgenic inflorescence, showing GUS staining in the anthers. (C) Transgenic mature pollen grains with GUS staining at the floral developmental stage 13. (D) Transgenic mature pollen grains with GUS staining. (E) The subcellular localization of the WRKY34–GFP fusion protein in Nicotiana benthamiana epidermal cells. Images in the left column show the control plasmid expressing only the green fluorescent protein (GFP) and those in the right column show the WRKY34–GFP fusion protein expressed in N. benthamiana epidermal cells. The cells were examined with brightfield (top) and UV fluorescence (middle) microscopy, and as a merged image (bottom) showing either the diffused (control plasmid) or the nuclear localization of the proteins. (F) Quantitative RT-PCR comparison of the WRKY34 mRNA expression levels in different tissues from the WT plants grown at 22 °C. (G) Quantitative RT-PCR comparison of WRKY34 RNA expression levels in cold-treated (4 °C) and untreated (22 °C) mature pollen of the WT and wrky34 mutant. After cold treatment, WRKY34 RNA expression levels significantly increased in the WT, and showed no distinct change in the wrky34 mutants. Error bars indicate standard deviations of three independent biological samples. Differences between the untreated and treated plants with cold stress are significant at P <0.01 (**).
As shown in Fig. 1, the fluorescence signal of the WRKY34-eGFP construct was detected in the nuclei of the Nicotiana benthamiana cells (Fig. 1E).
Quantitative RT-PCR revealed that WRKY34 transcription in mature pollen increased by more than 7-fold after 24 h or 48 h cold stress compared to the control (Fig. 1G).
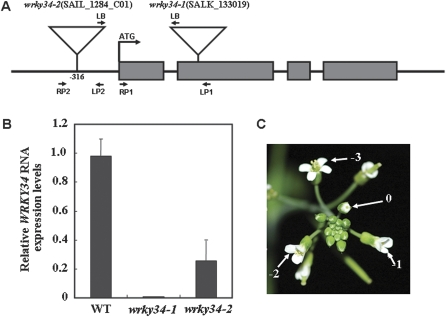
Identification of homozygous wrky34 mutants
Characterization of two homozygous wrky34 mutants is shown in Fig. 2. Mature pollen of the wrky34-1 (SALK_133019) mutant, which contained T-DNA at the second exon (Fig. 2A), produced no WRKY34 transcripts, in contrast to the WT (Fig. 2B). In the wrky34-2 (SAIL_1284_C01) mutant, which contained T-DNA at position –316 relative to the ATG of WRKY34 (Fig. 2A), WRKY34 expression in mature pollen was significantly reduced (Fig. 2B). WRKY34 transcripts were also detected in the wrky34 mutants after cold treatment and the level of WRKY34 transcripts for both mutants did not change distinctly in response to cold stress (Fig. 1G). The wrky34 mutant lines were sensitive to kanamycin since it harboured the T-DNA, which necessitated PCR-based analyses of the genotypes. No morphological abnormalities were observed in the wrky34 mutants after growth at 22 °C (data not shown).
Fig. 2.
Characterization of wrky34-1 and wrky-2 mutants, and developmental stage of the inflorescence at the time of cold treatment. (A) Exon and intron structure of the Arabidopsis WRKY34 gene, showing the locations of the T-DNA insertion sites in both mutants. Boxes indicate exons. (B) Relative WRKY34 RNA expression in mature pollen of the wrky34 mutants. Error bars indicate standard deviations of three independent determinations. Three independent experiments were shown by re-extracting RNA from other samples. Each experiment was also executed three times. (C) Flowers at stage 13 at the time of cold treatment are numbered ‘0’; flowers at more advanced stages are indicated by negative numbers.
Segregation ratios in progeny of self-fertilized WRKY34/wrky34 heterozygous mutants harbouring a T-DNA insertion allele showed distorted segregation ratios after cold stress
The ratio of w34 (WRKY34/wrky34 and wrky34/wrky34) genotypes to W34 (WRKY34/wrky34 and WRKY34/WRKY34) genotypes in the progeny from greenhouse-grown WRKY34/wrky34 heterozygous mutants was accorded with a predicted ratio of 1:1 (Table 1). For progeny from the cold-treated heterozygous mutant plants, the w34 to W34 ratio for both lines differed significantly from 1:1 (χ2 test, P <0.01). The ratios of w34 to W34 plants were 1.33:1 and 1.31:1 for WRKY34/wrky34-1 and WRKY34/wrky34-2, respectively, after 24 h cold treatment; divergence from the 1:1 was even greater after 48 h cold treatment (Table 1). Thus, among the progeny, plants harbouring a wrky34 allele were significantly more frequent than plants harbouring a WRKY34 allele (the WT), which indicated that differential gametophytic viability existed after exposure to cold stress.
Table 1.
Segregation analysis of progeny of self-fertilized heterozygous WRKY34/wrky34 plants with cold treatment
| Parent (self-fertilized) | Cold treatment | w34 | W34 | Ratio | χ2 |
| WRKY34/wrky34-1 | Control | 163 | 147 | 1.11:1 | 0.8 (P >0.05) |
| WRKY34/wrky34-1 | 24 h, 4oC | 153 | 115 | 1.33:1 | 5.4 (P <0.05) |
| WRKY34/wrky34-1 | 48 h, 4oC | 161 | 80 | 2.01:1 | 27.2 (P <0.01) |
| WRKY34/wrky34-2 | Control | 161 | 151 | 1.07:1 | 0.3 (P >0.05) |
| WRKY34/wrky34-2 | 24 h, 4oC | 149 | 114 | 1.31:1 | 4.7 (P <0.05) |
| WRKY34/wrky34-2 | 48 h, 4oC | 156 | 85 | 1.84:1 | 20.9 (P <0.01) |
The inheritance of wrky34 was analysed using PCR-based genotyping. w34 is the number of plants containing the wrky34 allele (plants with the WRKY34/wrky34 and wrky34/wrky34 genotypes), W34 is the the number of plants containing the WRKY34 allele (plants with the WRKY34/wrky34 and WRKY34/WRKY34 genotypes), Ratio is w34:W34. The χ2 test was used to compare the observed ratios with a predicted ratio of 1:1. The untreated greenhouse-grown plants were used as the control.
Pollen harbouring the wrky34 allele showed higher viability than that with the WRKY34 allele after cold stress
When the WRKY34/wrky34 heterozygote with or without cold treatment was used as the maternal parent in reciprocal crosses between untreated WT and cold-treated heterozygous plants, the ratios for WT and heterozygous plants did not differ significantly from 1:1 (P >0.05; Table 2). These results indicated that the wrky34 mutation did not affect female gametophytic viability. When the WRKY34/wrky34 heterozygote without cold treatment was the paternal parent, the ratios also accorded with 1:1, indicating that the wrky34 mutation did not affect male gametophytic viability in the absence of cold treatment.
Table 2.
Analysis of the genetic transmission of WRKY34/wrky34 after Cold treatment
| Cold treatment | Parentage (female×male) | Wild type | Heterozygous | Ratio | χ2 |
| Control | WRKY34/wrky34-1×WT | 90 | 95 | 0.95:1 | 0.05 (P>0.05) |
| Control | WT×WRKY34/wrky34-1 | 101 | 99 | 1.02:1 | 0.01 (P>0.05) |
| 48 h, 4 oC | WRKY34/wrky34-1×WT | 77 | 84 | 0.92:1 | 0.12 (P>0.05) |
| 48 h, 4 oC | WT×WRKY34/wrky34-1 | 22 | 97 | 0.23:1 | 11.2 (P<0.01) |
| Control | WRKY34/wrky34-2×WT | 88 | 95 | 0.93:1 | 0.11 (P>0.05) |
| Control | WT×WRKY34/wrky34-2 | 94 | 98 | 0.96:1 | 0.04 P>0.05) |
| 48 h, 4 oC | WRKY34/wrky34-2×WT | 79 | 83 | 0.95:1 | 0.05 (P>0.05) |
| 48 h, 4 oC | WT×WRKY34/wrky34-2 | 33 | 107 | 0.31:1 | 8.11 (P<0.01) |
The inheritance of wrky34 was analysed by genotyping the F1 progeny of the specified crosses. Ratio is the observed wild-type (WT) to heterozygous frequencies. The WT parental plants were grown at 22 °C without cold treatment. The χ2 test was used to compare the observed ratios with a predicted ratio of 1:1. The untreated greenhouse-grown parental plants were used as the control.
By contrast, when the WRKY34/wrky34 heterozygote with cold treatment was the paternal parent, the ratios of WT and heterozygous plants in the WRKY34/wrky34-1 and WRKY34/wrky34-2 progeny populations were 0.26:1 and 0.31:1, respectively, and differed significantly from 1:1 (P <0.01; Table 2). Thus, male gametophytes with a wrky34 allele exhibited greater competitive ability than gametophytes with a WRKY34 allele after cold treatment.
Cold sensitivity of mature pollen in wrky34 mutants
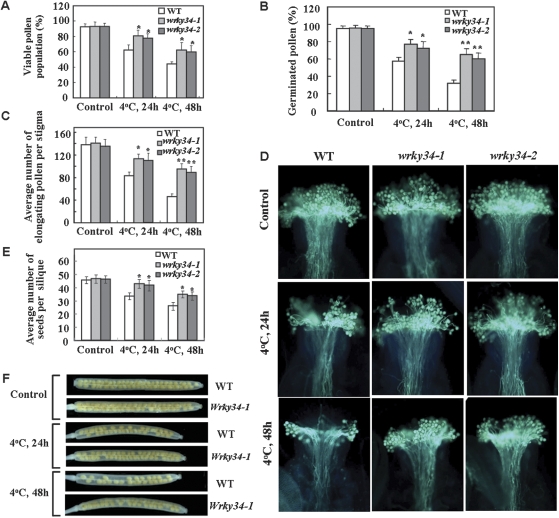
Without cold treatment, nearly all of the mature pollen from homozygous WT and wrky34 plants exhibited green fluorescence after staining with FDA. After cold treatment, the percentage of viable pollen grains from both mutants was distinctly higher than that of WT (P <0.05; Fig. 3A). After 48 h cold treatment, the percentage of viable pollen grains was 44.3%, 62.5%, and 62.8% in WT, wrky34-1, and wrky34-2 plants, respectively.
Fig. 3.
Mature pollen viability and seed set of wrky34-1 and wrky34-2 plants compared with the wild-type (WT) after cold treatment. (A) The percentage of viable pollen grains determined by staining with fluorescein diacetate. In each replicate, over 100 pollen grains were counted. (B) Percentage pollen germination in vivo. In each replicate, more than 100 pollen grains were counted. (C) Average number of elongated pollen tubes per stigma from self-fertilized plants. In each replicate, more than 30 stigmas were counted. (D) Germinated pollen in vivo stained with aniline blue. After cold treatment, elongated pollen tubes on the stigma were more frequent in the wrky34 mutants than the WT. (E) Seed set by flowers cold-treated at stage 13 and subsequently transferred to normal growing conditions until seeds had matured. The number of seeds per silique was counted. (F) Representative examples of seed set within mature siliques from cold-stressed wild-type and wrky34-1 plants. Because the seed production difference between the wrky34-1 and wrky34-2 was slight, a representative example of wrky34-1 seed sets was used to present the wrky34-1 and wrky34-2. Error bars indicate standard deviations of three replicate experiments. In each replicate, over 30 siliques were sampled. Differences between the WT and wrky34 mutants after cold stress were significant at P <0.05 (*) or P <0.01 (**). The untreated greenhouse-grown plants were used as the control.
Without cold stress, the percentage pollen germination for the wrky34 mutants was indistinguishable from that of WT pollen at 30 min after artificial pollination (Fig. 3B). After 24 h cold treatment, the percentage of germinated pollen grains was higher in the mutants than in the WT: 76.9% and 71.8% in wrky34-1 and wrky34-2, respectively, compared with 57.2% in the WT (P <0.05). With 48 h cold stress, the difference between the wrky34 mutants and WT was enhanced (P <0.01; Fig. 3B).
At 5 h after self-pollination, the number of elongated pollen tubes per stigma did not differ significantly between the WT and wrky34 mutants in the absence of cold treatment (Fig. 3C, D). After cold treatment, the number of elongated pollen tubes per stigma in the wrky34 mutants was considerably higher than that of the WT. With 48 h cold treatment, the number of elongated pollen tubes per stigma was 46.2±5.3, 96.1±9.5, and 85.7±10.3 in the WT, wrky34-1, and wrky34-2 plants, respectively (P <0.01; Fig. 3C, D). These data indicate that cold stress did not completely abolish pollen germination or pollen tube elongation, but more severely inhibited these processes in the WT than in the wrky34 mutants.
The average seed number per silique in all unstressed plants was about 46 (Fig. 3E, F). The seed number was severely reduced in WT plants, but only slightly reduced in wrky34 plants, treated at 4 °C for 24 h (Fig. 3E). With 48 h cold stress, the average seed number per silique was 25.9±2.9, 34.8±2.6, and 33.8±3.1 for the WT, wrky34-1 and wrky34-2, respectively (Fig. 3E). In the mature siliques of wrky34 plants exposed to cold stress, the gaps in the silique were fewer than those in the WT (Fig. 3F). Moreover, when unstressed WT pollen was crossed onto cold-stressed stigmas of WT and wrky34 mutant plants, no differences in seed set were recorded (data not shown). This indicated that reduced fertility of the plants was primarily due to defective pollen, and that cold stress more strongly affected pollen of the WT than the wrky34 mutant. Although exposure of mature pollen to cold stress resulted in reduced seed set, the seed set of wrky34 mutants was significantly higher than that of the WT, which indirectly indicated that the mature pollen of homozygous wrky34 mutants is less sensitive to cold stress. It also showed that even without competing pollen, wrky34 mutant pollen was more tolerant to cold stress and had a greater chance of achieving successful fertilization than WT pollen after cold treatment.
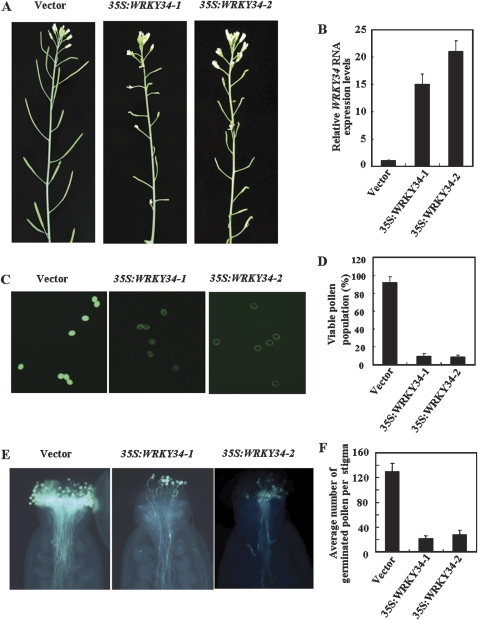
Effect of WRKY34 over-expression on fertility of mature pollen
Most of the T3 transgenic lines showing a high level of WRKY34 transcription were almost sterile (data not shown). Two of these lines were selected to elucidate whether reduction in male gametophytic fertility caused the sterility. WRKY34 expression in 35S:WRKY34 plants was detected using quantitative RT-PCR with gene-specific primers. In mature pollen, the levels of WRKY34 transcripts in the transgenic plants, 35S::WRKY34-1 and 35S::WRKY34-2, were 15-fold and 21-fold higher than that in vector transgenic plants, respectively (Fig. 4B), and both transformants were almost sterile (Fig. 4A). Scanning electron microscopic examination of the pollen surface and staining of the nuclei of mature pollen did not reveal obvious differences compared to that of the vector control (see Supplementary Fig. S1 at JXB online).
Fig. 4.
Phenotype of 35S::WRKY34 compared with the vector transgenic plant (control). (A) Inflorescence of a vector transgenic, 35S::WRKY34-1 and 35S::WRKY34-2 plants under normal growing conditions. (B) Relative WRKY34 RNA expression levels in mature pollen of 35S::WRKY34-1 and 35S::WRKY34-2 plants. (C) Fluorescence of mature pollen grains from vector transgenic plant, 35S::WRKY34-1 and 35S::WRKY34-2 plants stained with fluoroscein diacetate (FDA). (D) The proportion of viable pollen grains as determined by staining with FDA. (E) Elongated pollen tubes from vector transgenic, 35S::WRKY34-1 and 35S::WRKY34-2 plants stained with aniline blue. (F) Average number of elongated pollen tubes per stigma of 35S::WRKY34-1 and 35S::WRKY34-2 plants. Error bars indicate standard deviations of three independent biological samples.
Pollen viability and pollen tube growth of the 35S::WRKY34-1 and 35S::WRKY34-2 plants were examined, using the vector transgenic plants as the control. Less than 10% of the pollen grains was viable (Fig. 4C, D), and only a few pollen tubes elongated on the stigma of 35S::WRKY34 plants (Fig. 4E, F). Male and female fertility of 35S::WRKY34 plants were also investigated by performing reciprocal crosses between homozygous and vector transgenic plants. In vector transgenic plants pollinated by mature pollen of 35S::WRKY34, and self-fertilized 35S::WRKY34 plants, the fertilization aborted (see Supplementary Fig. S2 at JXB online). By contrast, fertilization was successful when the pistil of 35S::WRKY34 was pollinated with mature pollen of the vector transgenic plant (see Supplementary Fig. S2 at JXB online). These results indicated that the sterility of 35S::WRKY34 plants was caused by defective pollen. Thus, over-expression of WRKY34 in mature pollen of transgenic plants greatly reduced fertility.
Expression analysis of WRKY34 transcripts in three MIKC* gene mutants under cold stress
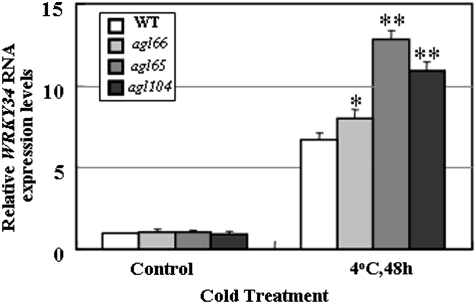
In the absence of cold stress, expression of WRKY34 in the agl66, agl65, and agl104 mutants did not differ significantly from that of the WT (Fig. 5). After cold treatment, the level of WRKY34 in agl66, agl65, and agl104 was 1.2, 1.9, and 1.6 times, respectively, that in the WT. On the other hand, cold treatment led to a 6.7, 8.0, 12.8, and 10.9-fold increase in accumulation of WRKY34 in the WT, agl66, agl65, and agl104, respectively (Fig. 5). Thus, mutation of AGL65, AGL66, and AGL104 enhanced the cold-induced expression of WRKY34. Previous studies have shown that MIKC* proteins exhibit functional redundancy, and a single MIKC* mutation does not significantly affect their function (Verelst et al., 2007a, b; Adamczyk and Fernandez, 2009). This might explain why WRKY34 expression in agl66, agl65, and agl104 showed no obvious difference from the WT without cold treatment.
Fig. 5.
WRKY34 RNA expression in agl66, agl65, and agl104 mutants. WRKY34 RNA expression was determined in RNA extracted from mature pollen after treatment. Error bars indicate standard deviations of determinations from three independent RNA extracts. Differences between the wild-type (WT) and wrky34 mutants after cold treatment were significant at P <0.05 (*) or P <0.01 (**).The untreated greenhouse-grown plants were used as the control.
Expression analysis of MIKC* gene transcripts in wrky34 mutants under cold stress
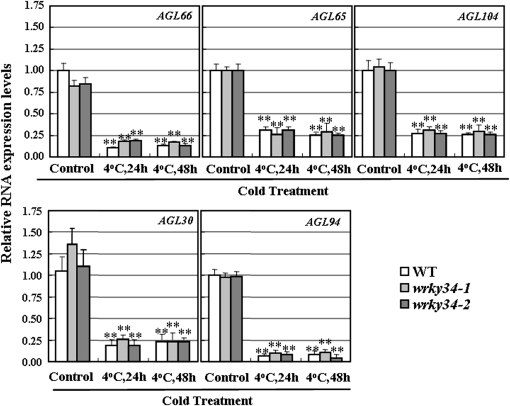
In untreated WT and wrky34 mutant plants, the levels of MIKC* transcripts showed no significant difference between the WT and wrky34 mutants. After cold treatment, expression of these genes decreased considerably in both the WT and wrky34 mutants (Fig. 6). Cold treatment strongly reduced accumulation of AGL66, AGL65, AGL104, AGL30, and AGL94 transcripts in all plants, and the accumulation of transcripts of each gene in cold-treated wrky34 mutants did not differ significantly from that in the WT. In addition, expression of the five MIKC* genes in 35S:WRKY34 transgenic plants did not differ significantly from that of vector transgenic plants (see Supplementary Fig. S3 at JXB online). Thus, the expression of MIKC* genes was independent of WRKY34 expression.
Fig. 6.
RNA levels of MIKC* genes in mature pollen of wrky34 mutants and wild-type (WT) plants. MIKC* RNA expression was determined in RNA extracted from mature pollen after treatment with 4 °C for 24 h or 48 h. Error bars indicate standard deviations from three independent RNA extracts. Differences between the untreated and cold-stressed plants were significant at P <0.01 (**).
Effect of WRKY34 on expression of transcriptional activator CBF genes
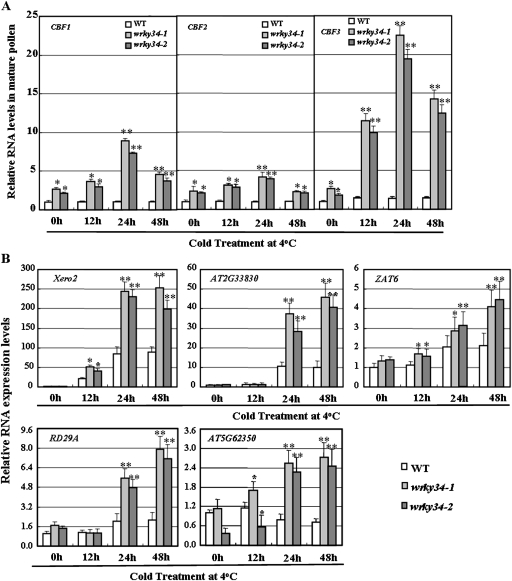
Without cold treatment, expression of CBF1, CBF2, and CBF3 increased about 2-fold in wrky34 mutants relative to that in the WT (Fig. 7A). Quantitative RT-PCR revealed that CBFs transcription was induced by cold treatment in wrky34 mutants and reached a maximum level after about 24 h of cold stress, and thereafter began to decrease slightly (Fig. 7A). CBF transcription in mature pollen of the WT was not induced by cold treatment when compared to wrky34 mutants. In mature pollen of wrky34 mutants, 24 h cold treatment led to a 10-fold increase in the accumulation of CBF3 transcripts, and a 3.4-fold increase in the accumulation of CBF1 transcripts, which was consistent with microarray analysis (Table 3). In addition, five COR genes, which are target genes of CBFs (Lee et al., 2002; Maruyama et al., 2004), displayed greater induction in mature pollen of wrky34 mutants than that in WT following cold treatment (Fig. 7B).
Fig. 7.
Expressionof COR RNA in mature pollen of wild-type (WT) and wrky34 plants. (A) Relative RNA levels for CBF1, CBF2, and CBF3. (B) Relative RNA levels for five COR genes. Relative RNA levels were analysed using gene-specific primers by real-time PCR. Error bars indicate standard deviations of determinations from three independent RNA extracts. Differences between the wild-type (WT) and wrky34 mutants after cold treatment were significant at P <0.05 (*) or P <0.01 (**).
Table 3.
Microarray analysis of the wrky34-1 mutant and wild-type (WT)
| AGI | Annotation | wrky34 mutants versus WT |
| Cold response pathway genes | ||
| AT4G25480 | ATCBF3: involved in response to low temperature and abscisic acid | 5.3 |
| AT4G25490 | ATCBF1: involved in response to low temperature and abscisic acid | 2.0 |
| AT5G04340 | ZAT6: C2H2 Zinc Finger 6, transcription factor/zinc ion binding | 3.1 |
| AT5G62350 | MMI9.21: invertase/pectin methylesterase inhibitor family protein | 4.2 |
| AT3G50970 | Xero2: Low tempature-induced 30 | 2.8 |
| AT5G52310 | RD29A: Low tempature-induced 78 | 3.4 |
| AT2G33830 | Dormancy/auxin associated family protein | 3.7 |
| TCG/MPG-specific genes | ||
| AT3G02480 | ABA-responsive protein-related | 6.1 |
| AT3G28770 | Unknown protein | 5.3 |
| AT1G78980 | SRF5:ATP binding/kinase/protein serine/threonine kinase | 2.6 |
| AT1G24110 | Peroxidase activity, response to oxidative stress. | 2.2 |
Relative RNA levels of cold-related and MPG-specific genes in microarray results. RNA was extracted from mature pollen of the wrky34-1 mutant and wild-type plants after treatment at 4 °C for 48 h. Gene expression levels were also measured by qRT-PCR.
Discussion
In the present study, the wrky34 allele was identified as a novel mutation that affects mature pollen viability under cold stress. Genetic analysis showed that mature pollen of the wrky34-1 and wrky34-2 mutants was less sensitive to cold stress compared with that of the WT. WRKY34 is expressed in the early stages of male gametophyte development and low levels of transcripts are present in mature pollen (Verelst et al., 2007,b; Honys et al., 2006). Without cold stress, knock-down of WRKY34 function did not affect pollen viability. Cold stress significantly induced WRKY34 expression in the WT, and pollen harbouring the wrky34 allele showed greater competitiveness than pollen containing the WRKY34 allele after cold treatment (Tables 1, 2). Mature pollen of wrky34 mutants showed higher cold-stress tolerance than that of the WT following cold treatment (Fig. 3), and over-expression of WRKY34 conferred much reduced fertility on mature pollen even under normal growing conditions (Fig. 4). All of these results suggest that WRKY34 functions as a negative regulator of cold sensitivity in mature pollen.
Mature pollen of wrky34 mutants is less sensitive to cold stress at floral developmental stage 13. At this stage, the pollen grains are mature, the stigma is receptive, and anthesis occurs (Smyth et al., 1990). Mature pollen grains contain mRNAs whose protein products appear to function during the late-maturation stages of pollen tube growth and germination (Mascarenhas, 1975). Thus, differences in the gene expression profiles between wrky34 and WT provide valuable information for understanding why mature pollen of wrky34 mutants is more tolerant to cold stress relative to that of the WT.
In mature pollen, the MIKC* transcription factors, including AGL66, AGL65, AGL30, AGL104, and AGL94, are known to be important regulators of pollen germination and tube growth, and regulate gene expression by binding to the MEF2 motif (Verelst et al., 2007a, b; Adamczyk and Fernandez, 2009). Two MEF2 motifs are present in the putative promoter of WRKY34 (see Supplementary Fig. S4 at JXB online), and WRKY34 expression in MIKC* double mutants is significantly increased, indicating that MIKC* genes are negative regulators of WRKY34 expression (Verelst et al., 2007b). Therefore, it is possible that MIKC* genes are involved in the cold response of mature pollen. Consistent with this hypothesis, expression of the five MIKC* genes was similarly decreased in both the WT and wrky34 plants in response to cold treatment (Fig. 6), and expression of WRKY34 in agl66, agl65, and agl104 was significantly higher than that of the WT (Fig. 5), supporting the suggestion that WRKY34 acts as a downstream gene of MIKC* transcription factors, and that cold stress affects mature pollen viability by inhibiting expression of MIKC* genes. These results also implied that the cold-sensitivity of mature pollen in the WT might result from the accumulation of WRKY34 transcripts.
The MIKC* genes affect a large suite of tricellular and mature pollen grains (TCP/MPG) specific genes, including WRKY34 (Honys and Twell, 2003; Verelst et al., 2007b; Adamczyk and Fernandez, 2009). The mature pollen of wrky34 mutants was cold-insensitive, which implied that WRKY34 was involved in the regulation of MIKC* gene-mediated pollen viability under cold stress. To test this hypothesis, the microarray results with the reference dataset of Honys and Twell were compared (Honys and Twell, 2003). In the wrky34-1 mutant, the expression levels of 147 MPG-specific genes, which are putative downstream genes of MIKC* transcription factors (Verelst et al., 2007b; Adamczyk and Fernandez, 2009), were significantly higher than those of the WT (Table 3; see Supplementary Table S2 at JXB online). Four of these genes were analysed by qRT-PCR, which revealed that the transcript levels of SRF5, AT3G02480, AT1G24110, and AT3G28770 displayed reduced inhibition in wrky34 mutants than in WT following cold treatment (see Supplementary Fig. S5 at JXB online). Interestingly, the putative peroxidase gene AT1G24110 showed greater induction in mature pollen of wrky34 mutants than in the WT (see Supplementary Fig. S5 at JXB online). The enhanced expression of these genes in the wrky34 mutants might be partly responsible for the lower decrease in pollen viability than in the WT under cold stress. All of these results suggested that WRKY34 and MIKC* transcription factors may be important regulators controlling the mature pollen response to cold stress.
The CBF cold-response pathway has a prominent role in cold acclimation, and its transcriptional network in Arabidopsis has been well investigated (Thomashow, 1999; Chinnusamy et al., 2007). The pathway includes activity of three transcription factors, namely CBF1, CBF2, and CBF3 (also known as DREB1b, c, and a, respectively), that are rapidly induced in response to low temperature followed by expression of the CBF-targeted genes (the CBF regulon) that act in concert to increase plant freezing tolerance (Stockinger et al., 1997; Fowler and Thomashow, 2002; Maruyama et al., 2004; Chinnusamy et al., 2007). Therefore, it is possible that CBF genes might be associated with the cold tolerance of mature pollen in wrky34 mutants. Surprisingly, CBFs were significantly induced by cold stress in wrky34 mutants rather than in the WT, and disruption of WRKY34 also enhanced induction of CBF-targeted genes (Fig. 7; Table 3), implying that the cold-induced expression of WRKY34 in the WT might repress expression of cold-induced CBF genes in mature pollen. On the other hand, other reports have shown that WRKY proteins (ABF1 and ABF2) bind to the box2/W-box of the GA-regulated α-Amy2 promoter (Rushton et al., 1995), and GaWRKY1 strongly activated the CAD1-A promoter by binding to the W-box (Xu et al., 2004). A barley WRKY gene, HvWRKY38, and its rice (Oryza sativa) orthologue, OsWRKY71, act as transcriptional repressors of gibberellin-responsive genes in aleurone cells (Zou et al., 2008). The putative 3 kb promoter zone of CBF3 and CBF1 has five and four TTGAC(C/T) W-boxes, respectively, which raise the possibility that WRKY34 regulates transcription of CBF3 and CBF1. These results suggested that the cold-tolerance of mature pollen in the wrky34 mutants was partly attributable to elevated expression of CBFs and CBFs-targeted genes. It was reported previously that poor expression of cold-responsive genes that play a role in stress tolerance might be why Arabidopsis pollen is cold-sensitive (Lee and Lee, 2003). These results might explain why the transcripts of cold-responsive genes do not accumulate in mature pollen of the WT.
In summary, this study confirmed that the WRKY34 transcription factor negatively mediates cold sensitivity in mature pollen of Arabidopsis. Pollen-specific MIKC* transcription factors were negative regulators of the transcripts of WRKY34 during the cold response, and the WRKY34 transcription factor was involved in mature pollen development regulated by the pollen-specific MIKC* class of MADS-domain transcription factors under cold stress. Cold insensitivity of mature wrky34 pollen is partly attributable to the induction of CBFs and the up-regulation of COR genes in the mutants, indicating that the WRKY34 transcription factor might be involved in the CBF signal cascade in mature pollen. Elucidation of the physiological functions of WRKY34 requires further investigation.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table S1. List of quantitative RT-PCR primer sequences.
Supplementary Table S2. Mircoarray data for expression of 147 mature pollen grains (MPG) specific genes.
Supplementary Fig. S1. Nuclear staining and pollen surface of vector and 35S:WRKY34 plants.
Supplementary Fig. S2. Example of the reciprocal crosses between 35S:WRKY34 and vector plants.
Supplementary Fig. S3. Levels of MIKC* RNA in mature pollen of vector and 35S:WRKY34 plants.
Supplementary Fig. S4. Positions of two MEF2 motifs in the putative promoter of WRKY34.
Supplementary Fig. S5. RNA levels of mature pollen specific genes after cold treatment.
Supplementary Material
Acknowledgments
We thank the Arabidopsis Biological Resource Center at Ohio State University, USA, for the wrky34-2, agl65, agl66, and agl104 T-DNA insertion mutants. We are grateful to Dr Zhixiang Chen (Department of Botany and Plant Pathology, Purdue University, West Lafayette, Indiana, USA) for the Arabidopsis wrky34-1 mutant. This work was supported by the Science Foundation of the Ministry of Agriculture of the People's Republic of China (2009ZX08009-066B) and the Natural Science Foundation of China (90817003).
References
- Adamczyk BJ, Fernandez DE. MIKC* MADS domain heterodimers are required for pollen maturation and tube growth in Arabidopsis. Plant Physiology. 2009;149:1713–1723. doi: 10.1104/pp.109.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. Journal of Biological Chemistry. 2006;281:37636–37645. doi: 10.1074/jbc.M605895200. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K. A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Molecular Biology. 2003;53:107–116. doi: 10.1023/B:PLAN.0000009269.97773.70. [DOI] [PubMed] [Google Scholar]
- Asai S, Ohta K, Yoshioka H. MAPK signalling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. The Plant Cell. 2008;20:1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Benedict C, Geisler M, Trygg J, Huner N, Hurry V. Consensus by democracy. Using meta-analyses of microarray and genomic data to model the cold acclimation signalling pathway in Arabidopsis. Plant Physiology. 2006;141:1219–1232. doi: 10.1104/pp.106.083527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. The Plant Cell. 2009;21:3554–3566. doi: 10.1105/tpc.108.064980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhun T, Aya K, Asano K, et al. Gibberellin regulates pollen viability and pollen tube growth in rice. The Plant Cell. 2007;19:3876–3888. doi: 10.1105/tpc.107.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes and Development. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene expression in plants. Trends in Plant Science. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dellagi A, Heilbronn J, Avrova AO, et al. A potato gene encoding a WRKY-like transcription factor is induced in interactions with Erwinia carotovora subsp. atroseptica and Phytophthora infestans and is coregulated with class I endochitinase expression. Molecular Plant–Microbe Interactions. 2000;13:1092–1101. doi: 10.1094/MPMI.2000.13.10.1092. [DOI] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defence response. Plant Molecular Biology. 2003;51:21–37. doi: 10.1023/a:1020780022549. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends in Plant Science. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE. Networks of WRKY transcription factors in defence signalling. Current Opinion in Plant Biology. 2007;10:366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Fowler S, Thomashow MF. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. The Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SG, Fowler SG, Thomashow MF. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Molecular Biology. 2004;54:767–781. doi: 10.1023/B:PLAN.0000040902.06881.d4. [DOI] [PubMed] [Google Scholar]
- Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN. RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiology. 2008;147:852–863. doi: 10.1104/pp.108.118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MA, Heyer AG, Hincha DK. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genetics. 2005 doi: 10.1371/journal.pgen.0010026. 1, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yagi M, Kusano T, Sano H. Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Molecular and General Genetics. 2000;263:30–37. doi: 10.1007/pl00008673. [DOI] [PubMed] [Google Scholar]
- Honys D, Oh SA, Reňák D, Donders M, Šolcová B, Johnson JA, Boudová R, Twell D. Identification of microspore-active promoters that allow targeted manipulation of gene expression at early stages of microgametogenesis in Arabidopsis. BMC Plant Biology. 2006;6:31. doi: 10.1186/1471-2229-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiology. 2003;132:640–652. doi: 10.1104/pp.103.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Duman JG. Cloning and characterization of a thermal hysteresis (antifreeze) protein with DNA-binding activity from winter bittersweet nightshade, Solanum dulcamara. Plant Molecular Biology. 2002;48:339–350. doi: 10.1023/a:1014062714786. [DOI] [PubMed] [Google Scholar]
- Ishida T, Hattori S, Sano R, et al. Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. The Plant Cell. 2007;19:2531–2543. doi: 10.1105/tpc.107.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K. Arabidopsis MALE STERILITY1 encodes a PHD Type transcription factor and regulates pollen and tapetum development. The Plant Cell. 2007;19:3549–3562. doi: 10.1105/tpc.107.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Jiang W, Yu D. Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biology. 2009;9:96. doi: 10.1186/1471-2229-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Zhou X, Song Y, Yu D. Heterologous expression of OsWRKY23 gene enhances pathogen defence and dark-induced leaf senescence in Arabidopsis. Plant Growth Regulation. 2009;58:181–190. [Google Scholar]
- Johnson CS, Kolevski B, Smyth DR. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. The Plant Cell. 2002;14:1359–1375. doi: 10.1105/tpc.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace M, Matton DP. Characterization of a WRKY transcription factor expressed in late torpedo-stage embryos of Solanum chacoense. Planta. 2004;219:185–189. doi: 10.1007/s00425-004-1253-2. [DOI] [PubMed] [Google Scholar]
- Lai Z, Vinod K, Zheng Z, Fan B, Chen Z. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biology. 2008;8:68. doi: 10.1186/1471-2229-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Henderson DA, Zhu JK. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. The Plant Cell. 2005;17:3155–3175. doi: 10.1105/tpc.105.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Guo Y, Ohta M, Xiong LM, Stevenson B, Zhu JK. LOS2, a genetic locus required for cold responsive transcription encodes a bi-functional enolase. EMBO Journal. 2002;21:2692–2702. doi: 10.1093/emboj/21.11.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee DH. Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiology. 2003;132:517–529. doi: 10.1104/pp.103.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim ES, Choi Y, Hwang I, Staiger CJ, Chung YY, Lee Y. The Arabidopsis phosphatidylinositol 3-kinase is important for pollen development. Plant Physiology. 2008;147:1886–1897. doi: 10.1104/pp.108.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Fu Q, Huang W, Yu D. Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Reports. 2009;28:683–693. doi: 10.1007/s00299-008-0666-y. [DOI] [PubMed] [Google Scholar]
- Luo M, Dennis ES, Berger F, Peacock WJ, Chaudhury A. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2005;102:17531. doi: 10.1073/pnas.0508418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM. Chilling injury in plants. Annual Review of Plant Physiology. 1973;24:445–466. [Google Scholar]
- Mascarenhas JP. The biochemistry of angiosperm pollen development. Botanical Review. 1975;41:259–314. [Google Scholar]
- Mayer E, Gottsberger G. Pollen viability in the genus Silene (Caryophyllaceae) and its evaluation by means of different test procedures. Flora. 2000;195:394–353. [Google Scholar]
- Maruyama K, Sakuma Y, Kasuga M, et al. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. The Plant Journal. 2004;38:982–993. doi: 10.1111/j.1365-313X.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- Miao Y, Zentgraf U. The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. The Plant Cell. 2007;19:819–830. doi: 10.1105/tpc.106.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nature Cell Biology. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- Park SK, Howden R, Twell D. The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development. 1998;125:3789–3799. doi: 10.1242/dev.125.19.3789. [DOI] [PubMed] [Google Scholar]
- Patterson BD, Mutton L, Paull RE, Nguyen VQ. Tomato pollen development: stages sensitive to chilling and a natural environment for the selection of resistant genotypes. Plant, Cell and Environment. 1987;10:363–368. [Google Scholar]
- Qiu Y, Yu D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environmental and Experimental Botany. 2009;65:35–47. [Google Scholar]
- Rizhsky L, Davletova S, Liang H, Mittler R. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. Journal of Biological Chemistry. 2004;279:11736–11743. doi: 10.1074/jbc.M313350200. [DOI] [PubMed] [Google Scholar]
- Robatzek S, Somssich IE. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. The Plant Journal. 2001;28:123–133. doi: 10.1046/j.1365-313x.2001.01131.x. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Macdonald H, Huttly AK, Lazarus CM, Hooley R. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of α-Amy2 genes. Plant Molecular Biology. 1995;29:691–702. doi: 10.1007/BF00041160. [DOI] [PubMed] [Google Scholar]
- Sataka T, Koike S. Sterility caused by cooling treatment at the flowering stage in rice plants. Japanese Journal of Crop Science. 1983;52:207–213. [Google Scholar]
- Seki M, Narusaka M, Ishida J, et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. The Plant Journal. 2002;31:279–292. doi: 10.1046/j.1365-313x.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. The Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcription activator that binds to the C repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings of the National Academy of Sciences, USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Boren M, Ahlandsberg S, Jansson C. A novel WRKY transcription factor, SUSIBA2, participates in sugar signalling in barley by binding to the sugar-responsive elements of the iso1 promoter. The Plant Cell. 2003;15:2076–2092. doi: 10.1105/tpc.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Ulker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Current Opinion in Plant Biology. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Verelst W, Saedler H, Munster T. MIKC* MADS-Protein complexes bind motifs enriched in the proximal region of late pollen-specific Arabidopsis promoters. Plant Physiology. 2007a;143:447–460. doi: 10.1104/pp.106.089805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verelst W, Twell D, de Folter S, Immink R, Saedler H, Munster T. MADS-complexes regulate transcriptome dynamics during pollen maturation. Genome Biology. 2007b;8:R249. doi: 10.1186/gb-2007-8-11-r249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YH, Wang JW, Wang S, Wang JY, Chen XY. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-{delta}-cadinene synthase-A. Plant Physiology. 2004;135:507–515. doi: 10.1104/pp.104.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang L, Sundaresan V, Ye D. Tapetum determinant1 is required for cell specialization in the Arabidopsis anther. The Plant Cell. 2003;15:2792–2804. doi: 10.1105/tpc.016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Mosher SL, Fan B, Klessiq DF, Chen Z. Functional analysis of Arabidopsis WRKY25 transcription factor in plant defence against Pseudomonas syringae. BMC Plant Biology. 2007;7:2. doi: 10.1186/1471-2229-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Neuman D, Shen QJ. Interactions of two transcriptional repressors and two transcriptional activators in modulating gibberellin signalling in aleurone cells. Plant Physiology. 2008;148:176–186. doi: 10.1104/pp.108.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.