Abstract
Photosynthesis is a process that inevitably produces reactive oxygen species, such as hydrogen peroxide, which is reduced by chloroplast-localized detoxification mechanisms one of which involves 2-Cys peroxiredoxins (2-Cys Prxs). Arabidopsis chloroplasts contain two very similar 2-Cys Prxs (denoted A and B). These enzymes are reduced by two pathways: NADPH thioredoxin reductase C (NTRC), which uses NADPH as source of reducing power; and plastidial thioredoxins (Trxs) coupled to photosynthetically reduced ferredoxin of which Trx x is the most efficient reductant in vitro. With the aim of establishing the functional relationship between NTRC, Trx x, and 2-Cys Prxs in vivo, an Arabidopsis Trx x knock-out mutant has been identified and a double mutant (denoted Δ2cp) with <5% of 2-Cys Prx content has been generated. The phenotypes of the three mutants, ntrc, trxx, and Δ2cp, were compared under standard growth conditions and in response to continuous light or prolonged darkness and oxidative stress. Though all mutants showed altered redox homeostasis, no difference was observed in response to oxidative stress treatment. Moreover, the redox status of the 2-Cys Prx was imbalanced in the ntrc mutant but not in the trxx mutant. These results show that NTRC is the most relevant pathway for chloroplast 2-Cys Prx reduction in vivo, but the antioxidant function of this system is not essential. The deficiency of NTRC caused a more severe phenotype than the deficiency of Trx x or 2-Cys Prxs as determined by growth, pigment content, CO2 fixation, and Fv/Fm, indicating additional functions of NTRC.
Keywords: Chloroplast, oxidative stress, peroxiredoxin, thioredoxin
Introduction
Oxygenic photosynthesis converts light energy into organic material, being a process of primary importance for the production of biomass and oxygen in the biosphere (Nelson and Ben-Shem, 2004; Rascher and Nedbal, 2006). Because photosynthesis involves the transport of electrons in the presence of oxygen, it is a process that inevitably produces reactive oxygen species (ROS), which act as oxidants and may cause cell damage (Apel and Hirt, 2004; Mittler et al., 2004). In addition to this toxic effect, ROS have an important signalling function (Van Breusegem et al., 2008). This is the case for hydrogen peroxide (H2O2), which is well established as a signalling molecule in eukaryotes (Wood et al., 2003; Mullineaux et al., 2006), and singlet oxygen, which acts as a signal to activate gene expression in response to environmental stress in plants (Op den Camp et al., 2003; Wagner et al., 2004; Kim et al., 2008).
In photosynthetic plant cells chloroplasts are a major source of ROS production (Pitzschke et al., 2006), which increases under adverse environmental conditions (Apel and Hirt, 2004; Ledford et al., 2007). Therefore, the different antioxidant mechanisms, non-enzymatic and enzymatic, that operate in the chloroplast are important not only to avoid toxic ROS effects but also to modulate their signalling function. Non-enzymatic antioxidants may be classified into those which are hydrophilic, including glutathione and ascorbate (AsA), which reach millimolar concentrations in the chloroplast, and those which are lipophilic. Lipophilic antioxidants, such as β-carotene, zeaxanthine, and α-tocopherol, have an important scavenging role and protect thylakoid membranes and photosynthetic structures (Havaux et al., 2005; Kruk et al., 2005; Peñuelas and Munne-Bosch, 2005).
Enzymatic antioxidant systems include superoxide dismutases (SODs), ascorbate peroxidases (Apxs), catalases, glutathione peroxidases (Gpxs), and peroxiredoxins (Prxs) (Foyer and Noctor, 2009). In plants, these enzymes are encoded by gene families and, for most of them, excluding catalases, some of the isoforms are targeted to chloroplasts. Among the different SODs, Fe-SOD and CuZn-SOD are present in chloroplasts and their overexpression produces enhanced tolerance to different environmental stresses (Lee et al., 2007). Apxs are haem-containing peroxidases, of which the chloroplast contains two isoforms localized in the stroma (sApx) and associated with the thylakoid (tApx), respectively (Shigeoka et al., 2002). These peroxidases play an important protective role in the chloroplast because they participate in the AsA-dependent water–water cycle able to reduce H2O2 using AsA as reductant (Asada, 2006). Interestingly, Arabidopsis mutants devoid of both sApx and tApx showed phenotypic differences from wild-type plants only under severe oxidative stress imposed by treatment with methyl viologen (Kangasjärvi et al., 2008). Gpxs are encoded by a gene family composed of eight genes in Arabidopsis with different cellular localization (Rodríguez-Milla et al., 2003). Though these enzymes were initially described as glutathione dependent, their reduction by thioredoxins (Trxs) has recently been reported (Navrot et al., 2006). An Arabidopsis mutant deficient in one of the isoforms, AtGpx3, is sensitive to oxidative stress (Miao et al., 2006), thus supporting the antioxidant function of these enzymes.
The most recently described peroxidases in plants are Prxs, thiol-based enzymes encoded by a gene family of 10 members in Arabidopsis (Dietz, 2003). According to their reaction mechanism and biochemical properties, Prxs are classified into four types: typical 2-Cys Prx, atypical Prx Q and type II Prx, and 1-Cys Prx. The Arabidopsis chloroplast contains four Prxs: Prx Q, Prx IIE, and two very similar 2-Cys Prxs, namely 2-Cys Prx A and 2-Cys Prx B (Bréhélin et al., 2003; Horling et al., 2003; Dietz et al., 2006). The highly conserved reaction mechanism involves a cysteine residue, termed peroxidatic, which attacks the peroxide and becomes transiently oxidized to sulphenic acid. This intermediate is subsequently attacked by a second cysteine residue, termed resolving, yielding water or the corresponding alcohol, and the two cysteine residues become oxidized, forming a disulphide bridge (Dietz, 2003; Konig et al., 2003; Hall et al., 2009) that needs to be reduced for a new catalytic cycle. In chloroplasts, two pathways have been proposed to reduce typical 2-Cys Prxs. One is based on plastidial Trxs, such as Trx x, which is the most efficient reductant (Collin et al., 2003), and CDSP32, which has been shown to interact with 2-Cys Prx in vitro and in vivo (Broin et al., 2002; Rey et al., 2002). The other pathway is based on a peculiar type of NADPH thioredoxin reductase (NTR), termed NTRC, with a joint Trx domain at the C-terminus (Serrato et al., 2002, 2004). NTRC conjugates NTR and Trx activities to reduce 2-Cys Prxs with high efficiency (Pérez-Ruiz et al., 2006; Pérez-Ruiz and Cejudo, 2009). Whereas the Trx-dependent pathway obtains reducing power from ferredoxin (Fd) reduced by the photosynthetic electron chain and mediated by ferredoxin-dependent thioredoxin reductase (FTR), NTRC uses NADPH as the source of reducing power. This difference is important because NADPH can be produced in darkness from glucose 6-phosphate via the oxidative pentose phosphate pathway, thus supplying reducing power under conditions when the levels of reduced Fd are lower (Spínola et al., 2008). Previously, analysis based on in vitro assays with purified recombinant proteins showed that NTRC is more efficient than Trx x or CDSP32 as a reductant of 2-Cys Prx (Pérez-Ruiz et al., 2006). The present study undertakes an in vivo assessment of these redox pathways. Following the identification of Arabidopsis mutants deficient in 2-Cys Prxs and Trx x, a comparative analysis with the previously reported NTRC-deficient mutant was performed with the aim of identifying the contribution of these Prxs and the two major pathways, NTRC dependent and Trx x dependent, to the overall mechanism of chloroplast detoxification of peroxides and maintenance of chloroplast redox homeostasis.
Materials and methods
Growth conditions and plant material
Arabidopsis thaliana wild-type (ecotype Columbia) and mutant plants were grown in soil supplemented with Hoagland medium in growth chambers under long-day conditions (16 h light/8 h darkness) at 22 °C during the day and 20 °C during the night. The light intensity was set at 140 μE m−2 s−1. The 2-Cys Prx A–2-Cys Prx B double mutant, denoted Δ2cp, was obtained by manual crossing of the single mutants 2cpA, line SALK_065264, and 2cpB, line SALK_017213, previously characterized (Kirchsteiger et al., 2009). Seeds resulting from this cross were checked for heterozygosity of T-DNA insertions in the 2cpA and 2cpB genes. The plants were then selfed and double homozygous plants were detected in the progeny by PCR analysis of genomic DNA using oligonucleotides a (5′-GAGAAGTTGAACACCGA-3′) and b (5′-GGGGACAAAGTGAGAATC-3′) for the 2cpA gene, and a′ (5′-CCACCTGAACCAAGAAAG-3′) and b′ (5′-CCTGCAAGACAACATCAC-3′) for the 2cpB gene in conjunction with the oligonucleotide T (5′-TGGTTCACGTAGTGGGCCATCG-3′) located in the T-DNA. A T-DNA homozygous line SALK_128914 (Alonso et al., 2003) in the single gene encoding Trx x of Arabidopsis (At1g50320 locus) was selected by PCR analysis of genomic DNA with oligonucleotides c (5′-GCCATGGACTCTATCGTCTC-3′) and d (5′-CCTTCCCTTCTGCTCCCT-3′) in conjunction with the T oligonucleotide. The NTRC knock-out mutant was described previously (Serrato et al., 2004).
Treatment with methyl viologen
Leaf discs, 6 mm in diameter, were excised from equivalent leaves of wild-type and mutant plants that had been grown for 21–25 d. Discs were immediately incubated in sterile distilled water supplemented with methyl viologen up to 10 μM concentration and incubated during 48 h under continuous light (30 μE m−2 s−1).
Quantitative PCR (qPCR) and western blot analysis
Total RNA (1 μg) extracted from ground leaf tissue using Trizol™ was retro-transcribed by means of a QuantiTect™ RT-kit (Qiagen). Real-time PCR was performed in a total reaction volume of 20 μl containing primers (4 μM), cDNA (40 ng), and 10 μl of iQ™ SYBR Green Supermix (Bio-Rad). The results obtained from three independent biological samples (three analytical replicates each) are represented as 2–ΔΔCT (threshold cycle) as described by Livak and Schmittgen (2001). Ubiquitin 10 was used as housekeeping gene. Gene-specific primers used are described in Supplementary Table S1 available at JXB online. Fluorescence of PCR products was determined continuously by the iQ5 cycler (Bio-Rad).
Western blot analysis was performed as previously described (Kirchsteiger et al., 2009) using as probes anti-NTRC, anti-NTRB, and anti-2-Cys Prx antibodies, previously described. Anti-Trx x polyclonal antibodies were produced after immunization of rabbits with purified recombinant Trx x at the Servicio de Protección Animal (Sevilla University, Spain). The anti-Prx Q antibody was purchased (Agrisera, Vännäs, Sweden), and anti-Prx IIE was kindly provided by Dr N. Rouhier (University of Nancy, France). For optimized resolution, SDS–PAGE was performed with 12% or 15% acrylamide/bisacrylamide gels and loading of 15 μg of total protein extract, or 7.5 μg for 2-Cys Prx, respectively, under reducing conditions.
Determination of the photosynthetic rate and PSII photochemical efficiency
Photosynthetic gas exchange was measured using a portable infrared gas analyser (model LI-6400, LI-COR Biosciences, Inc., Lincoln, NE, USA), which allows environmental conditions inside the chamber to be precisely controlled. Air temperature in the chamber was set at 25 °C, and the relative humidity was maintained at 50%. The CO2 assimilation rate was determined at 1000 μE m−2 s−1 with the upper leaf of wild-type and mutant plants grown for 3 weeks as described above. Plants treated with continuous light were grown in a growth chamber at 22 °C at a constant light intensity of 140 μE m−2 s−1. For the treatment of prolonged darkness, plants grown under a 16 h/8 h photoperiod were subjected to darkness during 72 h before the determination of CO2 assimilation rate, which was performed at 1000 μE m−2 s−1.
Parameters of chlorophyll fluorescence emission were measured at 22 °C with the chlorophyll fluorometer PAM 2000 (Walz, Effeltrich, Germany). The maximal quantum yield of PSII (Fv/Fm) was calculated from the parameters using the following equation: Fv/Fm=(Fm–Fo)/Fm, where Fo is the initial minimal fluorescence emitted from leaves dark-adapted for 15 min and Fm is the maximal fluorescence elicited by saturating actinic light.
Determination of photosynthetic pigments, carbonyl groups, H2O2, AsA, and glutathione
The content of total chlorophyll and carotenoids was determined according to the method of Lichtenthaler and Wellburn (1983), as previously reported (Pérez-Ruiz et al., 2006). Carbonyl groups were assayed using the dinitrophenyl hydrazine method, according to Romero-Puertas et al. (2002). H2O2 was analysed by using PeroXOquant Quantitative Peroxide Assay Kits (Pierce). AsA and dehydroascorbate (DHA) were analysed in microplates using the α-α′ bipyridyl method according to Gillespile and Ainsworth (2007). Total AsA was measured after incubation of the samples with dithiothreitol (DTT) and the oxidized form (DHA) was determined as the difference between total and reduced AsA. To quantify the glutathione contents, leaf tissues frozen with liquid nitrogen were homogenized in cold extraction buffer containing 0.1 N HCl and 1 mM EDTA. Homogenates were centrifuged at 15 000 g for 15 min at 4 °C. Thiols were reduced at 4 °C for 15 min by mixing 400 μl of extracted samples with 600 μl of 200 mM CHES (pH 9.2) and 100 μl of 250 mM NaBH4. For derivatization, a 330 μl aliquot was added to 20 μl of 15 mM monobromobimane and kept in the dark at room temperature for 15 min. The reaction was stopped by adding 250 μl of 0.25% (v/v) methanesulphonic acid. Derivatized thiols were separated and quantified by reverse-phase HPLC as described in Domínguez-Solís et al. (2001).
Determination of SOD and Apx activities
SOD was assayed by native isoelectric focusing (IEF) in 5% acrylamide vertical slab gels (pH 3–7). The anode and cathode solutions were 20 mM acetic acid and 25 mM NaOH, respectively, and the proteins were focused sequentially at 150 V for 30 min, 200 V for 2 h, and 400 V for 2 h, at 4 °C. Individual isoenzymes were stained by a photochemical method using NBT and identified by their sensitivity to H2O2 and KCN (Sandalio and del Río, 1988). Isoenzyme activity was quantified by densitometry in the Quantity One (Bio-Rad), recording the area under the peaks, and the total SOD activity was obtained by addition of the areas of all the isoforms. Results are expressed as a percentage of each isoform from the mutants in comparison with the wild type, arbitrarily considered 100%. For determination of soluble Apx activity, leaf tissues were ground in liquid nitrogen and homogenized in potassium phosphate buffer pH 7.6, supplemented with 20% (w/v) sorbitol, 1 mM EDTA, 5 mM AsA, and 2% (w/v) polyvinylpyrrolidone. Extracts were then centrifuged at 12 000 g for 30 min and activity was measured in the supernatant as described by Maruta et al. (2010) by the oxidation of AsA at 290 nm.
Results
Comparative analysis of Arabidopsis mutants deficient in chloroplast 2-Cys Prxs, Trx x, and NTRC
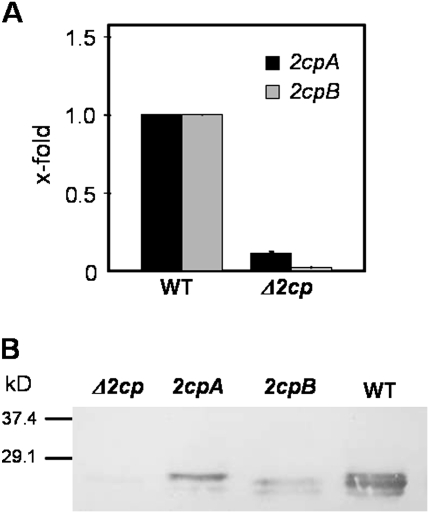
Previous results based on in vitro assays established that chloroplast 2-Cys Prxs are reduced by different Trxs, of which the x-type was the most efficient electron donor (Collin et al., 2003). However, NTRC, a bimodular protein conjugating both NTR and Trx activity, showed even higher catalytic efficiency than Trx x as reductant of 2-Cys Prx (Pérez-Ruiz et al., 2006). The Arabidopsis genome contains two genes encoding very similar isoforms of typical 2-Cys Prx, namely A and B. Single T-DNA insertion mutants of Arabidopsis deficient in either 2-Cys Prx A or 2-Cys Prx B have been described and do not show a phenotype significantly different from that of the wild-type plants, when grown under standard conditions (Kirchsteiger et al., 2009). To analyse further the function of chloroplast 2-Cys Prxs, a double mutant deficient in both 2-Cys Prx A and 2-Cys Prx B was obtained by manual crossing of the corresponding single mutants and selection of lines homozygous for the T-DNA insertions in both genes, as determined by PCR analysis of genomic DNA (Supplementary Fig. S1 at JXB online). The double mutant, termed Δ2cp, did not contain detectable amounts of 2-Cys Prx B transcripts and had only minimal amounts of 2-Cys Prx A transcripts, as determined by qPCR analysis (Fig. 1A). Accordingly, the content of 2-Cys Prx protein was severely reduced in the double mutant, being almost undetectable in western blot analysis of leaf protein extracts (Fig. 1B). Though not visible in Fig. 1B, when western blots were overloaded a faint band could be detected, which corresponded to 2-Cys Prx A as deduced from the electrophoretical mobility of 2-Cys Prx A and 2-Cys Prx B in the respective single mutants (Kirchsteiger et al., 2009). Therefore, it was concluded that the Δ2cp mutant was a severe knock-down containing <5% of the wild-type content of 2-Cys Prx A and no 2-Cys Prx B.
Fig. 1.
Characterization of the Arabidopsis Δ2cp double mutant. (A) qPCR analysis of 2cpA and 2cpB transcripts in wild-type and Δ2cp double mutant plants. The amount of transcripts in the Δ2cp double mutant was represented as arbitrary units relative to the respective levels in the wild-type plant, which was set to 1.0. (B) Western blot analysis of the content of 2-Cys Prx in the Δ2cp double mutant as compared with the respective single mutants 2cpA and 2cpB. Protein samples (7.5 μg of protein) extracted from leaves of the wild type, 2cpA and 2cpB single mutants, and the Δ2cp double mutant were subjected to SDS–PAGE (15% acrylamide) under reducing conditions, electrotransferred onto nitrocellulose sheets, and probed with anti-2-Cys Prx antibody. Molecular mass markers, in kD, are indicated on the left.
The second objective of this study was to establish the physiological relevance of NTRC and Trx x, previously identified as the most efficient reductants of 2-Cys Prx in vitro (Collin et al., 2003; Pérez-Ruiz et al., 2006). To analyse the function of Trx x and make a comparison with the NTRC-dependent pathway, a T-DNA insertion mutant, SALK_128914, in the single gene encoding Trx x of Arabidopsis (At1g50320 locus) was isolated (Supplementary Fig. S2 at JXB online). The homozygous line for the T-DNA, the trxx mutant, contained no detectable Trx x mRNA (Fig. 2A) and, accordingly, no Trx x protein in leaf extracts (Fig. 2B). Based on these results it is concluded that the trxx mutant is a knock-out for Trx x.
Fig. 2.
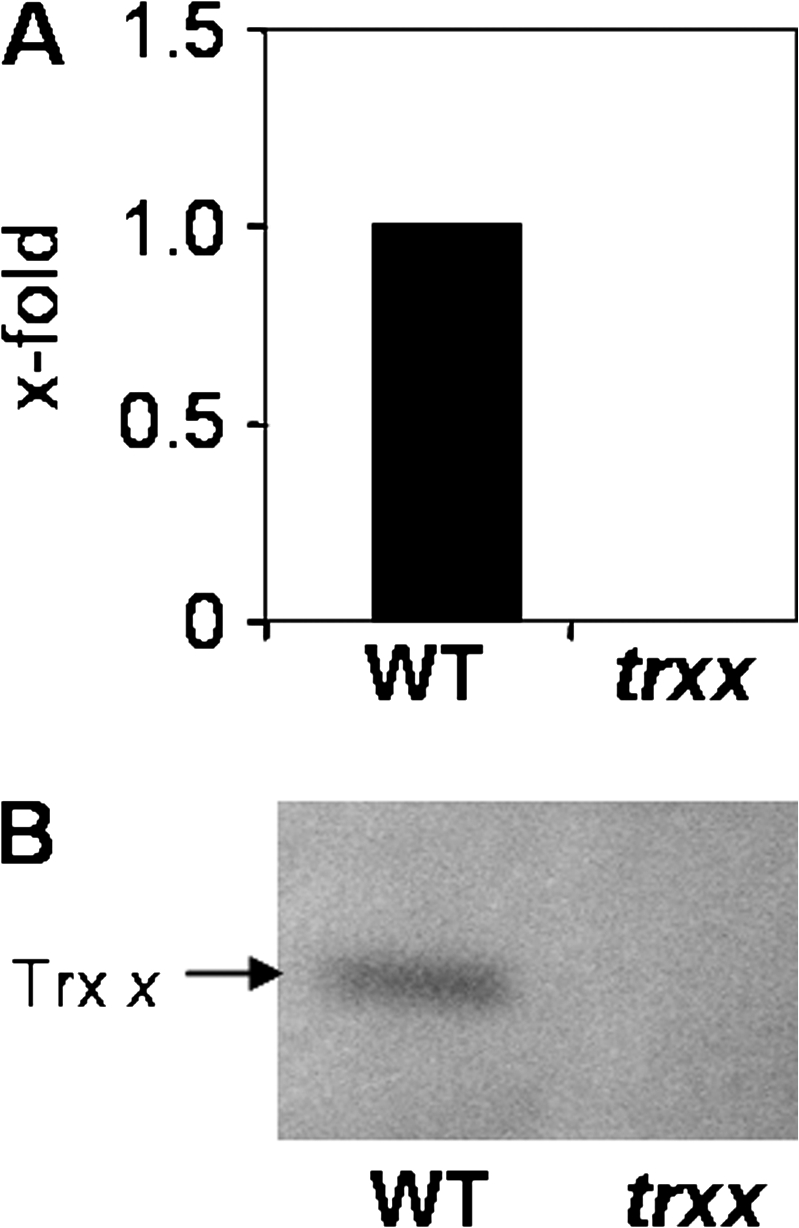
Characterization of the Arabidopsis T-DNA insertion mutant in the Trx x gene. (A) qPCR analysis of Trx x transcripts in wild-type and trxx mutant plants. The amount of transcripts in the mutant was represented as arbitrary units relative to the respective level in the wild-type plant, which was set to 1.0. (B) Western blot analysis of the Trx x protein content. Protein samples (15 μg of protein) extracted from leaves of the wild type and trxx mutant were subjected to SDS–PAGE (15% acrylamide) under reducing conditions, electrotransferred onto nitrocellulose sheets, and probed with anti-Trx x antibody.
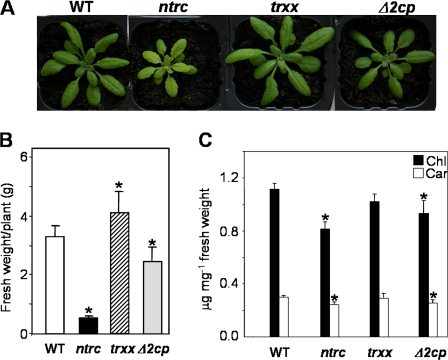
When grown under standard long-day conditions, the NTRC knock-out mutant showed the previously reported characteristic phenotype (Serrato et al., 2004; Pérez-Ruiz et al., 2006) of retarded growth, lower fresh weight, and pale green leaves with a lower content of photosynthetic pigments than wild-type plants (Fig. 3A–C). In comparison, the 2-Cys Prx-deficient mutant, Δ2cp, showed a slightly lower fresh weight and photosynthetic pigment content, total chlorophyll, and carotenoids, though the phenotype of these plants resembled more that of wild type than that of NTRC-deficient plants (Fig. 3A–C). No difference from the wild type in the chlorophyll a:chlorophyll b ratio was detected in these mutants (not shown). Similarly, the Trx x knock-out mutant exhibited a phenotype more similar to the wild type than to NTRC-deficient plants (Fig. 3A). However, the size of the rosette leaves was larger in the Trx x knock-out than in the wild-type plants (Fig. 3A). This result was confirmed by an increase of fresh weight of the rosette leaves (Fig. 3B) although there was no significant difference in the photosynthetic pigment content (Fig. 3C).
Fig. 3.
Growth and contents of photosynthetic pigments in Arabidopsis wild-type and mutant lines grown under standard long-day conditions. (A) Wild-type and mutant plants, as indicated, after 24 d of growth under long-day conditions. (B) Fresh weight of the rosette leaves of wild-type and mutant plants after 33 d of growth. Mean values plus standard errors are represented. (C) Content of photosynthetic pigments (total chlorophyll and carotenoids) in leaves from Arabidopsis wild-type and mutant plants after 33 d of growth. Pigments were extracted from four independent plant samples and the mean values ±SE are indicated. Asterisks mark significant differences from the wild type (P ≤0.025, t-Student).
2-Cys Prxs, Trx x, and NTRC are not essential components of the mechanism of response to oxidative stress
To test the antioxidant and metabolic significance of 2-Cys Prxs, Trx x, and NTRC, several markers of oxidative stress and photosynthetic activity were analysed in plants grown under standard conditions and in response to different treatments. All three mutants showed altered redox homeostasis as revealed by the increase in the levels of protein carbonylation, which was duplicated in the ntrc and trxx mutants and reached up to an almost 5-fold increase in the 2-Cys Prx knock-down mutant (Table 1). The increase in protein carbonylation might be produced by the accumulation of H2O2 due to the deficiency of 2-Cys Prx in the Δ2cp mutant, or the pathways of 2-Cys Prx reduction in ntrc and trxx mutants. The Δ2cp mutant and, to a lesser extent, the ntrc mutant showed significant increases in H2O2 in leaves (Table 1). However, the content of H2O2 was not significantly affected by the deficiency of Trx x (Table 1). These results indicate the involvement of these enzymes in the maintenance of the redox homeostasis of the chloroplast. However, unlike the deficiency of NTRC, which has a clear effect on plant growth, the deficiency of 2-Cys Prxs A and B and Trx x had only a slight effect, at least under standard growth conditions.
Table 1.
Content of carbonyl groups and hydrogen peroxide in leaves from Arabidopsis wild-type and mutant lines
| Line | Carbonyl groups (nmol DNPH mg−1 protein) | H2O2 (nmol g−1 fresh weight) |
| Wild type | 68.7±13.1 c | 769.5±24.6 c |
| ntrc | 157.6±17.3 b | 991.1±7.6 b |
| trxx | 162.4±43.1 b | 767.7±11.4 c |
| Δ2cp | 321.8±62.4 a | 1116.1±37.9 a |
Values are means ±SE of at least two replicates of three independent samples. Values with different letters are significantly different (P ≤0.05) as determined by Tukey's multiple range test.
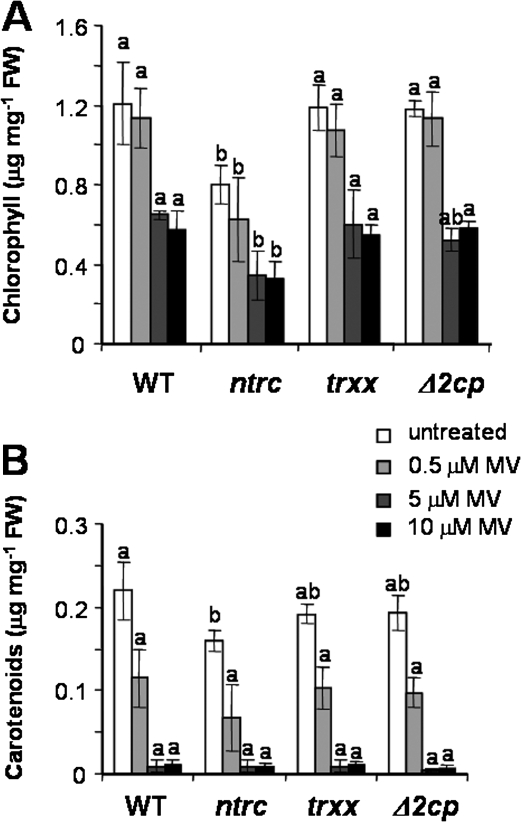
To analyse further the involvement of these enzymes in the response to oxidative stress, leaf discs from the three mutants were incubated in the presence of the photooxidative agent methyl viologen under continuous light. Treatment with increasing concentrations of methyl viologen provoked a decrease in the total chlorophyll content (Fig. 4A) and dramatically affected the carotenoid content (Fig. 4B). However, the loss of photosynthetic pigments was similar in discs from the wild type and the mutants, hence indicating that 2-Cys Prxs, Trx x, and NTRC are not essential components of the protection mechanism against oxidative stress.
Fig. 4.
Effect of oxidative stress treatments on leaf discs. Leaf discs from wild-type and mutant plants were incubated in the presence of increasing concentrations of methyl viologen, as indicated, for 48 h under continuous light (30 μE m−2 s−1), and the contents of total chlorophyll (A) or carotenoids (B) were determined. The assay was performed three times, and the values are presented as the mean ±SE. Letters indicate significance of difference (P ≤0.05) as determined by Tukey's multiple range test comparing the four Arabidopsis lines for each methyl viologen treatment.
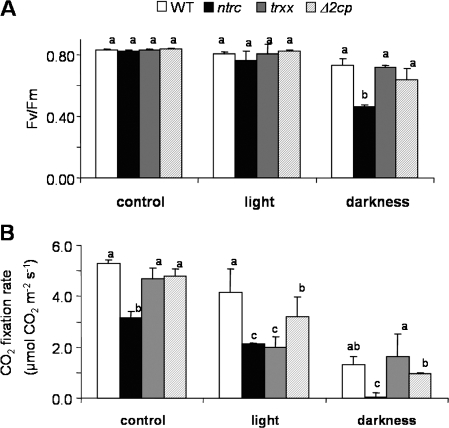
NTRC and Trx x use NADPH and reduced Fd, respectively, as the source of reducing power for 2-Cys Prx reduction. Whereas during the day reduced Fd and NADPH are products of the photosynthetic electron transport chain, during the night, when the level of reduced Fd is low, NADPH can be produced from sugars by the oxidative pentose phosphate pathway. Thus, the NADPH-dependent NTRC pathway becomes more important in the dark, as suggested by the hypersensitivity of the ntrc mutant to prolonged darkness (Pérez-Ruiz et al., 2006). To analyse further the function of both pathways, the effect of treatments affecting the source of reducing power, prolonged darkness and continuous light, on photosynthetic parameters of wild-type and mutant plants was determined. Under control conditions or in continuous light the photochemical efficiency (Fv/Fm) did not show any significant difference between the three mutants and the wild-type plants, whereas prolonged darkness significantly decreased Fv/Fm only in the ntrc mutant (Fig. 5A). CO2 fixation of the ntrc mutant was inhibited under control conditions, as previously reported by Pérez-Ruiz et al. (2006), but not in the Δ2cp or trxx mutants (Fig. 5B). The rate of CO2 fixation was affected by continuous light in the ntrc and trxx mutants and, to a lower extent, in the Δ2cp mutant (Fig. 5B). The treatment with prolonged darkness, which reduced the CO2 fixation activity of the wild-type plants, completely abolished this activity in the NTRC-deficient plants, whereas the trxx mutant did not differ significantly from the wild type (Fig. 5B). Therefore, although the continuous light treatment had a significant effect on the CO2 fixation activity of the trxx mutant, the relative changes in Fv/Fm and CO2 fixation parameters showed that photosynthetic activities are more affected by the deficiency of NTRC than Trx x or 2-Cys Prx.
Fig. 5.
PSII photochemical efficiency and CO2 fixation in Arabidopsis wild-type and mutant lines. Plants were either grown under standard long-day conditions (control), with continuous light (light), or grown as in control conditions and then subjected to 3 d of continuous darkness (darkness). Then six leaves from different plants of wild-type and mutant lines were used for determination of the initial PSII photochemical efficiency (Fv/Fm) (A) and CO2 fixation rate, which was determined as described in the Materials and methods at a light intensity of 1000 μE m−2 s−1 (B). Values are represented as the means ±SE. Letters indicate significance of difference (P ≤0.05) as determined by Tukey's multiple range test.
NTRC and Trx x have different effects on the redox status of 2-Cys Prxs
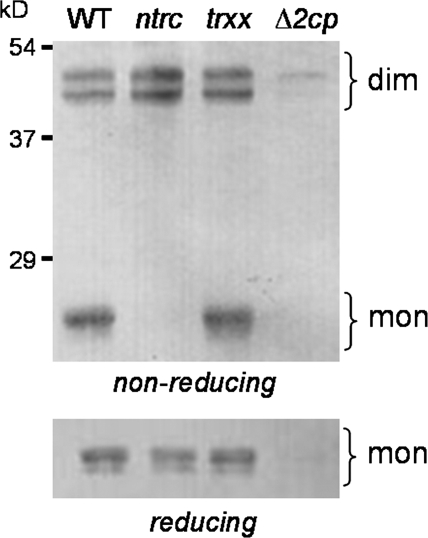
Kirchsteiger et al. (2009) reported recently that the redox status of plastidial 2-Cys Prx is imbalanced in NTRC knock-out plants. Thus, the availability of the trxx mutant allowed testing of which of the two pathways, Trx x or NTRC, enables the most efficient reduction in vivo. For this purpose, the redox status of 2-Cys Prx of leaves harvested during the day from both the ntrc and trxx mutants was analysed. The ratio of reduced (monomeric) to oxidized (dimeric) forms of 2-Cys Prxs in the trxx mutant was indistinguishable from that of the wild-type plants. In clear contrast, reduced 2-Cys Prx was almost undetectable in the ntrc mutant (Fig. 6). These results indicate that the redox status of 2-Cys Prx is imbalanced in the absence of NTRC, whereas it is not affected by the absence of Trx x, thus suggesting that NTRC is the prevailing pathway for 2-Cys Prx reduction in vivo.
Fig. 6.
Redox status of 2-Cys Prx in Arabidopsis wild-type and mutant lines. Protein extracts from leaves of the wild type, and trxx, ntrc, and Δ2cp mutants were prepared during the day and subjected to SDS–PAGE (15% acrylamide) under non-reducing (15 μg of protein) and reducing (7.5 μg of protein) conditions, as indicated, electrotransferred onto nitrocellulose sheets, and probed with the anti-2-Cys Prx antibody. Positions of molecular mass markers, in kD, are marked on the left. dim, dimeric partially or fully oxidized form; mon, monomeric reduced forms of 2-Cys Prx.
Analysis of compensatory effects between different antioxidant systems
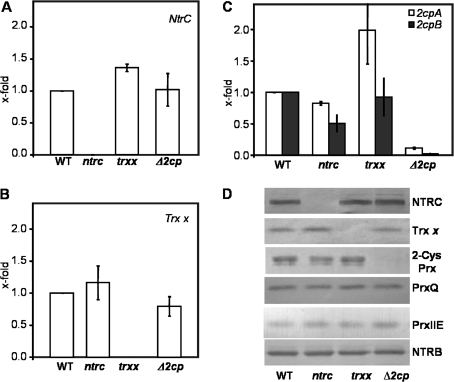
The last set of experiments addressed the possibility that the deficiency of NTRC, Trx x, or 2-Cys Prxs could alter the expression of alternative antioxidant mechanisms. The amount of NtrC transcripts, undetectable in the NTRC knock-out mutant, was not significantly affected in the Δ2cp mutant and only slightly increased in the trxx mutant (Fig. 7A). Similarly, the amount of Trx x transcripts was only slightly affected in the ntrc or Δ2cp mutants, as compared with wild-type plants (Fig. 7B). The most significant variations were observed for the levels of 2-Cys Prx transcripts: the ntrc mutant contained a lower amount of 2-Cys Prx transcripts, significantly reduced for 2-Cys Prx B, whereas the trxx mutant showed wild-type levels of 2-Cys Prx B but an ∼2-fold increase in 2-Cys Prx A transcripts (Fig. 7C). In addition, no significant variation of the content of the respective proteins, NTRC, 2-Cys Prxs, or Trx x, was observed, except for the total content of 2-Cys Prx in the ntrc mutant, which was lower (Fig. 7D). Furthermore, the amounts of the other plastidial Prxs, Prx IIE and Prx Q, were not altered in any of the three mutants analysed (Fig. 7D). Therefore, despite minor variations of the transcript levels in each of the mutants, no compensatory mechanisms based on the induction of NTRC, Trx x, or chloroplast-localized Prxs were observed.
Fig. 7.
Analysis of NtrC, Trx x, and 2-Cys Prx expression in Arabidopsis wild-type and mutant lines. (A–C) qPCR analysis of NtrC, Trx x, and 2cpA and 2cpB transcripts in leaves from wild-type and mutant lines, as indicated. The transcript amounts are represented as arbitrary units relative to the respective levels in the wild-type plants set to 1.0. Mean values of three independent determinations and the standard errors are represented. (D) Western blot analysis of protein contents of NTRC, Trx x, and the four chloroplast-localized Prxs in Arabidopsis wild-type and mutant lines, as indicated. Protein samples (15 μg of protein, except for 2-Cys Prx where 7.5 μg were loaded) extracted from leaves of wild-type and mutant lines were subjected to SDS–PAGE (12–15% acrylamide) under reducing conditions, electrotransferred onto nitrocellulose sheets, and probed with the corresponding antibody shown on the right. The anti-NTRB antibody was used to check equal loading.
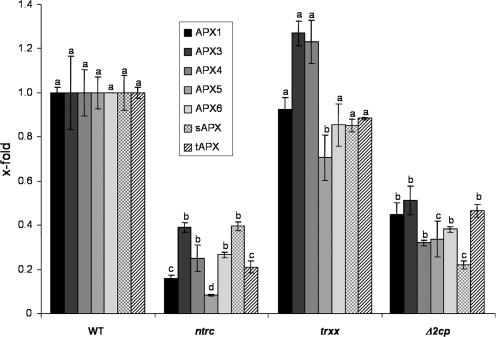
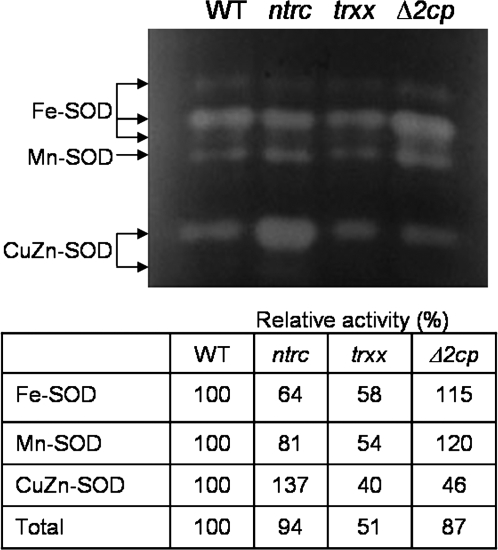
In addition, the possibility was addressed that compensatory mechanisms could involve other antioxidants such as Apx or SOD activities. Total soluble Apx activity in leaves from the three mutants was lower than in wild-type plants (Table 2). As Apx is encoded by a gene family in Arabidopsis, the expression of the different Apx genes was analysed by qPCR with gene-specific primers. Both the ntrc and Δ2cp mutants contained reduced amounts of transcripts of all the Apx genes analysed (Fig. 8), which was more pronounced than the observed reduction of Apx activity. The trxx mutant showed the lowest level of Apx activity (Table 2) despite having a higher content of Apx transcripts than the ntrc and trxx mutants (Fig. 8). The analysis of SOD activity was performed by native IEF, which enabled separation of the six different isoforms. On the basis of their inhibition by KCN and H2O2 (not shown), three of the isoforms were identified as Fe-SODs, one as Mn-SOD, and two as CuZn-SODs (Fig. 9). Total SOD activity was considerably reduced in the trxx mutant, which showed reduced levels of all isoforms (Fig. 9). SOD activity of the ntrc mutant was similar to that of the wild type, because the reduced level of Fe-SOD and Mn-SOD was compensated for by the increase in CuZn-SOD, in contrast to the Δ2cp mutant, which showed reduced CuZn-SOD but increased Fe-SOD and Mn-SOD (Fig. 9).
Table 2.
Apx activity, glutathione, and AsA pools from Arabidopsis wild type and mutant lines
| Wild type | ntrc | trxx | Δ2cp | |
| Apx activity (μmol AsA min−1 mg−1 protein) | 1.7±0.03 a | 1.5±0.1 ab | 1.0±0.05 c | 1.33±0.04 b |
| AsA+DHA (μmol AsA g−1 FW) | 1.60±0.12 c | 2.78±0.73 a | 2.70±0.72 a | 2.44±0.13 b |
| Reduced AsA/(AsA+DHA) | 0.56±0.027 a | 0.61±0.040 b | 0.69±0.022 c | 0.52±0.033 a |
| Total GSH (nmol g−1 FW) | 213±9.8 a | 252±15.9 a | 210±16.1 a | 252±22.6 a |
| Reduced GSH/total GSH | 0.83±0.07 a | 0.79±0.05 ab | 0.75±0.006 ab | 0.68±0.007 b |
Values are expressed as means± SE of four different extracts from two independent experiments. Values with different letters are significantly different (P ≤0.05) as determined by Tukey's multiple range test.
Fig. 8.
Expression analysis of the Apx gene family in Arabidopsis wild-type and mutant lines. RNA samples isolated from leaves of the corresponding Arabidopsis wild-type and mutant lines were retrotranscribed and the amount of transcripts of the Apx genes, as indicated, was determined by qPCR. Values are represented as arbitrary units relative to the respective levels in the wild-type plants set to 1.0. Mean values of three independent determinations and the standard errors are represented. Letters indicate significance of difference (P ≤0.05) as determined by Tukey's multiple range test comparing each gene in wild-type and mutant lines.
Fig. 9.
In-gel assay of SOD activity in Arabidopsis wild-type and mutant lines. Protein extracts from leaves (80 μg of protein) were subjected to IEF (pH 3–7) and stained with NBT. SOD isoforms are indicated on the left and each band was quantified and expressed as a percentage of each isoform from the mutants in comparison with the wild type, which was arbitrarily considered 100%. The assay was repeated at least three times, and a representative gel is shown.
Finally, the content and redox state of AsA and glutathione was also analysed. For unknown reasons, wild-type plants showed a high degree of oxidation of the AsA pool (Table 2), which might be due to the time (hour of the day) of harvesting and the age of the plants, both factors showing an influence on the AsA redox state. Nevertheless, results obtained for wild-type and mutant plants were comparable because the extracts were obtained from plants grown under the same conditions and harvested at the same time. The total amount of AsA was significantly increased in the three mutants, but the proportion of reduced AsA was increased only in the ntrc and trxx mutants and not in the Δ2cp mutant (Table 2). The redox state of glutathione was altered in all of the three mutants; however, the most significant change was observed in the 2-Cys Prx-deficient plants (Table 2).
Discussion
The reducing power necessary for Prx-dependent peroxide reduction is provided either by NADPH, in a reaction catalysed by NTRC (Moon et al., 2006; Pérez-Ruiz et al., 2006; Alkilfioui et al., 2007), or by reduced Fd through the action of different plastidial Trxs, of which Trx x is the most efficient (Broin et al., 2002; Collin et al., 2003, 2004; Rey et al., 2005). Previous in vitro activity assays suggested that NTRC is a more efficient catalyst than Trx x in terms of kinetic parameters (Pérez-Ruiz et al., 2006). While the catalytic activity, the conformational dynamics, the reductive regeneration, and the function in the context of photosynthesis have been described (Baier and Dietz, 1999; König et al., 2002; Collin et al., 2004; Dietz et al., 2006; Pérez-Ruiz et al., 2006), the precise role of 2-Cys Prxs in chloroplast physiology still awaits clarification. Likewise, the physiological significance of the two pathways of reducing power transfer to 2-Cys Prx is not known. The aim of this work was to analyse this relationship by a comparative analysis of Arabidopsis mutants.
First, the functional relevance of chloroplast 2-Cys Prxs was addressed. Among the different peroxide detoxification systems of the chloroplast, Prxs are characterized by a low overall catalytic efficiency due to slow regeneration, which is compensated for by their high abundance and the capacity of these enzymes to reduce not only H2O2 but also organic peroxides (König et al., 2002; Rouhier and Jacquot, 2002). These characteristics suggest an important role for Prxs in chloroplast peroxide detoxification. This notion is further supported by the fact that four of the 10 Prxs encoded by the Arabidopsis genome are targeted to the chloroplast (Bréhélin et al., 2003; Horling et al., 2003). Arabidopsis mutants deficient in individual chloroplast-localized Prxs (2-Cys Prx A, 2-Cys Prx B, Prx Q, and Prx IIE) show no significant difference in phenotype as compared with wild-type plants when grown under standard conditions (Lamkemeyer et al., 2006; Petersson et al., 2006; Romero-Puertas et al., 2007; Kirchsteiger et al., 2009), thus suggesting redundant functions for these and other peroxidases. The function of 2-Cys Prx was previously addressed by the construction of antisense plants, which showed a phenotype with alterations in developmental and photosynthetic parameters during early development of seedlings on agar plates (Baier and Dietz, 1999; Baier et al., 2000). However, the altered phenotype disappeared during further plant development as the antisense plants accumulated 2-Cys Prx protein to wild-type amounts during leaf maturation. As a consequence, the effects of 2-Cys Prx deficiency on performance of mature photosynthesizing leaves still need clarification. Thus compared with the studies with leaky antisense constructs, the newly generated double mutant, Δ2cp, which contains no 2-Cys Prx B and only trace amounts of 2-Cys Prx A (Fig. 1), represents a novel genetic model to study the physiological significance of 2-Cys Prx since the genetic defect is stable. The deficiency of 2-Cys Prx provoked alterations in the redox homeostasis as shown by the accumulation of H2O2 in leaves and the higher level of protein carbonylation (Table 1), which is indicative of the involvement of these enzymes in the mechanism of peroxide detoxification of the chloroplast. However, despite these symptoms of altered redox homeostasis, only minor differences were detected in the fresh weight and pigment content of leaves of the Δ2cp mutant plants (Fig. 3). Moreover, photosynthetic efficiency, as determined by Fv/Fm and the CO2 assimilation rate, was either not altered under control conditions or slightly reduced in Δ2cp mutant plants treated with continuous light or prolonged darkness (Fig. 5). Therefore, though 2-Cys Prxs are involved in the maintenance of the redox homeostasis of the chloroplast, the redox imbalance observed can be compensated for by alternative chloroplast antioxidant and repair systems without a significant loss of fitness. Apparently, these enzymes are not essential to protect the chloroplast against oxidative damage, at least under standard growth conditions. This notion was further supported by the similar effect of increasing concentrations of methyl viologen on leaf discs from wild-type and 2-Cys Prx-deficient plants (Fig. 4).
The major objective of this work was to establish which of the two pathways of 2-Cys Prx reduction, NTRC or Trx x, is more relevant in vivo. The analysis focused on the comparison of the previously reported ntrc mutant (Serrato et al., 2004) with a mutant characterized in this work, trxx, which is a knock-out for Trx x (Fig. 2). The deficiency of each of these components provoked alterations in redox homeostasis as shown by the higher level of protein carbonylation, though to a lesser extent than in the 2-Cys Prx-deficient plants (Table 1). The deficiency of NTRC caused a dramatic effect on plant growth (Fig. 3), in concordance with the significant loss of photosynthetic efficiency of the ntrc mutant both under standard growth conditions and in response to continuous light or darkness treatments (Fig. 5). In a converse manner, the absence of Trx x did not affect Fv/Fm and modified the CO2 assimilation rate only during continuous light treatment (Fig. 5). The limited effect of Trx x deficiency on the photosynthetic parameters analysed here is in agreement with the lack of negative effect on growth or pigment content of the mutant plants (Fig. 3). Therefore, the comparison of the two mutants suggests a higher overall physiological relevance of NTRC.
Concerning the specific roles of NTRC and Trx x in 2-Cys Prx reduction, the redox status of 2-Cys Prx in the respective mutants was analysed by gel electrophoresis under non-reducing conditions. The reduced 2-Cys Prx, which is detected as a monomeric form in denaturing gels, was hardly detectable in the ntrc mutant. In contrast, the level of reduced 2-Cys Prx in the trxx mutant was similar to that of the wild-type plants (Fig. 6), thus showing that the deficiency of NTRC, but not of Trx x, caused the imbalance in the redox status of this enzyme. In agreement with these results, the level of hydrogen peroxide, which was increased in the ntrc and Δ2cp mutants, was unaltered in the trxx mutant (Table 1). Altogether, the results suggest that the NTRC pathway is more important than the FTR/Trx x pathway for 2-Cys Prx reduction in vivo, in agreement with the higher catalytic regeneration efficiency of NTRC observed in vitro with the purified recombinant enzymes (Pérez-Ruiz et al., 2006). The recent finding, based on FRET (fluorescence resonance energy transfer) analysis, that NTRC interacts with 2-Cys Prx A but not with Trx x in protoplasts from Arabidopsis leaves (Muthuramalingam et al., 2009), lends further support to this proposal.
Though the 2-Cys Prx-deficient mutant showed slightly retarded growth compared with the wild-type plant, its phenotype was less severe than the phenotype shown by the ntrc mutant (Fig. 3). Moreover, the ntrc mutant was more sensitive than the Δ2cp mutant to continuous light and prolonged darkness in terms of Fv/Fm and CO2 fixation (Fig. 5). The different phenotypes of the ntrc and Δ2cp mutants suggest that beside the function of NTRC exerted through the efficient reduction of 2-Cys Prx, the enzyme may play additional roles not related to the 2-Cys Prx. One of the functions already established of NTRC in conjunction with 2-Cys Prx is the activation of protochlorophyllide synthesis, which takes place through the stimulation of an aerobic cyclase by the NTRC/2-Cys Prx system, as observed in vitro (Stenbaek et al., 2008). Beyond the functions of NTRC in the context of 2-Cys Prx reduction, NTRC was recently implicated in the mechanism of redox activation of ADP-glucose pyrophosphorylase (AGPase), and thus in starch metabolism both in leaves and in roots (Michalska et al., 2009). In addition, transcript and metabolite profiling revealed altered contents of amino acids and auxin in the ntrc mutant, a phenotype which was more severe under short-day conditions (Lepistö et al., 2009). It is therefore likely that the activity of NTRC as a protein disulphide reductase (Pérez-Ruiz and Cejudo, 2009; Pérez-Ruiz et al., 2009) adjusts the redox status of several target proteins involved in different metabolic and/or signalling pathways, thus explaining the pleiotropic phenotype of the NTRC knock-out plant.
The deficiency of NTRC, but not of Trx x or 2-Cys Prx, provoked a lower rate of CO2 fixation under control conditions (Fig. 5), which is additional evidence of the unique role of NTRC in different aspects of chloroplast function. As expected, a pre-treatment of plants with prolonged darkness, which affected the rate of CO2 fixation in all mutants, showed a more severe effect in the ntrc mutant, in agreement with previous data showing hypersensitivity of this mutant to prolonged darkness (Pérez-Ruiz et al., 2006). Surprisingly, continuous light, which might favour the FTR/Trx x pathway, provoked a similar decrease of the CO2 fixation rate in the ntrc and the trxx mutants, suggesting that Trx x may become important under certain environmental conditions.
Despite the different symptoms of imbalanced redox homeostasis detected in the three mutants, ntrc, trxx, and Δ2cp, only the ntrc mutant showed a clear phenotypic variation. The results suggest that the antioxidant function of these proteins might be compensated for by alternative chloroplast antioxidant systems. Despite minor variations in the expression of the different chloroplast-localized Prxs, NTRC, and Trx x, no significant induction of these systems was detected in any of the mutants (Fig. 7). Moreover, although CuZn-SOD activity was increased in the ntrc mutant, total SOD and Apx activities did not increase in any of the three mutants (Table 2, Fig. 9). In the case of the ntrc and the Δ2cp mutants, the decrease of Apx activity was expected since these plants showed a lower content of transcripts of the Apx gene family (Fig. 8). Thus, the possible compensatory effect exerted by additional detoxification systems seems not to require the induction of such systems. Similarly, Arabidopsis mutants deficient in stromal or thylakoid Apx (Maruta et al., 2010) or the double mutant deficient in both chloroplast-localized Apxs (Kangasjärvi et al., 2008) show no altered phenotype when grown under standard conditions, nor induction of the chloroplast-localized Prxs unless plants are subjected to treatments such as high light and, particularly, high light combined with high temperature (Kangasjärvi et al., 2008).
Despite the lack of induction of alternative enzymatic antioxidants, all three mutants showed increased levels of the AsA pool, and the reduction state (ratio AsA/AsA+DHA) was increased in the ntrc and trxx mutants (Table 2). Moreover, although no significant differences were detected in the total content of glutathione, its redox balance was altered, showing a lower proportion of reduced glutathione, which was most significant in the Δ2cp mutant (Table 2). Therefore, the redox buffer capacity of non-enzymatic antioxidants such as glutathione or AsA may be important to compensate for the deficiency of any of the enzymatic antioxidant systems analysed here.
In summary, the results reported here show that the deficiency of NTRC, Trx x, or 2-Cys Prx affects the redox homeostasis of the chloroplast. However, the moderate differences in phenotype caused by the deficiency of Trx x and 2-Cys Prx, in contrast to the dramatic effect caused by the deficiency of NTRC, strongly supports the central role of NTRC in the redox regulation of the chloroplast, not only related to the response of the plant to oxidative stress.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. (A) Schematic representation of the structure of 2cpA and 2cpB genes from Arabidopsis showing the location of the T-DNA insertions, as previously reported (Kirchsteiger et al., 2009), used for construction of the double mutant. The position of the oligonucleotides used for PCR analysis is marked with arrows. (B) PCR analysis of the presence of the T-DNA insertions in genomic DNA isolated from the wild type and homozygous double mutant (Hom) for both insertions. DNA size markers, in kbp, were loaded on the left. T denotes the primer specific for the T-DNA insertion, while a, a', b, and b' represent the gene-specific primers as shown in A.
Figure S2. (A) Schematic representation of the structure of the Trx x gene from Arabidopsis showing the position of the T-DNA insertion in line SALK_125897 and the oligonucleotides used for PCR analysis. (B) PCR analysis of genomic DNA isolated from the wild type and a homozygous line for the T-DNA insertion in the trxx gene. DNA size markers, in kbp, are shown on the left. T denotes the primer specific for the DNA insertion; c and d represent the gene-specific primers as shown in A.
Table S1. Gene-specific oligonucleotides used for qPCR analysis.
Supplementary Material
Acknowledgments
This work was supported by grant BIO2007-60644 from Ministerio de Educación y Ciencia, and grants P06-CVI-01578 and BIO-182 from Junta de Andalucía (Spain). K-JD acknowledges support by the DFG (Di346). PP, KK, and MG were supported by pre-doctoral fellowships from Seville University, CSIC, and Junta de Andalucía, respectively. We are deeply grateful to Dr N. Rouhier (University of Nancy, France) for the anti-Prx IIE antibody. The technical assistance of C. Parejo and critical reading of the manuscript by M. Lindahl are gratefully acknowledged.
References
- Alkhalfioui F, Renard M, Vensel WH, Wong JH, Tanaka CK, Hurkman WJ, Buchanan BB, Montrichard F. Thioredoxin-linked proteins are reduced during germination of Medicago truncatula seeds. Plant Physiology. 2007;144:1559–1579. doi: 10.1104/pp.107.098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Dietz KJ. Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis. Evidence from transgenic Arabidopsis. Plant Physiology. 1999;119:1407–1414. doi: 10.1104/pp.119.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Noctor G, Foyer CH, Dietz KJ. Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. Plant Physiology. 2000;124:823–832. doi: 10.1104/pp.124.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehelin C, Meyer EH, de Souris JP, Bonnard G, Meyer Y. Resemblance and dissemblance of Arabidopsis type II peroxiredoxins: similar sequences for divergent gene expression, protein localization, and activity. Plant Physiology. 2003;132:2045–2057. doi: 10.1104/pp.103.022533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broin M, Cuiné S, Eymery F, Rey P. The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. The Plant Cell. 2002;14:1417–1432. doi: 10.1105/tpc.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin J-M, Knaff DB, Miginiac-Maslow M. The Arabidopsis plastidial thioredoxins. New functions and new insights into specificity. Journal of Biological Chemistry. 2003;278:23747–23752. doi: 10.1074/jbc.M302077200. [DOI] [PubMed] [Google Scholar]
- Collin V, Lamkemeyer P, Miginiac-Maslow M, Hirasawa M, Knaff DB, Dietz K-J, Issakidis-Bourguet E. Characterization of plastidial thioredoxins from Arabidopsis belonging to the new y-type. Plant Physiology. 2004;136:4088–4095. doi: 10.1104/pp.104.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K-J, Horling F, Konig J, Baier M. The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. Journal of Experimental Botany. 2002;53:1321–1329. [PubMed] [Google Scholar]
- Dietz K-J. Plant peroxiredoxins. Annual Review of Plant Biology. 2003;54:93–107. doi: 10.1146/annurev.arplant.54.031902.134934. [DOI] [PubMed] [Google Scholar]
- Dietz K-J, Jacob S, Oelze M-L, Laxa M, Tognetti V, Nunes de Miranda SM, Baier M, Finkemeier I. The function of peroxiredoxins in plant organelle redox metabolism. Journal of Experimental Botany. 2006;57:1697–1709. doi: 10.1093/jxb/erj160. [DOI] [PubMed] [Google Scholar]
- Domínguez-Solís JR, Gutiérrez-Alcalá G, Vega JM, Romero LC, Gotor C. The cytosolic O-acetylserine(thiol)lyase gene is regulated by heavy metals and can function in cadmium tolerance. Journal of Biological Chemistry. 2001;276:9297–9302. doi: 10.1074/jbc.M009574200. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signalling, acclimation, and practical implications. Antioxidant Redox Signaling. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Gillespile K, Ainsworth EA. Measurement of reduced, oxidized and total ascorbate content in plants. Nature Protocols. 2007;2:871–874. doi: 10.1038/nprot.2007.101. [DOI] [PubMed] [Google Scholar]
- Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins—structures, mechanisms and functions. FEBS Journal. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Eymery F, Porfirova S, Rey P, Dormann P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. The Plant Cell. 2005;17:3451–3469. doi: 10.1105/tpc.105.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horling F, Lamkemeyer P, Konig J, Finkemeier I, Kandlbinder A, Baier M, Dietz K- J. Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiology. 2003;131:317–325. doi: 10.1104/pp.010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangasjärvi S, Lepistö A, Hannikainen K, Piippo M, Luomala EM, Aro EM, Rintamaki E. Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochemical Journal. 2008;412:275–285. doi: 10.1042/BJ20080030. [DOI] [PubMed] [Google Scholar]
- Kim C, Meskauskiene R, Apel K, Laloi C. No single way to understand singlet oxygen signalling in plants. EMBO Reports. 2008;9:435–439. doi: 10.1038/embor.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchsteiger K, Pulido P, González MC, Cejudo FJ. NADPH thioredoxin reductase C controls the redox status of chloroplast 2-Cys peroxiredoxins in Arabidopsis thaliana. Molecular Plant. 2009;2:298–307. doi: 10.1093/mp/ssn082. [DOI] [PubMed] [Google Scholar]
- König J, Baier M, Horling F, Kahmann U, Harris G, Schurmann P, Dietz K-J. The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redox hierarchy of photosynthetic electron flux. Proceedings of the National Academy of Sciences, USA. 2002;99:5738–5743. doi: 10.1073/pnas.072644999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J, Lotter K, Plessow R, Brockhinke A, Baier M, Dietz K-J. Reaction mechanism of plant 2-Cys peroxiredoxin. Journal of Biological Chemistry. 2003;278:24409–24420. doi: 10.1074/jbc.M301145200. [DOI] [PubMed] [Google Scholar]
- Kruk J, Hollander-Czytko H, Oettmeier W, Trebst A. Tocopherol as singlet oxygen scavenger in photosystem II. Journal of Plant Physiology. 2005;162:749–757. doi: 10.1016/j.jplph.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Lamkemeyer P, Laxa M, Collin V, et al. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. The Plant Journal. 2006;45:968–981. doi: 10.1111/j.1365-313X.2006.02665.x. [DOI] [PubMed] [Google Scholar]
- Ledford HK, Chin BL, Niyogi KK. Acclimation to singlet oxygen stress in Chlamydomonas reinhardtii. Eukaryotic Cell. 2007;6:919–930. doi: 10.1128/EC.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, Kwon SY, Kim TH, Lee BH. Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. Journal of Plant Physiology. 2007;16:1626–1638. doi: 10.1016/j.jplph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lepistö A, Kangasjärvi S, Luomala EM, Brader G, Sipari N, Keränen M, Keinänen M, Rintamäki E. Choloroplast NADPH-thioredoxin reductase interacts with photoperiodic development in Arabidopsis. Plant Physiology. 2009;149:1261–1276. doi: 10.1104/pp.108.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions. 1983;603:591–592. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maruta T, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant and Cell Physiology. 2010;51:190–200. doi: 10.1093/pcp/pcp177. [DOI] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. The Plant Cell. 2006;18:2749–2766. doi: 10.1105/tpc.106.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. NTRC links built in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proceedings of the National Academy of Sciences, USA. 2009;106:9908–9913. doi: 10.1073/pnas.0903559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Moon JC, Jang HH, Chae HB, et al. The C-type Arabidopsis thioredoxin reductase ANTR-C acts as an electron donor to 2-Cys peroxiredoxins in chloroplasts. Biochemical and Biophysical Research Communications. 2006;348:478–484. doi: 10.1016/j.bbrc.2006.07.088. [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Baker NR. Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiology. 2006;141:346–350. doi: 10.1104/pp.106.078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuramalingam M, Seidel T, Laxa M, Nunes de Miranda SM, Gärtner F, Ströher E, Kandlbinder A, Dietz K-J. Multiple redox and non-redox interactions define 2-Cys peroxiredoxin as a regulatory hub in the chloroplast. Molecular Plant. 2009;2:1273–1288. doi: 10.1093/mp/ssp089. [DOI] [PubMed] [Google Scholar]
- Navrot N, Collin V, Gualberto J, Gelhaye E, Hirasawa M, Rey P, Knaff DB, Issakidis E, Jacquot JP, Rouhier N. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiology. 2006;142:1364–1379. doi: 10.1104/pp.106.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N, Ben-Shem A. The complex architecture of oxygenic photosynthesis. Nature Reviews in Molecular Cell Biology. 2004;5:1–12. doi: 10.1038/nrm1525. [DOI] [PubMed] [Google Scholar]
- op den Camp RG, Przybyla D, Ochsenbein C, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. The Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J, Munne-Bosch S. Isoprenoids: an evolutionary pool for photoprotection. Trends in Plant Science. 2005;10:166–169. doi: 10.1016/j.tplants.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, Cejudo FJ. A proposed reaction mechanism for rice NADPH thioredoxin reductase C, an enzyme with protein disulfide reductase activity. FEBS Letters. 2009;583:1399–1402. doi: 10.1016/j.febslet.2009.03.067. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, González MC, Spínola MC, Sandalio LM, Cejudo FJ. The quaternary structure of NADPH thioredoxin reductase C is redox sensitive. Molecular Plant. 2009;2:457–467. doi: 10.1093/mp/ssp011. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, Spínola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. The Plant Cell. 2006;18:2356–2368. doi: 10.1105/tpc.106.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson UA, Kieselbach T, Garcia-Cerdan JG, Schroder WP. The Prx Q protein of Arabidopsis thaliana is a member of the luminal chloroplast proteome. FEBS Letters. 2006;580:6055–6061. doi: 10.1016/j.febslet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxidant Redox Signaling. 2006;8:1757–1764. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- Rascher U, Nedbal L. Dynamics of photosynthesis in fluctuating light. Current Opinion in Plant Biology. 2006;9:671–678. doi: 10.1016/j.pbi.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Rey P, Cuiné S, Eymery F, Garin J, Court M, Jacquot J-P, Rouhier N, Broin M. Analysis of the proteins targeted by CDSP32, a plastidic thioredoxin participating in oxidative stress responses. The Plant Journal. 2005;41:31–42. doi: 10.1111/j.1365-313X.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez Milla MA, Maurer A, Rodríguez Huete A, Gustafson JP. Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signalling pathways. The Plant Journal. 2003;36:602–615. doi: 10.1046/j.1365-313x.2003.01901.x. [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Laxa M, Matté A, Zanonotto F, Finkemeier I, Jones AME, Perazolli M, Vandelle E, Dietz KJ, Delledonne M. S-Nitrosylation of peroxiredoxin IIE promotes peroxynitrite-mediated tyrosine nitration. The Plant Cell. 2007;19:4120–4130. doi: 10.1105/tpc.107.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas MC, Palma JM, Gómez M, del Río LA, Sandalio LM. Cadmium causes the oxidative modification of proteins in pea plants. Plant, Cell and Environment. 2002;25:677–686. [Google Scholar]
- Rouhier N, Jacquot JP. Plant peroxiredoxins: alternative hydroperoxide scavenging enzymes. Photosynthesis Research. 2002;74:259–268. doi: 10.1023/A:1021218932260. [DOI] [PubMed] [Google Scholar]
- Sandalio LM, del Río LA. Intraorganellar distribution of superoxide dismutase in plant peroxisomes (glyoxysomes and leaf peroxisomes) Plant Physiology. 1988;88:1215–1218. doi: 10.1104/pp.88.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrato AJ, Pérez-Ruiz JM, Cejudo FJ. Cloning of thioredoxin h reductase and characterization of the thioredoxin reductase–thioredoxin h system from wheat. Biochemical Journal. 2002;217:392–399. doi: 10.1042/BJ20020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrato A, Pérez-Ruiz JM, Spínola MC, Cejudo FJ. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. Journal of Biological Chemistry. 2004;279:43821–43827. doi: 10.1074/jbc.M404696200. [DOI] [PubMed] [Google Scholar]
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K. Regulation and function of ascorbate peroxidase isoenzymes. Journal of Experimental Botany. 2002;53:1305–1319. [PubMed] [Google Scholar]
- Spínola MC, Pérez-Ruiz JM, Pulido P, Kirchsteiger K, Guinea M, González MC, Cejudo FJ. NTRC: new ways of using NADPH in the chloroplast. Physiologia Plantarum. 2008;133:516–524. doi: 10.1111/j.1399-3054.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- Stenbaek A, Hansson A, Wulff RP, Hansson M, Dietz K-J, Jensen PE. NADPH-dependent thioredoxin reductase and 2-Cys peroxiredoxins are needed for the protection of Mg-protoporphyrin monomethyl ester cyclase. FEBS Letters. 2008;582:2773–2778. doi: 10.1016/j.febslet.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Bailey-Serres J, Mittler R. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiology. 2008;147:978–984. doi: 10.1104/pp.108.122325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Przybyla D, Op den Camp R, et al. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signalling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.