Abstract
In C4 plants, acclimation to growth at low irradiance by means of anatomical and biochemical changes to leaf tissue is considered to be limited by the need for a close interaction and coordination between bundle sheath and mesophyll cells. Here differences in relative growth rate (RGR), gas exchange, carbon isotope discrimination, photosynthetic enzyme activity, and leaf anatomy in the C4 dicot Flaveria bidentis grown at a low (LI; 150 μmol quanta m2 s−1) and medium (MI; 500 μmol quanta m2 s−1) irradiance and with a 12 h photoperiod over 36 d were examined. RGRs measured using a 3D non-destructive imaging technique were consistently higher in MI plants. Rates of CO2 assimilation per leaf area measured at 1500 μmmol quanta m2 s−1 were higher for MI than LI plants but did not differ on a mass basis. LI plants had lower Rubisco and phosphoenolpyruvate carboxylase activities and chlorophyll content on a leaf area basis. Bundle sheath leakiness of CO2 (ϕ) calculated from real-time carbon isotope discrimination was similar for MI and LI plants at high irradiance. ϕ increased at lower irradiances, but more so in MI plants, reflecting acclimation to low growth irradiance. Leaf thickness and vein density were greater in MI plants, and mesophyll surface area exposed to intercellular airspace (Sm) and bundle sheath surface area per unit leaf area (Sb) measured from leaf cross-sections were also both significantly greater in MI compared with LI leaves. Both mesophyll and bundle sheath conductance to CO2 diffusion were greater in MI compared with LI plants. Despite being a C4 species, F. bidentis is very plastic with respect to growth irradiance.
Keywords: C4 photosynthesis, carbon isotope discrimination, CO2 leakiness, Flaveria bidentis, gas exchange, image analysis, leaf anatomy
Introduction
As light is a fundamental requirement for photosynthesis, growing plants in a light-limiting environment can affect the development and physiology of leaves. In C3 species these adaptive changes have been well documented and can include structural, biochemical, and physiological modifications to leaves. At low irradiance, C3 plants tend to allocate less energy to root production and direct it instead to increase leaf and stem biomass (Poorter and Perez-Soba, 2001). Changes in the size, shape, and number of leaf mesophyll cells reduce leaf thickness and increase specific leaf area (SLA; the leaf area to leaf dry mass ratio), acting to maximize light absorption and reduce the diffusion pathway of CO2 to photosynthetic tissue (Bjorkman, 1981). The amount of the essential CO2-fixing enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) decreases with reduced growth irradiance as cellular allocation of nitrogen switches away from soluble proteins towards pigment–protein complexes (Hikosaka and Terashima, 1995; Evans and Poorter, 2001). As a result of these changes, plants grown at low irradiance have a reduced photosynthetic capacity per unit leaf area, yet photosynthetic rates do not differ greatly per unit leaf dry mass. Plants conducting C4 photosynthesis are generally considered to be less phenotypically plastic with respect to low light acclimation due to their defining anatomical features (Sage and McKown, 2006) although they have also been shown to respond in similar ways to C3 plants. In an early comparison between the C4 plant Zea mays (maize) and the C3 plant Phaseolus vulgaris (bean), both species had thinner leaves and a lower leaf mass per unit area when grown at lower irradiances (Louwerse and Zweerde, 1977). However, these responses were consistently greater in the C3 plant, corresponding to a smaller percentage difference in photosynthetic CO2 assimilation rate between plants grown at high and low irradiance compared with that in the C4 plant. A study between the sun-adapted C4 Z. mays and the shade-tolerant C4 grass Paspalum conjugatum showed that the response to low irradiance can differ greatly between C4 plants from different habitats (Ward and Woolhouse, 1986b). At low irradiance, the activity of the C4 photosynthetic enzymes phosphoenolpyruvate carboxylase (PEPC) and pyruvate Pi dikinase were decreased in both species, yet only in the sun-adapted species did levels of Rubisco, the major component of cellular protein content, fall.
The efficiency of the C4 photosynthetic concentrating mechanism is intimately linked to bundle sheath leakiness (ϕ) defined as that fraction of CO2 generated by C4 acid decarboxylation in the bundle sheath that subsequently leaks out (Farquhar, 1983). Bundle sheath leakiness depends on both the bundle sheath conductance to CO2 diffusion and the relative biochemical capacities of the C4 and C3 cycles, but estimation of these factors remains difficult. Since the C4 cycle consumes energy in the form of ATP during the regeneration of phosphoenolpyruvate (PEP), leakage of CO2 out of the bundle sheath is an energy cost to the leaf. Estimates of leakiness of CO2 from bundle sheath cells have been shown to increase when measured at lower irradiances (Henderson et al., 1992; Tazoe et al., 2008), contributing to the overall decrease in CO2 assimilation rate. However, Tazoe et al. (2008) also reported that in the C4 dicot Amaranthus cruentus, there was less variation in leakiness in plants grown at low irradiance, suggestive of some acclimation.
The aim of the present study was to measure the response of the C4 model plant Flaveria bidentis, a sun-adapted species, to growth at low irradiance. The potential of a novel 3D growth imaging system to correlate changes in growth with expected physiological, biochemical, and anatomical changes was examined. The specific effects of growth at low irradiance on several factors key to the efficiency of C4 photosynthesis, namely bundle sheath cell leakiness and both mesophyll and bundle sheath cell conductance to CO2 diffusion, were also investigated. Concurrent measurements of gas exchange and carbon isotope discrimination are used to estimate bundle sheath leakiness, and anatomical measurements of F. bidentis grown at differing irradiances are utilized to estimate mesophyll and bundle sheath conductance to CO2 diffusion.
Materials and methods
Plant material and growth conditions
Seeds of F. bidentis were sown in clear plastic containers measuring 10 cm by 10 cm, and 5 cm in height, containing a commercial seed raising mix (Debco, Melbourne, Australia) and 1 g l−1 of a slow release fertilizer (Osmocote, Scotts, Australia). Seeds were germinated in growth cabinets under a 12/12 h day–night cycle at 28/25 °C, 70% humidity at an irradiance of 400 μmol quanta m−2 s−1. Following germination, seedlings were transplanted to individual black plastic pots 5 cm by 5 cm, and 10 cm in height containing an identical soil mix and placed in partially water-filled plastic trays. Plants were grown within the same growth cabinet under two light conditions, a medium irradiance of 500 μmol PAR quanta m−2 s−1 (MI), in which plants were left uncovered, and a low irradiance of 150 μmol PAR quanta m−2 s−1 (LI), in which plants were continuously covered by a light shade-cloth frame. Forty plants were grown at each irradiance, 15 of which were used for imaging (and subsequent physiological, biochemical, and anatomical measurements) and 25 for destructive harvesting.
Plant imaging and destructive harvesting
Plants were removed from the growth cabinet for imaging every other day for a period of 5–6 weeks. Plants were individually photographed in a Scanalyser growth imaging system (LemnaTec) comprising two cameras to obtain a top view, 0 ° side view, and a 90 ° rotated side view per imaging session. Individual images taken throughout the growth period were used to create and refine an automated analysis algorithm using the Scanalyser software package. The analysis grid was subsequently used to analyse the complete set of images, separating plant from non-plant pixels to give a two-dimensional plant area for each image. Images were calibrated to convert image areas from pixel2 to mm2. For a single plant the three areas taken at each imaging session were combined to give a plant volume at each time point as shown in Equation 1:
| (1) |
Relative growth rates (RGRs) calculated from image analysis for single plants over the entire growth period were obtained as the slope of an exponential curve fitted to a plot of plant volumes over time. RGRs for dry matter harvests were calculated as in Equation 2:
| (2) |
At four time points throughout the growth period (13, 20, 27, and 34 d), 5–10 plants were taken from both light conditions for destructive harvest. Fresh masses of leaves and stems were measured immediately after harvest. Total leaf area (TLA) of each plant was determined using an LI-3000A leaf area meter (LI-COR, Lincoln, NE, USA). Dry masses of leaves and stem were measured after 48 h at 80 °C. Specific leaf area (SLA), leaf dry matter content (LDMC), and leaf dry mass per unit area (LMA) were calculated from destructive harvest data taken from 10 plants after 34 d.
Gas exchange
Gas exchange was measured on the highest fully expanded leaf using a LI-6400 equipped with a blue–red light-emitting diode (LED) light source (LI-COR). Due to the substantial differences in growth rate at different irradiances, plants grown at 500 μmol quanta m−2 s−1 were measured between 17 d and 30 d after transplantation of seedlings, whereas plants grown at 150 μmol quanta m−2 s−1 were measured between 40 d and 50 d after transplantation on an equivalent leaf size and at an equivalent position. Measurements were conducted ∼2–3 h into the day cycle. Measured leaves were initially equilibrated for 20 min in a standard environment of 50 μmol mol−1 CO2, 25 °C leaf temperature, flow rate of 500 μmol s−1, and either 150, 500, or 1500 μmol quanta m−2 s−1 blue–red irradiance depending on the subsequent measurement condition. Photosynthetic CO2 response (A–Ci) curves were conducted by imposing a stepwise increase in CO2 partial pressure from 30 μmol mol−1 to 600 μmol mol−1, maintaining temperature and irradiance conditions.
Following gas exchange measurements. 0.5 cm2 leaf discs were removed from the tested leaves and snap-frozen in liquid nitrogen for measurement of photosynthetic enzyme activities and chlorophyll content. The entire opposite leaf was also taken and oven dried at 80 °C for 48 h for later measurement of total nitrogen and carbon isotope composition.
Enzyme activities and chlorophyll measurement
The activities of the photosynthetic enzymes Rubisco and PEPC were measured as previously described by Cousins et al. (2007), with some changes. Frozen leaf tissue was processed in ice-cold glass homogenizers with 500 μl of extraction buffer (50 mM HEPES-KOH pH 7.8, 1 mM EDTA, 0.1% Triton-X, 10 mM dithiothreitol, and 1% polyvinylpolypyrrolidone) and 10 μl of protease inhibitor cocktail (Sigma). The homogenate was briefly centrifuged and the supernatant used for assays. For PEPC, 10 μl of leaf extract was combined with 980 μl of assay buffer (50 mM EPPS-NaOH pH 8, 10 mM MgCl2, 0.5 mM EDTA, 0.2 mM NADH, 5 mM glucose-6-phosphate 1 mM NaHCO3, and 1 U ml−1 malate dehydrogenase) and the reaction initiated by the addition of 10 μl of 400 mM PEP. For Rubisco, 10 μl of leaf extract was combined with 970 μl of assay buffer (50 mM EPPS-NaOH pH 8, 10 mM MgCl2, 0.5 mM EDTA, 1 mM ATP, 5 mM phosphocreatine, 20 mM NaHCO3, 0.2 mM NADH, 50 U ml−1 creatine phosphokinase, 0.2 mg carbonic anhydrase, 50 U ml−1 3-phosphoglycerate kinase, 40 U ml−1 glyceraldehyde-3-phosphate dehydrogenase, 113 U m;−1 Triose-phosphate isomerase, 39 U ml−1 glycerol 3 phosphate dehydrogenase) and the reaction initiated by the addition of 20 μl of 21.9 mM ribulose-1, 5-bisphosphate (RuBP). The activity of both enzymes was calculated by monitoring the decrease of NADH absorbance at 340 nm with a diode array spectrophotometer (Hewlett Packard) after initiation of the reaction.
Chlorophyll was extracted from frozen leaf discs in a glass homogenizer with 80% acetone. The chlorophyll a and b contents of extracts were measured in a quartz cuvette at 663.3 nm and 646.6 nm, and calculated according to Porra et al. (1989).
Leaf anatomical measurements
Multiple leaf pieces of ∼2 mm by 5 mm were taken from fully developed source leaves avoiding major veins. Slices were fixed in 5 ml of buffer containing 2.5% gluteraldehyde, 3.5% formaldehyde, 0.1 M Na cacodylate, 0.12 M sucrose, 10 mM ethylene glycol tetra-acetic acid (EGTA), and 2 mM MgCl2 under a vacuum for 4 h. Leaf pieces were subsequently washed in the same buffer lacking fixatives then post-fixed in 1% osmium tetroxide for 2 h. Fixed leaf sections were dehydrated in an ethanol series (50, 60, 70, 75, 80, 90, 95, and 100%) followed by two further rinses in 100% ethanol and two rinses in 100% acetone. Leaf sections were then floated in a 2:1 mix of 100% acetone:araldite resin for 1 h, a 1:1 mix for a further hour, a 1:2 mix for a further hour, followed by three further washes in pure araldite resin for 2 h each. Leaf sections were finally placed in plastic moulds filled with fresh araldite resin and baked at 60 °C for 24 h.
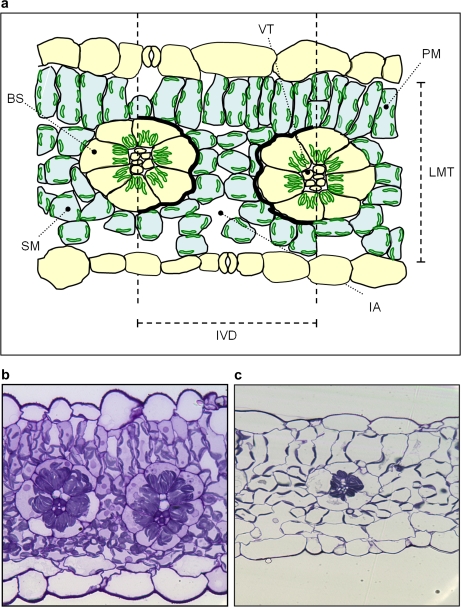
Leaf sections of 0.5 μm thickness were removed from embedded slices using glass knives on an ultramicrotome, stained with toluidine blue, and heat-fixed to glass slides. Slides were viewed using a Zeiss Axioskop light microscope at ×400 magnification. From each slide, three images were produced for analysis, each containing a leaf cross-section in the same orientation, showing at least two vascular bundles. Regions of interest (ROIs) from each cross-section image were selected manually (Fig. 5) using Image J quantification software (NIH, USA).
Fig. 5.
(a) Diagram representing regions of interest (ROIs) in cross-sections of Flaveria bidentis leaves. ROIs include bundle sheath cells (BS), palisade mesophyll cells (PM), spongy mesophyll cells (SM), and total vascular tissue (VT) which were manually outlined and measured to give area and perimeter data. Interveinal distances (IVD) were measured between two adjacent vascular bundles. Leaf mesophyll thickness (LMT) was measured between epidermal layers at four points in each cross-section. Intercellular airspaces (IA) within the interveinal distance were individually outlined and combined to give a total length of mesophyll cells exposed to intercellular airspace (Sm). Black stripes represent the perimeter of the bundle sheath tissue within the interveinal zone (Sb). Representative light microscope images (×400 magnification) of leaf sections of plants grown at 150 μmol quanta m−2 s−1 (b) and 500 μmol quanta m−2 s−1 (c). (This figure is available in colour at JXB online.)
Calculations of mesophyll surface area exposed to intercellular airspace (Sm) and bundle sheath surface area per unit leaf area (Sb) are given in Equations 3 and 4, respectively. The curvature correction factor (CCF) of 1.43 was taken from Evans et al. (1994).
| (3) |
| (4) |
Leaf vein density analysis
Vein density was measured on leaf sections taken from three plants grown at each irradiance. Leaves were initially cleared by immersion in a 95% ethanol, 5% NaOH solution for 4 d and re-hydrated in water for 1 h. Sections were cut from upper, mid, and lower sections of the leaf avoiding major veins, and digital images were taken at ×50 magnification. Vein density was determined from each image by measuring the total length of veins within a 2 cm2 quadrant using ImageJ quantification software.
Carbon isotope measurements
Plants were transferred from growth cabinets to the laboratory (room temperature 25 °C) and gas exchange measurements were carried out on one fully expanded leaf in the 6 cm2 leaf chamber of the LI-6400 using a red–blue LED light source (LI-COR). Measured leaves were initially left for 30 min at 25 °C, with an ambient CO2 of 400 μmol mol−1, an irradiance of 1500 μmol quanta m−2 s−1, a flow rate of 200 μmol s−1, and 21% O2. For subsequent measurements, ambient CO2 and O2 were changed to 380 μmol mol−1 and 2%, respectively. Gas exchange calculations were adjusted on the LI-6400 program to account for 2% O2 in the air according to Bunce (2002). Irradiance was adjusted stepwise from 80, to 125, 200, 500, 1500, 1800, 1000, 250, 150, 100, 80, and 0 μmol quanta m−2 s−1 at 30 min intervals.
The LI-6400 systems were coupled to a tuneable diode laser (TDL; model TGA100, Campbell Scientific, Inc., Logan, UT, USA) for online carbon isotope measurements as described by Bowling et al. (2003) and Griffis et al. (2004). Input gases (N2 and O2) were mixed using mass flow controllers (OMEGA Engineering). Part of the N2 and O2 mixture and gas from a compressed air tank were used to correct for gain drift throughout the day. A second calibration gas was created by mixing 10% CO2 (δ13C= –24.5‰) and part of the N2/O2 gas stream in a capillary gas mixing system to generate ∼1000 μmol mol−1 CO2. This was then used to generate six different CO2 concentrations of the same isotopic composition for 13CO2 calibration, allowing measurements to be made at different O2 concentrations. CO2 cylinders used in the LI-6400 had a carbon isotope composition of 13.1‰ with respect to PDB. The measurement sequence of the TDL consisted of 20 s measurements of zero and six calibration CO2 concentrations, the compressed air followed by the reference and sample gases of the two LI 6400 machines. The reference gas sampled the inlet gas to the leaf chamber and the sample gases were collected from the tube used to match the infrared gas analysers (IRGAs) in the LI-6400. These gases were dried by passing through a dryer assembly in the gas line, and δ13C of the gases was measured by the TDL.
Calculation of CO2 leakiness
Carbon isotope discrimination (Δ) was calculated using Equation 5 (Evans et al., 1986):
| (5) |
where δe and δo are the carbon isotope compositions of dry air entering and leaving the leaf chamber, respectively, measured by the TDL. ξ=Ce/(Ce–Co), and Ce and Co are the CO2 partial pressures of dry air entering and leaving the chamber, respectively, measured by the TDL. CO2 leakiness (ϕ) was estimated using the model of C4 carbon isotope discrimination proposed by Farquhar (1983):
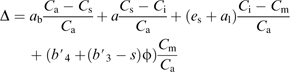 |
(6) |
where Ca, Cs, Ci, and Cm are the CO2 partial pressures in the air, at the leaf surface, in the intercellular airspace, and in the mesophyll cytoplasm. The symbol ab is the fractionation during diffusion through the boundary layer (2.9‰), a is the fractionation associated with diffusion of CO2 in air (4.4‰), es is the fractionation during dissolution of CO2 (1.1‰), al is the fractionation during aqueous diffusion (0.7‰), and s is the fractionation during CO2 leakage from the bundle sheath cells (1.8‰). The combined fractionation of Rubisco, respiration, and photorespiration (b3′) is given by
| (7) |
where Vc and Vo are the rates of Rubisco carboxylation and oxygenation, Mm and Ms are the rates of respiration occurring in the mesophyll and bundle sheath cells, respectively, b3 is the fractionation by Rubisco (30‰), and f is the fractionation associated with photorespiration [11.6‰ (Lanigan et al., 2008)]. The fractionation factor e (–5.1‰) associated with respiration was calculated from the difference between δ13C in the CO2 cylinder (–13.1‰) used during experiments and that in the atmosphere under growth conditions (–8‰) (Tazoe et al., 2009).
The combined fractionation of PEP carboxylation, respiration, and fractionation during dissolution of CO2 and conversion to HCO3– is given by
| (8) |
where Vp and Vh are the rates of PEP carboxylation and CO2 hydration; b4=bp+eb+es (–5.7 ‰ at 25 °C) is the combined fractionation of PEP carboxylation (bp=2.2‰) and the preceding isotopic equilibrium fractionation (eb= –9 ‰ at 25 °C) and dissolution of CO2 (es); and h is the fractionation of the catalysed CO2 hydration (1.1‰). References to the different fractionation factors can be found in Cousins et al. (2006) and Henderson et al. (1992)
To calculate ϕ, it was assumed that both the boundary layer conductance and the internal conductance to CO2 diffusion were large (Ca=Cs and Ci=Cm) and that there was sufficient carbonic anhydrase activity such that Vp/Vh=0. Since measurements were made at 2% O2 it was also assumed that Vo=0.
To account for the contribution of respiration in Equations (7) and (8), it was assumed that Mm+Ms=Rd, the rate of measured dark respiration, and that Mm=0.5Rd. Using the C4 photosynthesis model (von Caemmerer, 2000) Vc and Vp were approximated by Vc=A+Rd and Vp=(A+0.5Rd)/(1–ϕ), respectively, where A is the CO2 assimilation rate. With these simplifications
| (9) |
and
| (10) |
Using Equations 7–10, Equation 6 can be modified to form:
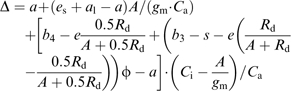 |
(11) |
where gm is the mesophyll conductance to CO2 diffusion and A/gm=Ci–Cm.
Rearranging Equation 11 gives an explicit expression of ϕ
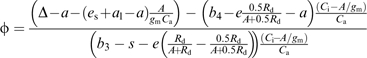 |
(12) |
Note that the inclusion of a finite gm has only a small effect on the estimates of ϕ.
Dry matter N content and δ13C
Oven-dried leaves were ground to a consistent powder and dried again at 80 °C for 30 min before a small sample was weighed for measurements. The percentage nitrogen was calculated by combustion of samples in an elemental analyser (EA1110, Carlo Erba) and the isotopic composition of CO2 determined by mass spectrometry. The δ13C was calculated as [(Rsample–Rstandard)/Rstandard]×1000, where Rsample and Rstandard are the 13C/12C ratio of the sample and the standard V-Pee Dee Belemnite, respectively.
Statistical analysis
The relationship between mean values of photosynthetic, anatomical, and biochemical data obtained throughout this study were tested using the Student's t-test (P <0.05). Significant differences are marked with asterisks.
Results
Measurement of growth over 36 days
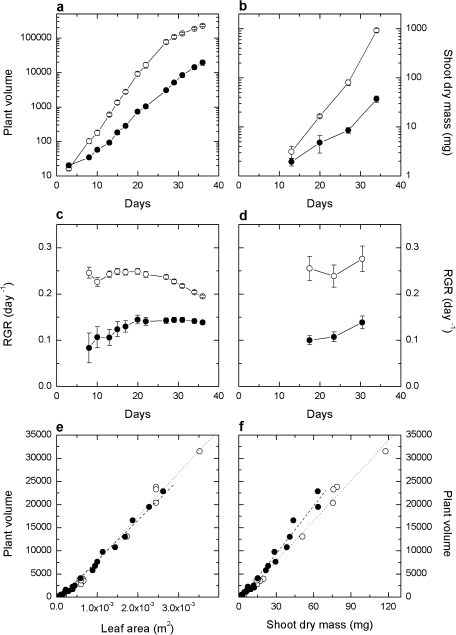
The growth of F. bidentis at LI (150 μmol quanta m−2 s) and MI (500 μmol quanta m−2 s) was compared using a novel image-based analysis over a 36 d period. Images taken from multiple aspects (see Fig. 1) were combined to give a representative plant volume, allowing the change over time to be measured non-destructively on individual plants. The utility of this approach was that it required a smaller subset of replicated experimental material and allowed individual plants to be measured non-destructively over the entire growth period, thus yielding a large data set sensitive to day-to-day changes in growth. The expected difference in growth between plants grown at the two irradiances was clear in terms of both the plant volume over time (Fig. 2a) and the RGR calculated progressively over time (Fig. 2c.). MI plants grew initially at an RGR of ∼0.24 d−1, declining slightly towards the end of the growth period. Conversely, plants grown at LI began with an RGR of ∼0.1 d−1, increasing to ∼0.14 d−1. This difference in RGR resulted in a 10-fold difference in plant volumes after 36 d of growth.
Fig. 1.
Top and side images of the Flaveria bidentis wild type grown at MI taken over a 36 d period with a 3D growth imaging system (Scanalyser, Lemnatech). Image backgrounds have been removed by the software analysis grid, leaving 2D pictures that are subsequently expressed as a plant area.
Fig. 2.
Comparison between growth measurements derived from image analysis or destructive harvest. Biomass over 36 d using (a) imaging volumes and (b) shoot dry mass. Cumulative relative growth rates from (c) imaging and (d) shoot dry mass. Points indicate the average of 5–10 plants. Correlation between computed volumes from imaging and (e) leaf area and (f) shoot dry mass. Plants grown at an irradiance of 150 μmol quanta m−2 s−1 (LI) and 500 μmol quanta m−2 s−1 (MI) are represented by filled and open circles, respectively. In (e) dashed (y=870.77x–803.48) and dotted (y=947.92x–1562.4) lines represent the linear regression for LI and MI irradiance treatments, respectively. In (f) dashed (y=341.91x–850.79) and dotted (y=288.24x–803.47) lines represent the linear regression for LI and MI irradiance treatments, respectively.
To validate the accuracy of this imaging system to measure growth in F. bidentis, volume measurements were compared with shoot dry mass (SDM) and TLA measurements made from a series of four destructive harvests of plants (Fig. 2b, d) over the 36 d period. RGRs calculated from TLA (data not shown) and SDM (Fig. 2d) were comparable with RGRs derived from volumes (Fig. 2c). Comparison between TLA and plant volume (Fig. 2e) indicated a similar linear correlation for both groups of plants. A linear correlation was also evident between plant volume and SDM (Fig. 2f) but, at a given volume, plants grown at LI exhibited a lower SDM than those grown at MI.
SLA, calculated from the destructive harvest of plants grown for 34 d, was significantly higher in plants grown at LI (Table 1). The LDMC and LMA were both higher in plants grown at MI. Furthermore, LI plants allocated 82% of shoot dry matter to leaves compared with 77% in MI plants. Changes in LMA were such that CO2 assimilation rates measured at high light expressed on a leaf dry mass basis were similar between LI- and MI-grown plants (Table 1).
Table 1.
Leaf properties of Flaveria bidentis grown at 150 μmol quanta m−2 s−1 and 500 μmol quanta m−2 s−1
| Light intensity (μmol quanta m−2 s−1) |
||
| 150 | 500 | |
| A, saturating irradiance (μmol CO2 m−2 s−1) | 19.3±0.5 | 31.4±0.4* |
| A, saturating irradiance (μmol CO2 g−1 s−1) | 0.92±0.05 | 0.88±0.04 |
| A, growth irradiance (μmol CO2 m−2 s−1) | 4.6±0.1 | 14.6±0.2* |
| A, growth irradiance (μmol CO2 g−1 s−1) | 0.22±0.1 | 0.41±0.08* |
| SLA leaf area/leaf dry mass (m2 kg−1) | 48.2±1.3 | 28.3±1.0* |
| LMA leaf dry mass/leaf area (g m−2) | 20.9±0.6 | 35.8±1.4* |
| LAR leaf area/shoot dry mass (m2 kg−1) | 39.2±0.9 | 21.7±1.1* |
| LDMC leaf dry/fresh mass (mg g−1) | 91.7±3.0 | 139.0±5.1* |
| LDM/SDM leaf dry mass/shoot dry mass (g g−1) | 0.81±0.01 | 0.77±0.01* |
Measurements represent averages and standard errors of 5–10 replicate observations. Asterisks indicate a significant difference in data for plants grown at the differing light intensities.
SLA, specific leaf area; LMA, leaf dry mass per unit area; LAR, leaf area ratio; LDMC, leaf dry matter content; saturating irradiance, 1500 μmol quanta m−2 s−1.
Photosynthetic CO2 assimilation
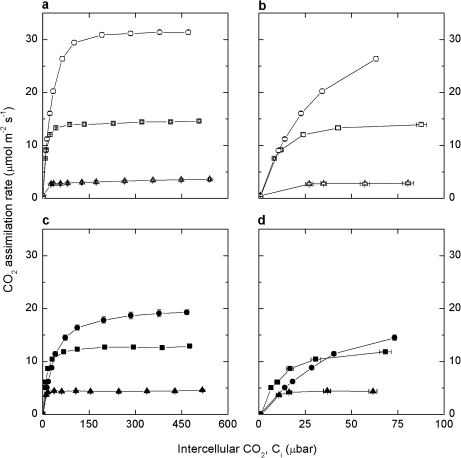
The response of CO2 assimilation rate (A) to increasing intercellular pCO2 (Ci) was measured in plants grown at both irradiances using the LI-6400 gas exchange system. To assess photosynthesis under both growth irradiances and at high irradiance, measurements were conducted at a static irradiance of 150, 500, and 1500 μmol quanta m−2 s−1 at a leaf temperature of 25 °C on all plants. Plants grown at MI had CO2-saturated assimilation rates of 3.6±0.2, 14.6±0.2, and 31.4±0.4 μmol m−2 s−1 at these three irradiances, respectively (Fig. 3a). Plants grown at LI had significantly lower CO2-saturated assimilation rates (Fig. 3c) under 500 μmol quanta m−2 s−1 and 1500 μmol quanta m−2 s−1 (12.6±0.2 μmol m−2 s−1 and 19.3±0.5 μmol m−2 s−1, respectively), but slightly higher rates at 150 μmol quanta m−2 s−1 (4.6±0.1 μmol m−2 s−1) than MI plants. MI plants exhibited a steep initial rise in A at low Ci (Fig. 3b) under both 500 μmol quanta m−2 s−1 and 1500 μmol quanta m−2 s−1 irradiance. This differed from plants grown at LI (Fig. 3d) where CO2 assimilation rate increased more slowly at low Ci when measured at 1500 μmol quanta m−2 s−1 compared with 500 μmol quanta m−2 s−1.
Fig. 3.
CO2 assimilation rate as a function of intercellular pCO2 (Ci) in Flaveria bidentis plants grown at 500 μmol quanta m−2 s−1 (a, b open symbols) and 150 μmol quanta m−2 s−1 (c, d filled symbols). Graphs (b) and (d) expand the scale at low Ci to highlight the difference between growth irradiance treatments. Gas exchange measurements were made at three irradiances, 150 (triangle), 500 (square), and 1500 (circle) μmol quanta m−2 s−1, and a leaf temperature of 25 °C.
Leaf characteristics: enzyme activity, chlorophyll, N, and dry matter δ13C
Rubisco and PEPC activities were measured from leaf disc extracts from plants grown at both irradiances (Table 2). LI plants had 61% lower PEPC activity and 52% lower Rubisco activity, resulting in a lower PEPC/Rubisco ratio. Chlorophyll content per leaf area and chlorophyll a/b ratio were less in plants grown at LI largely due to a significant difference in chlorophyll a. The δ13C of dried leaf samples was found to be lower in plants grown at LI (–19.8±0.2‰ compared with –16.9±0.1‰). N content per unit leaf area was 18% higher in MI plants (Table 2).
Table 2.
Leaf biochemical properties of Flaveria bidentis grown at 150 μmol quanta m−2 s−1 and 500 μmol quanta m−2 s−1
| Growth irradiance (μmol quanta m−2 s−1) |
||
| 150 | 500 | |
| PEPC (μmol CO2 m−2 s−1) | 49.8±4.5 | 127.0±17.8* |
| Rubisco (μmol CO2 m−2 s−1) | 23.9±1.7 | 50.2±3.3* |
| PEPC/Rubisco | 2.1±0.1 | 2.5±0.2* |
| Chlorophyll a+b (mmol m−2) | 0.49±0.1 | 0.63±0.1* |
| Chlorophyll a/b | 4.7±0.1 | 5.2±0.1* |
| Dry matter δ13C (‰) | –19.8±0.2 | –16.9±0.1* |
| Leaf nitrogen (mmol m−2) | 77.6±2.0 | 95.4±5.6* |
Measurements represent averages and standard errors of 5–10 replicate observations. Asterisks indicate a significant difference between plants grown at the two irradiances (P <0.05). Dry matter δ13C and leaf nitrogen were calculated from destructive harvest measurements of 34-d-old plants.
Real-time carbon isotope and CO2 assimilation rate measurements
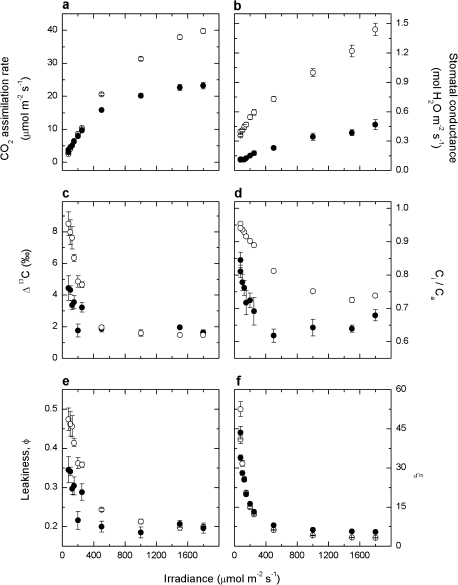
Carbon isotope discrimination (Δ13C) and CO2 assimilation rate (A) were measured in response to real-time changes in irradiance using a system coupling the LI-6400 to a TDL. Measurements were conducted at an ambient CO2 (Ca) of ∼360 μbar with irradiance increasing in steps from 80 μmol quanta m−2 s−1 to 1800 μmol quanta m−2 s−1 then decreasing back down. Plants grown at LI had lower CO2 assimilation rates at higher irradiances compared with plants grown at MI (23.3±1.0 μmol m−2 s−1 versus 39.9±0.7 μmol m−2 s−1 at 1800 μmol quanta m−2 s−1, Fig. 4a). Stomatal conductance (gs) increased from low to high irradiance (Fig. 4b) in both sets of plants and was significantly higher in MI plants at any given irradiance. Ci/Ca decreased as irradiance increased, again being greater in MI plants (Fig. 4d). Δ13C was similar for both groups of plants at >400 μmol quanta m−2 s−1 (∼2‰), yet a greater discrimination was observed in MI plants when measured below 400 μmol quanta m−2 s−1 (Fig. 4c). Similarly, leakiness (ϕ), calculated using Equation 12, was considerably different between the two groups of plants at low irradiance (Fig. 4e). A measure of uncertainty in Δ13C (and ϕ) calculations, ξ (Equation 5), remained below 10 at irradiances >400 μmol quanta m−2 s−1, yet increased at lower irradiances (Fig. 4f).
Fig. 4.
CO2 assimilation rate, A (a), stomatal conductance, gs (b), carbon isotope discrimination, Δ (c), the ratio of intercellular to ambient pCO2, Ci/Ca (d), bundle sheath leakiness, ϕ (e), and ξ (f), as a function of irradiance, in Flaveria bidentis grown at 150 μmol quanta m−2 s−1 (filled circles) and 500 μmol quanta m−2 s−1 (open circles) irradiance. Measurements were made at an ambient pCO2 of ∼360 μbar, and a leaf temperature of 25 °C.
Leaf characteristics: anatomy and vein density
The leaf anatomy and vein density of F. bidentis plants grown at LI and MI were different in several respects (Table 3). Leaf mesophyll thickness (LMT), as defined in Fig. 5a, was 21% greater in MI than LI plants. As no differences were observed between the average spongy mesophyll or palisade mesophyll cell areas in the two groups of plants, this difference in leaf thickness is partially due to extra mesophyll cell layers rather than an increase in cell size. No significant difference was observed in the width between vascular bundles, defined as the interveinal distance (IVD) in Fig. 5a, although both the bundle sheath cell area and vascular bundle area were higher in plants grown at MI, resulting in larger vascular bodies, as evidenced in Fig. 5b and c. Mesophyll surface area exposed to intercellular airspace (Sm), as defined in Fig. 5a and Equation 3, was substantially higher in MI plants (Table 3). This increase coupled with a small rise in the bundle sheath surface area per unit leaf area (Sb) (due to the increase in size of the vascular tissues and bundle sheath cells) in MI plants resulted in an increased Sm/Sb. The density of minor tertiary veins was measured in random transects of cleared leaves grown at both irradiances. Although no difference had been found in the IVD measured from leaf cross-sections, vein density was significantly higher in MI plants.
Table 3.
Leaf anatomical parameters of Flaveria bidentis grown at 150 μmol quanta m−2 s−1 and 500 μmol quanta m−2 s−1
| Growth irradiance (μmol quanta m−2 s−1) |
||
| 150 | 500 | |
| Leaf mesophyll thickness (μm) | 118.7±1.7 | 150.6±2.1* |
| Interveinal distance (μm) | 139.6±11.2 | 121.1±7.0 |
| Bundle sheath cell area (μm2) | 402.5±24.5 | 532.2±14.9* |
| Palisade mesophyll cell area (μm2) | 446.8±31.2 | 435.7±18.3 |
| Spongy mesophyll cell area (μm2) | 224.9±9.8 | 203.9±10.3 |
| Vascular bundle area (μm2) | 175.9±17.2 | 402.5±31.0* |
| Sm (m2 m−2) | 10.7±0.7 | 18.0±1.7* |
| Sb (m2 m−2) | 1.7±0.1 | 2.1±0.1* |
| Sm/Sb | 6.7±0.5 | 8.8±1.1 |
| Minor vein density (mm mm−2) | 9.2±0.3 | 11.2±0.4* |
Terminology is defined in Fig. 5a. Parameters represent means and standard errors of 5–20 replicate observations. Asterisks indicate a significant difference between plants grown at the two irradiances (P <0.05).
Discussion
The pros and cons of image-based growth analysis
Growth irradiance was used in this study as an easily manipulated stimulus to illustrate the accuracy and effectiveness of an image-based growth analysis in comparison with classical destructive growth measurements. Growth irradiance is known to have considerable effects on growth in C4 plants, including anatomical, biochemical, and physiological changes to the photosynthetic machinery and operation of the leaves (Ward and Woolhouse, 1986a, b; Araus et al., 1991; Tazoe et al., 2006). The experimental set-up included 15 plants grown at two irradiances over 34 d, during which imaging was conducted 13 times (Fig. 2a). In contrast, for an equivalent amount of data to be obtained from destructive harvesting, an initial population of 195 plants (at each irradiance) would have been required. In addition to logistical simplification, the imaging approach conferred the singular benefit of being able to match growth data with physiological and anatomical information taken from the same group of plants. Consistent with expectations, differing irradiances during growth produced plants that grew at substantially different rates, resulting in a large difference in final biomass after 36 d (Fig. 2). These growth differences were clearly observed and comparable in measurements made from either imaging or destructive harvest data. RGR is typically calculated from the increase in a plant component mass (leaf, stem, and root) or leaf area over a given time period. Classically, obtaining these biomass data required a destructive harvest in which plants were removed from the soil for measurement, almost unavoidably resulting in plant death (Atkin et al., 1998; Poorter et al., 2005). Less often employed are non-destructive measurement techniques that involve consecutive estimates of plant size through time. This is done through either imaging or painstaking measurement to avoid damaging and thus affecting the growth of plants (Brewster and Barnes, 1981; Weiner et al., 1990). Here, RGR was calculated through imaging, combining three separate images to correct for occlusion of leaves in any one view, giving a representative plant volume. Imaging has previously been used to measure growth in Arabidopsis thaliana and Nicotiana tabacum, using a single camera mounted above the plants. Leister et al. (1999) showed that in A. thaliana Col-0, measurement of leaf area using a single top-down image can be significantly affected by overlapping of leaves. Although overlap did not change leaf area estimates in younger plants of this study, in older plants the overlap hid up to 20% of the actual plant area as measured by destructive harvest. Subsequent image-based analyses have avoided this issue by only measuring seedlings early on in the growth period, typically 2–3 weeks after germination (El-Lithy et al., 2004; Walter et al., 2007). As F. bidentis seedlings have significant leaf overshadowing, a method was developed that allowed the accurate estimation of leaf area for the first 5 weeks after germination by combining data from a top-view and several side-view images. The plant volumes obtained correlated well with SDM and TLA. It is clear, however, that plant volumes calculated in this manner are not a direct substitute for biomass in situations that change leaf thickness. Linear regression of volume data and destructive data indicates that calculated volumes are still very much an approximated total plant area and do not take into account leaf thickness and thus, if it is reduced, will overestimate plant biomass (Fig. 2f). The estimation of RGR by imaging volumes is not influenced by this overestimation, as it is a relative figure. The technique should therefore be useful for growth analysis of segregating populations of transgenic plants in which replicate samples are not available to facilitate the linking of photosynthetic and growth phenotypes.
Growth response of F. bidentis to low irradiance
It has been suggested that C4 species may be less phenotypically plastic in their growth and photosynthetic response to low light because of the unique anatomical requirements of the C4 photosynthetic pathway (Sage and McKown, 2006). However F. bidentis showed a growth acclimation very similar to that observed in C3 species (Poorter and van der Werf, 1998). Destructive harvest measurements showed that plants grown at LI were significantly altered in how biomass was invested in leaves as evidenced by an increased LAR relative to plants grown at MI (Table 1) together with a reduced leaf thickness (Table 3). Leaves of F. bidentis plants grown at LI were 21% thinner than those grown at MI and had 42% less mass per unit area. In addition, the size of the vascular bundles decreased, as did the average size of the bundle sheath cell surrounding them (Table 3). A decrease in leaf thickness in response to low light intensity has been observed previously in studies on both C3 and C4 plants (Louwerse and Zweerde, 1977; Bjorkman, 1981; Ward and Woolhouse, 1986b; Araus et al., 1991; Sage and McKown, 2006). This trait has been argued to reduce the construction cost per unit leaf area of any new leaves to match the reduced energy input in a low light environment. As a consequence of the change in SLA, CO2 assimilation rates at high light were similar in LI and MI plants when expressed per leaf or plant dry mass, which has been commonly observed in C3 species. At growth irradiance, photosynthetic rates of LI plants were 31% of rates of MI plants on an area basis, but 54% on a mass basis, which was similar to the difference in observed relative growth rates (50–60%, Fig. 2c, d).
Leaf anatomical measurements suggest reduced mesophyll conductance to CO2 diffusion in F. bidentis grown at low irradiance
For photosynthesis to occur, CO2 has to diffuse from the atmosphere through stomata and intercellular air space into mesophyll cells. Mesophyll conductance (gm) to CO2 diffusion has been defined as the conductance to CO2 diffusion from the intercellular airspace to the chloroplast stroma in C3 species and to the mesophyll cytosol in C4 species (Evans and von Caemmerer, 1996). In C3 species, gm correlates with the chloroplast surface area appressed to the intercellular air space, and both gm and chloroplast surface area exposed to the intercellular airspace have been shown to correlate with photosynthetic capacity (von Caemmerer and Evans, 1991; Evans et al., 2009). C3 plants grown at low light have lower photosynthetic capacities and lower gm than high light-grown plants (Hanba et al., 2002; Warren et al., 2007; Yamori et al., 2010). Very little is known about the magnitude of gm and its variation with photosynthetic capacity in C4 species since neither carbon isotope discrimination nor fluorescence techniques commonly used to estimate gm in C3 species can be used in C4 species. Because the initial CO2 fixation occurs in the mesophyll cytosol during C4 photosynthesis, CO2 needs only to diffuse across the cell wall, the plasma membrane, and the cytoplasm, and Evans and von Caemmerer (1996) suggested that mesophyll surface area appressed to the intercellular airspace (Sm) would be the relevant parameter to correlate with gm for C4 photosynthesis. The present anatomical measurements show that Sm is reduced to a degree similar to photosynthetic capacity (assessed from the CO2 assimilation rate as measured at high light; Table 3). This suggests that gm should vary with growth irradiance in C4 species. Although there is uncertainty in the estimates of the CO2 permeability for both the cell wall and plasma membrane (Evans et al., 2009), assuming the values of cell wall thickness and permeabilities used by Evans et al. (1994) gm was estimated to be 0.92 mol m−2 s−1 bar−1 and 0.55 mol m−2 s−1 bar−1 in MI and LI plants, respectively.
Using leaf anatomy to estimate bundle sheath conductance to CO2 diffusion
The efficiency of the C4 pathway is dependent upon both the bundle sheath conductance to CO2 diffusion and the relative biochemical capacities of the C3 and C4 cycles. Unfortunately important parameters of the C4 concentrating mechanism, such as bundle sheath conductance, bundle sheath pCO2, and leakiness of the bundle sheath, cannot be measured directly, and estimates of bundle sheath conductance vary between 0.01 mol m−2 s−1 and 0.001 mol m−2 s−1 (for a review, see von Caemmerer and Furbank, 2003). Bundle sheath conductance expressed on a leaf area basis is dependent on the conductance across the mesophyll–bundle sheath interface and the bundle sheath surface area per unit leaf area (Sb). Estimates of Sb range between 0.6 m2 m−2 and 3.1 m2 m−2, and the present estimates fall within that range (Apel and Peisker, 1978; Brown and Byrd, 1993).
It is interesting to ask how bundle sheath conductance should vary with changes in photosynthetic capacity observed here under contrasting light environments. Mathematical models of C4 photosynthesis demonstrated that a low bundle sheath conductance is an essential feature of the C4 photosynthetic pathway (Berry and Farquhar, 1978; von Caemmerer and Furbank, 1999), and von Caemmerer et al. (1997) pointed out that bundle sheath conductance would need to co-vary with biochemical capacity to maintain similar bundle sheath leakiness unless the relative capacities of the C4 and C3 cycles also vary. Only a 20% reduction in Sb was observed in LI compared with MI plants, which is less than the 40% reduction observed in CO2 assimilation rate measured at high irradiance or the reductions observed in Rubisco and PEPC activities (Fig. 3, Tables 1–3).
No differences were observed between LI and MI plants in IVD, a critical parameter in C4 plants due to the spatial separation of CO2 fixation processes between mesophyll and bundle sheath cells (Dengler and Nelson, 1999; Ogle, 2003; McKown and Dengler, 2007). However, the smaller bundle sheath cross-sectional area in LI leaves increased separation between the surfaces of the bundle sheath cells and resulted in mesophyll cells that were elongated in the horizontal plane (Fig. 5c). Vein density, which is negatively correlated with IVD amongst Flaveria C3 and C4 species (McKown and Dengler, 2007), was significantly lower in LI leaves and may more accurately reflect the distance between vascular tissue as it was measured on a larger sample subset. A significant decrease in vein density and increase in IVD was observed in response to lower growth irradiance in the C4 Flaveria australasica (Sage and McKown, 2006). Sb was estimated in two ways: from leaf cross-sections and tracings of bundle sheath perimeters and by combining vein density measurements with estimates of bundle sheath area. The estimates of Sb by the two methods were similar, and reduced vein density as well as reduced bundle sheath area should both contribute to reducing bundle sheath conductance in LI plants.
Anatomical and biochemical acclimation responses to light result in lower bundle sheath leakiness at low irradiance in LI- compared with MI-grown plants
The efficiency of the CO2-concentrating mechanism is intimately linked to bundle sheath leakiness (ϕ) defined as that fraction of CO2 generated by C4 acid decarboxylation in the bundle sheath that subsequently leaks out (Farquhar, 1983). Since the C4 cycle consumes energy in the form of ATP during the regeneration of PEP, leakage of CO2 out of the bundle sheath is an energy cost to the leaf particularly pertinent at low light. Both dry matter δ13C and real-time measurements of Δ13C were used during gas exchange to assess leakiness in LI and MI plants. As previously reported, lower dry matter δ13C values were also observed in plants grown at LI, indicative of increased leakiness under low light conditions (Table 1) (Henderson et al., 1992; Cousins et al., 2006; Tazoe et al., 2006). Real-time measurements also showed an increase in leakiness for both LI- and MI-grown plants. Henderson et al. (1992) observed comparable results in Sorghum bicolor and Z. mays, and hypothesized that this increase in leakiness might be due to a change in the coordination of the C3 and C4 cycles. Leakiness was similar in LI- and MI-grown plants at irradiances >400 μmol quanta m−2 s−1 but, at irradiances below this, plants grown at LI maintained a lower leakiness than those grown at MI (Fig. 4). A similar result was reported in the C4 plant Amaranthus cruentus (Tazoe et al., 2008). It is thus noteworthy that reduced vein density did not reduce the efficiency of the C4 photosynthetic pathway in LI plants. It is possible that the lower bundle sheath conductance in LI-grown plants predicted from the change in leaf anatomy helped reduce leakiness at low light, but changes in the coordination of the C3 and C4 cycle may also be at play. Perhaps at high light the combination of a lower bundle sheath conductance and a lower ratio of PEPC to Rubisco allowed LI plants to maintain a similar leakiness to MI-grown plants, since a lower PEPC to Rubisco ratio lowers bundle sheath CO2 while a reduced bundle sheath conductance increases bundle sheath CO2.
The physiological response of F. bidentis leaves to growth under low light was very similar to responses observed in C3 species (Evans, 1988). For example, there was the commonly observed shift to lower chlorophyll a/b ratios (Table 3) and an increase in SLA (Table 1). When measured at a high irradiance of 1500 μmol quanta m−2 s−1 the rate of CO2 assimilation in response to increasing Ci was substantially lower in plants that had been grown at LI compared with MI. This reflected reduced activities of both carboxylating enzymes PEPC and Rubisco measured in LI plants. Interestingly, the initial slope of the CO2 response curve of the CO2 assimilation rate was steeper when measured at an irradiance of 500 μmol quanta m−2 s−1 compared with 1500 μmol quanta m−2 s−1 in LI plants (Fig. 3d), but the initial slopes were identical in MI plants (Fig. 3b). The initial slope of the curve has been shown in C4 plants to be limited by PEPC activity (Peisker et al., 1988; Pfeffer and Peisker, 1998), which itself is regulated in part by a reversible light-dependent phosphorylation event (Chollet et al., 1996). This result was unexpected and may again indicate a level of biochemical regulation of PEPC activity achieved by plants grown at LI which contributes to the lower leakiness observed at low light in comparison with MI plants. Further evidence for acclimation is seen under 150 μmol quanta m−2 s−1 where plants grown at LI reached a significantly higher CO2 assimilation rate than those grown at MI (Fig. 3a, c).
Conclusion
The growth and photosynthetic response of F. bidentis to growth at low light was investigated. Testing a novel three-dimensional imaging system, it was possible to monitor RGRs of individual plants and combine this with leaf physiological analysis. It is concluded that shifts in both leaf anatomy and biochemistry underpin acclimation of growth and photosynthesis to low light in F. bidentis, a C4 dicot. Changes in leaf anatomy predict reduced mesophyll and bundle sheath conductance to CO2 diffusion in LI plants. Concurrent measurements of gas exchange and carbon isotope analysis show that low light-grown plants are more efficient at low light and achieve lower leakiness under these conditions, suggesting that differences in the coordination of the C3 and C4 cycle may be involved.
Acknowledgments
This work was completed with funding from an ARC discovery grant (DP0878395) in both the Research School of Biology at the Australian National University and the Australian Plant Phenomics Facility High Resolution Plant Phenomics Centre, Canberra.
References
- Apel P, Peisker M. Einfluss hoher Sauerstoffkonzentrationen auf den CO2-Kompensationspunkt von C4-pflanzen. Kulturpflanze. 1978;26:99–103. [Google Scholar]
- Araus JL, Brown HR, Byrd GT, Serret MD. Comparative effects of growth irradiance on photosynthesis and leaf anatomy of Flaveris brownii (C4 like), Flaveria linearis (C3–C4), and their F1 hybrid. Planta. 1991;183:497–504. doi: 10.1007/BF00194270. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Schortemeyer M, McFarlane N, Evans JR. Variation in the components of relative growth rate in ten Acacia species from contrasting environments. Plant, Cell and Environment. 1998;21:1007–1017. [Google Scholar]
- Berry JA, Farquhar GD. The CO2 concentrating function of C4 photosynthesis: a biochemical model. In: Hall D, Coombs J, Goodwin T, editors. The Proceedings of the Fourth International Congress on Photosynthesis. London: Biochemical Society of London; 1978. pp. 119–131. [Google Scholar]
- Bjorkman O. Responses to different quantum flux densities. Berlin: Springer-Verlag; 1981. [Google Scholar]
- Bowling DA, Sargent SD, Tanner BD, Ehleringer JR. Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem–atmosphere CO2 exchange. Agricultural and Forest Meteorology. 2003;118:1–19. [Google Scholar]
- Brewster JL, Barnes A. A comparison of relative growth rates of different individual plants and different cultivars of onion of diverse geographic origin at two temperatures and two light intensities. Journal of Applied Ecology. 1981;18:589–604. [Google Scholar]
- Brown RH, Byrd GT. Estimation of bundle sheath cell conductance in C4 species and O2 insensitivity of photosynthesis. Plant Physiology. 1993;103:1183–1188. doi: 10.1104/pp.103.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JA. Sensitivity of infrared water vapor analyzers to oxygen concentration and errors in stomatal conductance. Photosynthesis Research. 2002;71:273–276. doi: 10.1023/A:1015529909457. [DOI] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O'Leary MH. PHOSPHOENOLPYRUVATE CARBOXYLASE: a ubiquitous, highly regulated enzyme in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis. Insights from antisense RNA in Flaveria bidentis. Plant Physiology. 2006;141:232–242. doi: 10.1104/pp.106.077776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Baroli I, Badger MR, Ivakov A, Lea PJ, Leegood RC, von Caemmerer S. The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiology. 2007;145:1006–1017. doi: 10.1104/pp.107.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler NG, Nelson T. Leaf structure and development in C4 plants. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego: Academic Press; 1999. pp. 133–172. [Google Scholar]
- El-Lithy ME, Clerkx EJ, Ruys GJ, Koornneef M, Vreugdenhil D. Quantitative trait locus analysis of growth-related traits in a new Arabidopsis recombinant inbred population. Plant Physiology. 2004;135:444–458. doi: 10.1104/pp.103.036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar G. Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher plants. Australian Journal of Plant Physiology. 1986;13:281–292. [Google Scholar]
- Evans JR. Acclimation by the thylakoid membranes to growth irradiance and the partitioning of nitrogen between soluble and thylakoid proteins. Australian Journal of Plant Physiology. 1988;15:93–106. [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany. 2009;60:2235–2248. doi: 10.1093/jxb/erp117. [DOI] [PubMed] [Google Scholar]
- Evans JR, Poorter H. Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximising carbon gain. Plant, Cell and Environment. 2001;24:755–767. [Google Scholar]
- Evans JR, von Caemmerer S. Carbon dioxide diffusion inside leaves. Plant Physiology. 1996;110:339–346. doi: 10.1104/pp.110.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Von Caemmerer S, Setchell BA, Hudson GS. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Australian Journal of Plant Physiology. 1994;21:475–495. [Google Scholar]
- Farquhar G. On the nature of carbon isotope discrimination in C4species. Australian Journal of Plant Physiology. 1983;10:205–226. [Google Scholar]
- Griffis TJ, Baker JM, Sargent SD, Tanner BD, Zhang J. Measuring field-scale isotopic CO2 fluxes with tunable diode laser absorption spectroscopy and micrometeorological techniques. Agricultural and Forest Meteorology. 2004;123:15–29. [Google Scholar]
- Hanba YT, Kogami H, Terashima I. The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant, Cell and Environment. 2002;25:1021–1030. [Google Scholar]
- Henderson SA, Von Caemmerer S, Farquhar GD. Short-term measurements of carbon isotope discrimination in several C4 species. Australian Journal of Plant Physiology. 1992;19:263–285. [Google Scholar]
- Hikosaka K, Terashima I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell and Environment. 1995;18:605–618. [Google Scholar]
- Lanigan GJ, Betson N, Griffiths H, Seibt U. Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiology. 2008;148:2013–2020. doi: 10.1104/pp.108.130153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister D, Varotto C, Pesaresi P, Niwergall A, Salamini F. Large-scale evaluation of plant growth in Arabidopsis thalina by non-invasive image analysis. Plant Physiology and Biochemistry. 1999;37:671–678. [Google Scholar]
- Louwerse W, Zweerde WVD. Photosynthesis, transpiration and leaf morphology of Phaseolus vulgaris and Zea mays grown at different irradiances in artificial and sunlight. Photosymthetica. 1977;11:11–21. [Google Scholar]
- McKown AD, Dengler NG. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae) American Journal of Botany. 2007;94:382–399. doi: 10.3732/ajb.94.3.382. [DOI] [PubMed] [Google Scholar]
- Ogle K. Implications of interveinal distance for quantum yield in C4 grasses: a modelling and meta-analysis. Oecologia. 2003;136:532–542. doi: 10.1007/s00442-003-1308-2. [DOI] [PubMed] [Google Scholar]
- Peisker M, Apel P, Bauwe H, Pospisilova J, Ticha I. Relationship between intracellular resistance for CO2 uptake and phosphoenolpyruvate carboxylase activity in leaves of Zea mays L. Biochemie und Physiologie der Pflanzen. 1988;183:351–354. [Google Scholar]
- Pfeffer M, Peisker M. CO2 gas exchange and phosphoenolpyruvate carboxylase activity in leaves of Zea mays L. Photosynthesis Research. 1998;58:281–291. [Google Scholar]
- Poorter H, Perez-Soba M. The growth response of plants to elevated CO2 under non-optimal environmental conditions. Oecologia. 2001;129:1–20. doi: 10.1007/s004420100736. [DOI] [PubMed] [Google Scholar]
- Poorter H, van der Werf A. Is inherent variation in RGR determined by LAR at low irradiance and NAR at high irradiance? A review of herbaceous species. In: Lambers H, Poorter H, van Vuuren MMI, editors. Inherent variation in plants growth. Physiological mechanisms and ecological consequences. Leiden: Backhuys Publishers; 1998. pp. 309–336. [Google Scholar]
- Poorter H, van Rijn CP, Vanhala TK, Verhoeven KJ, de Jong YE, Stam P, Lambers H. A genetic analysis of relative growth rate and underlying components in. Hordeum spontaneum. Oecologia. 2005;142:360–377. doi: 10.1007/s00442-004-1705-1. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedmann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta. 1989;975:384–394. [Google Scholar]
- Sage RF, McKown AD. Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? Journal of Experimental Botany. 2006;57:303–317. doi: 10.1093/jxb/erj040. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Hanba YT, Furumoto T, Noguchi K, Terashima I. Relationships between quantum yield for CO2 assimilation, activity of key enzymes and CO2 leakiness in Amaranthus cruentus, a C4 dicot, grown in high or low light. Plant and Cell Physiology. 2008;49:19–29. doi: 10.1093/pcp/pcm160. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Noguchi K, Terashima I. Effects of growth light and nitrogen nutrition on the organization of the photosynthetic apparatus in leaves of a C4 plant. Amaranthus cruentus. Plant, Cell and Environment. 2006;29:691–700. doi: 10.1111/j.1365-3040.2005.01453.x. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Von Caemmerer S, Badger M, Evans JR. Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. Journal of Experimental Botany. 2009;60:2291–2301. doi: 10.1093/jxb/erp035. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. Biochemical models of leaf photosynthesis. Canberra: CSIRO; 2000. Publishing. [Google Scholar]
- von Caemmerer S, Evans JR. Determination of the average partial pressure of CO2 in chloroplast from leaves of several C3 plants. Australian Journal of Plant Physiology. 1991;18:287–305. [Google Scholar]
- von Caemmerer S, Furbank RT. Modeling of C4 photosynthesis. In: Sage RF, Monson R, editors. C4 plant biology. San Diego, CA, USA: Academic Press; 1999. pp. 169–207. [Google Scholar]
- von Caemmerer S, Furbank RT. The C4 pathway: an efficient CO2 pump. Photosynthesis Research. 2003;77:191–207. doi: 10.1023/A:1025830019591. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiology. 1997;113:469–477. doi: 10.1104/pp.113.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Scharr H, Gilmer F, et al. Dynamics of seedling growth acclimation towards altered light conditions can be quantified via GROWSCREEN: a setup and procedure designed for rapid optical phenotyping of different plant species. New Phytologist. 2007;174:447–455. doi: 10.1111/j.1469-8137.2007.02002.x. [DOI] [PubMed] [Google Scholar]
- Ward DA, Woolhouse HW. Comparative effects of light during growth on photosynthetic properties of NADP-ME type C4 grasses from open and shaded habitats. II. Photosynthetic enzyme activities and metabolism. Plant, Cell and Environment. 1986a;9:271–277. [Google Scholar]
- Ward DA, Woolhouse HW. Comparative effects of light during growth on photosynthetic properties of NADP-ME type C4 grasses from open and shaded habitats. I. Gas exchange, leaf anatomy and ultrastructure. Plant, Cell and Environment. 1986b;9:261–270. [Google Scholar]
- Warren CR, Low M, Matyssek R, Tausz M. Internal conductance to CO2 transfer of adult Fagus sylvatica: variation between sun and shade leaves and due to free-air ozone fumigation. Environmental and Experimental Botany. 2007;59:130–138. [Google Scholar]
- Weiner J, Mallory EB, Kennedy C. Growth and variability in crowded and uncrowded populations of dwarf marigolds (Tagetes patula) Annals of Botany. 1990;65:513–524. [Google Scholar]
- Yamori W, Evans JR, Von Caemmerer S. Effects of growth and measurement light intensities on temperature dependence of CO2 assimilation rate in tobacco leaves. Plant, Cell and Environment. 2010;33:332–343. doi: 10.1111/j.1365-3040.2009.02067.x. [DOI] [PubMed] [Google Scholar]