Abstract
We have shown previously that components of the extracellular matrix (ECM) modulate neuronal development. Here, we searched for additional ECM elements that might play roles in retinal histogenesis and identified a secreted glycoprotein that is heavily expressed in the retina. This molecule, named by others Wnt Inhibitory Factor-1 (WIF-1), is expressed during and after the period of rod photoreceptor morphogenesis in the mouse. We show that a potential WIF-1 ligand, Wnt4, as well as a potential Wnt4 receptor, fzd4, and a potential Wnt4 coreceptor, LRP6, are expressed in the region of, and at the time of, rod photoreceptor genesis. WIF-1 and Wnt4 are coexpressed during retinal development and bind to each other; therefore, they are likely to interact during rod production. WIF-1 protein inhibits rod production, and anti-WIF-1 antibodies increase rod production; in contrast, Wnt4 promotes rod production. Together, these data suggest that WIF-1 and Wnt4, both components of the ECM, regulate mammalian photoreceptor development.
Introduction
Retinal progenitors have the capacity to give rise to more than one cell type (Holt et al., 1988; Turner and Cepko, 1987; Turner et al., 1990; Wetts and Fraser, 1988; Wetts et al., 1989). Nevertheless, the production of each of the cell types occurs in progressive, timed, overlapping waves of generation. These waves of generation proceed through the late embryonic and early postnatal period in the mouse. The molecular mechanisms that specify cell fate might be generally programmed and extrinsically triggered by environmental factors present at specific locations at precise times. In fact, both intrinsic and extrinsic mechanisms are involved in retinal cell fate determination (reviewed in Cepko, 1999).
One important aspect of retinal development is the production and maintenance of the primary sensory neuron, the photoreceptor. Photoreceptor development can be broken down into four developmental processes: (1) the progenitor cell's withdrawal from the cell cycle and adoption of the photoreceptor fate; (2) morphogenesis of the inner and outer segments; (3) synaptogenesis with retinal interneurons; and (4) continued maintenance of the photoreceptor phenotype (survival). Some of this development is controlled intrinsically (Belliveau and Cepko, 1999; Cepko, 1999; Morrow et al., 1998). Nevertheless, although genes provide the potential for progenitors to become particular cell types, the proper environment is required for specifying location, process outgrowth, and synapse formation.
The retinal environment includes components of the extracellular matrix (ECM). The retinal ECM is a highly organized meshwork of secreted macromolecules that play key roles in the development of multicellular organisms, influencing, for example, (1) when to start, continue, or stop dividing; (2) when to differentiate; (3) where to migrate; (4) how to polarize; (5) where to form a synapse; and (6) whether to die (reviewed in Libby et al., 2000a). Thus, ECM molecules, and molecules bound to them, play different roles in the development of complex tissues including the retina.
Laminins are key components of ECMs; we have demonstrated that several members of the laminin family are elements of retinal ECMs (Hunter et al., 1992; Libby et al., 1996, 1997, 2000b) and that they help to mediate differentiation in the retina. For example, we have shown that laminins containing the laminin β2 chain are required for the expression of the rod photoreceptor phenotype in an in vitro model system for rod photoreceptor development (Hunter et al., 1992), and the laminin β2 chain is critical for maintenance of the rod photoreceptor phenotype in vivo (Libby et al., 1999). Furthermore, laminins containing the laminin β2 chain can concurrently modulate production of two retinal cell types, the rod photoreceptor and the rod bipolar cell in vitro (Hunter and Brunken, 1997). These data demonstrate that components of the ECM are important in mediating the differentiation of retinal cell type.
Other components of the ECM affect neuronal behavior as well, as do other molecules that can interact with components of the ECM (reviewed in Libby et al., 2000a). Some of these factors are small, partly diffusible molecules. For example, rod photoreceptor development is enhanced by FGF-2 (Hicks and Courtois, 1992), whereas TGF-α inhibits rod production while promoting the production of the Müller cell, simultaneously generated by the retinal progenitor (Lillien, 1995). However, it is important to realize that many, if not all, of these molecules can exist bound to components of the ECM.
We, and others (Hsieh et al., 1999), have isolated an additional component of the ECM that is highly expressed in the retina, Wnt Inhibitory Factor-1 (WIF-1). We originally isolated WIF-1 based on its homology to the laminins and its presence in the retina. This molecule received its name because of its ability to inhibit the activity of a class of extracellular molecules, Wnts (Hsieh et al., 1999). The binding partners for WIF-1, the Wnts (Wingless and MMTV integration site), are likely to be expressed along with WIF-1. These morphogens are secreted into the extracellular space and are tightly bound to the ECM (Bradley and Brown, 1990; Papkoff and Schryver, 1990). Here, we document the expression of one Wnt, Wnt4, in the developing mouse retina, compare its expression to that of WIF-1, and demonstrate that both Wnt4 and WIF-1 are components of the retinal ECM.
We show that the spatial and temporal expression patterns of WIF-1 and Wnt4, as well as a potential Wnt4 receptor, fzd4, and its coreceptor, LRP6, are consistent with roles for these Wnt signaling components in the development of the mouse retina. Finally, we demonstrate that interactions between WIF-1 and Wnt4 can influence the production of photoreceptors in the mouse retina.
Results
WIF-1 identification and distribution
A hallmark of many ECM components is a repeating module known as the EGF-like repeat. We used an in silico screen for new molecules containing EGF-like repeats and isolated two novel matrix molecules. One was a novel netrin, β-netrin (Koch et al., 2000; also known as netrin-4). The second was highly expressed in the retina and lung: all cDNAs for this molecule that were present in an expressed sequence tag database (dbEST) were from retina and lung. Thus, this molecule seemed a good candidate for an important ECM component in the retina and lung. Full-length cDNAs encoding the novel molecule were isolated from mouse and human retinal cDNA libraries; the potential protein encoded by these cDNAs consists of (1) a signal sequence for secretion from the cell, (2) a novel WIF domain, (3) five laminin-like EGF repeats, and (4) a hydrophilic tail (Fig. 1A). These domains suggest that the encoded protein is a secreted component of the ECM. This molecule has been independently identified by others and named by them Wnt Inhibitory Factor-1 (WIF-1; Hsieh et al., 1999).
Fig. 1.
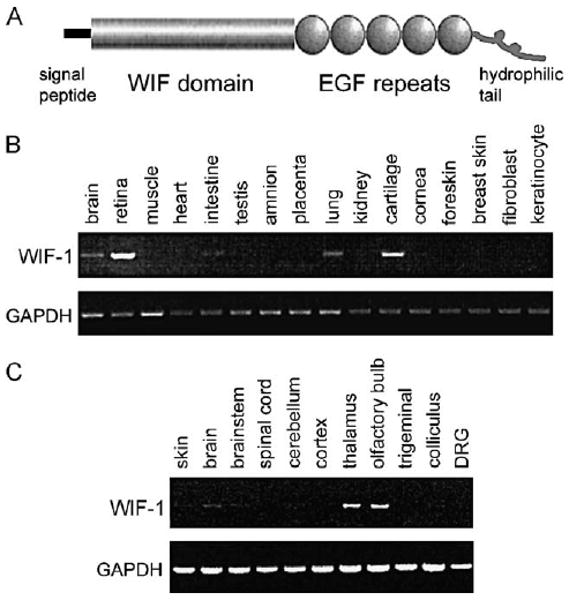
WIF-1: a putative extracellular matrix component that is expressed in multiple tissues and is prominent in the nervous system. (A) Predicted protein structure of WIF-1. WIF-1 modules include a signal peptide for secretion, the WIF domain unique to WIF-1, five EGF-like repeats typical of extracellular matrix molecules, and a hydrophilic C-terminus. (B) RT–PCR demonstrates WIF-1 RNA in many human tissues, including brain. WIF-1 RNA is particularly abundant in retina. (C) RT–PCR demonstrates WIF-1 RNA in several areas of the mouse nervous system including thalamus and olfactory bulb.
WIF-1 RNA is expressed in a wide variety of tissues in both human and mouse: notably, it is present in retina and lung (Fig. 1B), consistent with the prevalence of WIF-1 cDNAs in retinal and lung ESTs. WIF-1 RNA is also present in whole brain (Fig. 1B). The expression of WIF-1 RNA is spread across many different areas of the nervous system, but levels vary regionally and WIF-1 RNA appears particularly abundant in the thalamus and olfactory bulb (Fig. 1C).
Because of its abundance in the retina, we examined the expression and activity of WIF-1 in the retina in greater detail. We first focused on the expression of WIF-1 during the development of the mouse retina, which proceeds through a rapid series of developmental events during the late embryonic and early postnatal period. WIF-1 RNA is present during the peak of retinal differentiation, that is, embryonic day (E)19 to postnatal day (P)5, and remains present in the adult retina (Fig. 2A). The distribution of RNA encoding WIF-1 was also determined during this period by in situ hybridization. Early in retinal differentiation (E16), RNA encoding WIF-1 is present in the ganglion cell layer (GCL) and developing inner nuclear layer; in the mature retina, RNA encoding WIF-1 is spread through the retina but is particularly concentrated in the inner aspect of the inner nuclear layer and in the outer nuclear layer (ONL) (Fig. 2B).
Fig. 2.
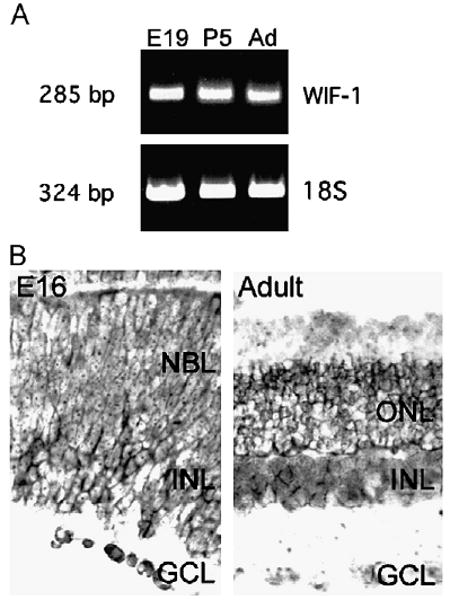
RNA encoding WIF-1 is expressed in the retina during retinal differentiation. (A) RT–PCR demonstrates WIF-1 RNA in extracts of retinae from embryonic day (E) 19, postnatal day (P) 5, and adult (Ad) mice. Equal amounts of RNA were loaded in each lane as shown by RT–PCR for 18S ribosomal RNA. (B) Sections of E16 and adult retinae were hybridized with antisense RNA probes for WIF-1. WIF-1 RNA is present in the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL), with little in the neuroblast layer (NBL).
To determine the distribution and timing of expression of WIF-1 protein, we made an antiserum to synthetic mammalian WIF-1 protein. This antiserum recognizes the 47-kDa WIF-1 in protein transfer blots of extracts of P5 and adult mouse retinae (Fig. 3A). We used this antiserum to identify the sites of distribution of WIF-1 in developing mouse retinae from E18 to P15 and in the adult.
Fig. 3.
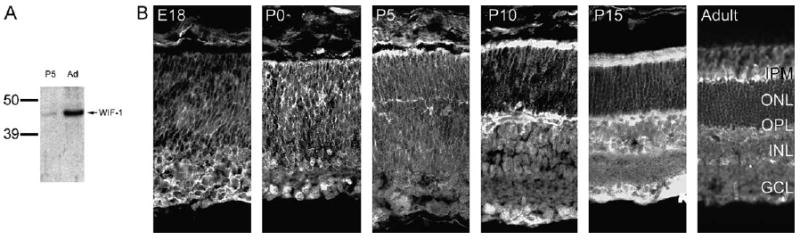
WIF-1 protein is expressed in the retina during retinal differentiation. (A) Protein transfer blots using anti-WIF-1 demonstrate the presence of WIF-1 protein in retinal matrix/membrane extracts of P5 and adult mice. (B) Immunohistochemical analyses using the same anti-WIF-1 serum demonstrate WIF-1 protein in retinal sections from E18, P0, P5, P10, P15, and adult mice. WIF-1 immunoreactivity is spread through the neuroepithelium at birth (P0), but becomes concentrated at the subretinal space by P5, remaining in this location (that becomes the interphotoreceptor matrix; IPM) through adulthood. In the adult, WIF-1 protein is also concentrated in the outer plexiform layer (OPL) but is largely excluded from the outer nuclear layer (ONL), some regions of the inner nuclear layer (INL), and the ganglion cell layer (GCL).
WIF-1 protein expression is localized to the inner retina before birth (Fig. 3B), consistent with the presence of RNA encoding WIF-1 in the inner retina (Fig. 2B). WIF-1 protein remains in the inner retina throughout photoreceptor development, that is, through P15 (Fig. 3B). WIF-1 expression becomes prominent in the outer plexiform layer (OPL) as it begins to form (P5) and becomes stabilized (P15), suggesting that it may play a role in stabilizing the rod photoreceptor synapse, which is forming at this time. Additionally, from P5 to P15, WIF-1 is concentrated in the subretinal space, the region of the developing retina that will become the interphotoreceptor matrix.
In the adult, WIF-1 is spread through the retina and is concentrated in the interphotoreceptor matrix; in particular, in the region surrounding the inner segments (IS) and at the distal tips of the photoreceptors (Fig. 3B). This expression pattern is consistent with the distribution of RNA encoding WIF-1 (Fig. 2B) and is similar to that for other components of the retinal ECM, the laminins, which are produced by Müller glial cells (Libby et al., 1997).
WIF-1 ligands
To begin to elucidate a role for WIF-1 in the developing retina, it is important to determine whether its presumed ligands, the Wnts, are present in the retina. Others have demonstrated the presence of several Wnts in the retina (e.g., Liu et al., 2003); here, we focus on one, Wnt4.
Reverse transcription–polymerase chain reaction (RT–PCR) on retinal RNA demonstrates that several Wnt RNAs are expressed during retinal differentiation (E19 to P5), including those encoding Wnt4 (Fig. 4A), Wnt1, and Wnt6 (not shown). In situ hybridizations demonstrate that RNA encoding Wnt4 is present in the retinal neuroepithelium and is concentrated in the ganglion cell layer early in retinal development (P0) (Fig. 4B). RNA encoding Wnt4 remains present in the adult retina, where it is spread through the retina but is particularly concentrated in the inner nuclear layer (Fig. 4B).
Fig. 4.
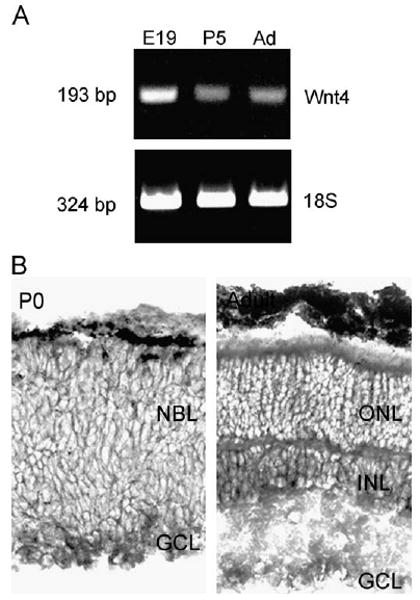
RNA encoding Wnt4 is expressed in the retina during retinal differentiation. (A) RT–PCR demonstrates Wnt4 RNA in extracts of retinae from embryonic day (E) 19, postnatal day (P) 5, and adult (Ad) mice. Equal amounts of RNA were loaded in each lane as shown by RT–PCR for 18S ribosomal RNA (18S data from same experiment as Fig. 2). (B) Sections of P0 and adult retinae were hybridized with antisense RNA probes for Wnt4. Wnt4 RNA is present in the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL), with little in the neuroblast layer (NBL).
We used an anti-Wnt4 serum that recognizes Wnt4 on protein transfer blots of a retinal membrane and matrix fraction to demonstrate the presence of Wnt4 during retinal differentiation (P5 to P12) and in the adult retina (Fig. 5A). Wnt4 protein is not detectable in adult kidney cortex, as previously reported (Surendran et al., 2002). This serum recognizes the 42-kDa Wnt4 on protein transfer blots not only of retina, but also of P0 skin, a tissue known to express Wnt4 (Saitoh et al., 1998); this band is absent in skin from Wnt4 homozygous null animals (Stark et al., 1994; obtained from the Jackson Laboratory) (Fig. 5B). Immunoreactivity for this serum in the P0 retina also disappears in the Wnt4 homozygous null (Fig. 5B).
Fig. 5.
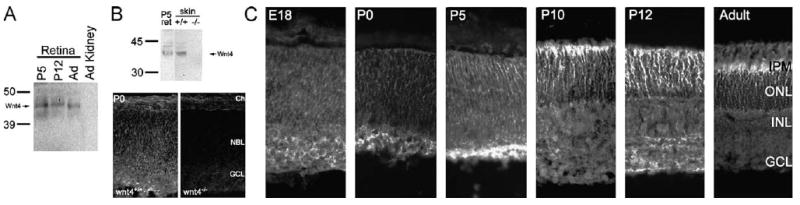
Wnt4 protein is expressed in the retina during retinal differentiation. (A) Protein transfer blots using anti-Wnt4 demonstrate the presence of Wnt4 protein in retinal matrix/membrane extracts of P5, P12, and adult (Ad) mice, and its absence in adult kidney cortex. (B) Wnt4 reactivity, present in P5 retina and P0 skin, is absent in P0 skin from Wnt4 homozygous null (−/−) animals. Wnt4 immunoreactivity, present in the ganglion cell layer (GCL) and the neuroblast layer (NBL) of P0 wild-type (+/+) retina, is absent in P0 retina from Wnt4 homozygous null (−/−) animals. Ch, choroid. (C) Immunohistochemical analyses demonstrate Wnt4 protein in retinal sections from E18, P0, P5, P10, P12, and adult mice. Wnt4 immunoreactivity is present in the inner retina at birth (P0) and becomes concentrated in the developing photoreceptor layer by P5, remaining in this location (the outer nuclear layer; ONL) through peak rod photoreceptor production (P10–12). In the adult, Wnt4 protein remains in the ONL and interphotoreceptor matrix (IPM) but is largely excluded from inner nuclear layer (INL) and ganglion cell layer (GCL).
Wnt4 is present early in the inner retina, near the developing ganglion cell layer (GCL); later, the expression spreads through the retina and becomes prominent in the outer retina as rod photoreceptor differentiation begins and rises, that is, between P5 and P10 (Fig. 5C). Wnt4 remains robust in the outer nuclear layer (ONL) through P12, paralleling peak photoreceptor differentiation. In the adult, Wnt4 remains spread through the ONL, as well as in the interphotoreceptor matrix, near the inner segments and the external limiting membrane (Fig. 5C).
The expression of Wnt4 in the absence of WIF-1 in the outer nuclear layer during the production and stabilization of photoreceptors at P10 suggests that free Wnt4 may act at the outer retina during production and stabilization of photoreceptor cells. In contrast, the coexpression of Wnt4 and WIF-1 in the outer retina in the adult (Figs. 3 and 5) suggests that Wnt4 may be inhibited by WIF-1 in the outer retina in the adult and that free Wnt4 is not required for continued maintenance of the photoreceptor.
Potential Wnt4 receptors and coreceptors
Others have demonstrated the presence of RNA encoding several Wnt receptors in the developing eye, specifically fzd3 (mouse, Wang et al., 1996), fzd4 (chicken, Kubo et al., 2003), and fzd5 (mouse, Wang et al., 1996). Of these, fzd4 has been suggested as a likely candidate for Wnt transduction during retinal development (Kubo et al., 2003). One obstacle for defining the role of these molecules during mouse retinal development has been a paucity of reagents for protein localization. Fortunately, an antiserum raised against fzd4 has been produced, and we used this antiserum to analyze the expression of this Wnt receptor during mouse retinal development.
Fzd4 protein is expressed in the outer retina during rod photoreceptor differentiation (Fig. 6). Fzd4 expression is not readily detectable before rod differentiation (e.g., at P0); rather, expression appears to parallel rod photoreceptor production, peaking at P8 to P10. Fzd4 protein remains in the outer retina through adulthood (Fig. 6). The presence of fzd4 protein in the photoreceptor layer is consistent with previous reports of extensive fzd4 transcription in adult mouse photoreceptors (assayed with β-galactosidase knocked into the fzd4 locus; Wang et al., 2001). Thus, fzd4 is present in a spatial and temporal pattern consistent with a role in production or stabilization of rod photoreceptors.
Fig. 6.
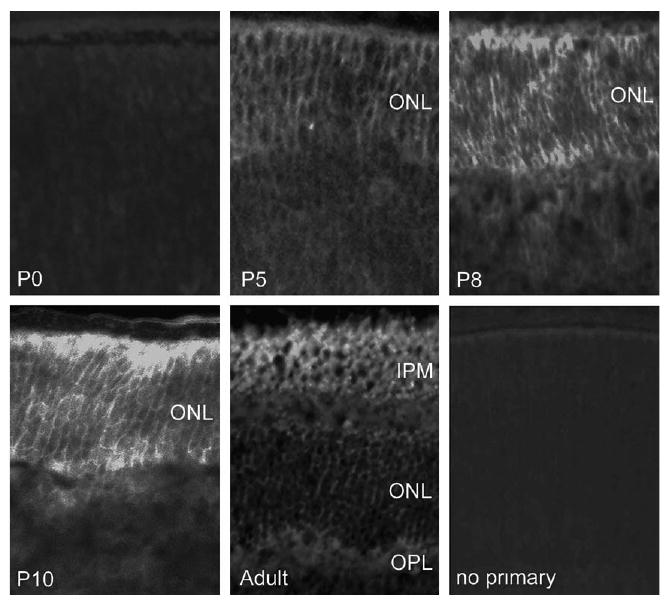
The Wnt receptor, fzd4, is expressed during photoreceptor differentiation and stabilization. Immunohistochemical analyses using an anti-fzd4 serum demonstrate fzd4 protein in retinal sections from P0, P5, P8, P10, and adult mice. Fzd4 immunoreactivity is weak in the neuroepithelium at birth (P0), but becomes concentrated in the developing photoreceptor layer, remaining in this location (the outer nuclear layer; ONL) through the period of peak rod photoreceptor production (P10). In the adult, Wnt4 protein remains in the outer retina, particularly in the interphotoreceptor matrix (IPM), but is largely excluded from the outer plexiform layer (OPL).
Fzds exert their actions in cooperation with a coreceptor, LRP6 (Liu et al., 2003; Pinson et al., 2000). We analyzed LRP6 expression in retinae from animals in which a β-galactosidase/neomycin resistance (“βgeo”) gene was knocked into the LRP6 locus (Pinson et al., 2000). In retinae from heterozygous animals, β-galactosidase immunoreactivity is present throughout the retina but is prominent in the inner retina just after birth (P3) and remains spread across the retina with notable immunoreactivity in the ganglion cell layer, inner nuclear layer, and inner segments of photoreceptors through the period of photoreceptor differentiation (P10–P12) (Fig. 7A). In the mature retina, β-galactosidase immunoreactivity (by inference, LRP6) is most prominent in the ganglion cell layer, but is also present in the inner nuclear layer (notably, the inner aspect of the inner nuclear layer), and in the inner segments of photoreceptors (Fig. 7A).
Fig. 7.
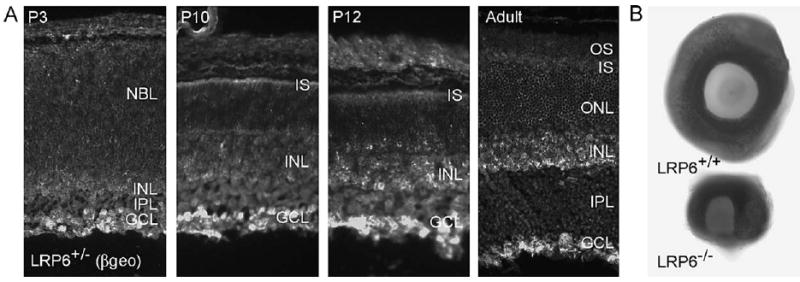
The Wnt coreceptor, LRP6, is expressed in the retina and is required for retinal development. (A) LRP6 expression detected by expression of the βgeo transgene in sections of developing mouse retina demonstrates LRP6 transcription in several layers of the retina, including the ganglion cell layer (GCL), inner nuclear layer (INL), and inner segments (IS) of the photoreceptors. There is little β-galactosidase immunoreactivity in the inner plexiform layer (IPL), outer nuclear layer (ONL), or outer segments (OS) of the photoreceptors. (B) Whole eyecups of E18 LRP6+/+ and LRP6−/− mice demonstrate the presence of a C-shaped retinal coloboma in the absence of LRP6.
Homozygous null LRP6 animals display microophthalmia and retinal coloboma (Pinson et al., 2000; Fig. 7B), suggesting that the loss of Wnt signaling leads to a massive disruption in retinal development. LRP6 −/− animals die at approximately E18, before most retinal differentiation occurs; therefore, we could not directly analyze retinal differentiation in these animals.
These data demonstrate that the Wnt coreceptor, LRP6, is present in the retina and that the function of LRP6 is required for proper retinal development. The massive disruption in retinal development that occurs in the absence of LRP6 that does not occur in the absence of individual fzds (i.e., fzd3; Wang et al., 2002, fzd4; Wang et al., 2001) suggests that retinal development is coordinated by an overlapping set of signals from several different fzds acting with LRP6.
WIF-1 and Wnt4 are coexpressed
Although others have demonstrated that Wnts and WIF-1 can interact in vitro (Hsieh et al., 1999), no direct biochemical interaction has been demonstrated in tissue. Antibodies directed against Wnt4 and WIF-1 suggest that these proteins are present in similar locations in the adult mouse retina; that is, in the IPM near the inner segments (IS) of photoreceptors (Fig. 8A). We used our Wnt4 and WIF-1 antisera to demonstrate the presence of a Wnt4/WIF-1 complex in the retina. Tissues were homogenized, and lysates were immunoprecipitated with an anti-Wnt4 antiserum, an anti-WIF-1 antiserum, or an anti-fzd4 antiserum, then complexes were detected for the presence of Wnt4 and WIF-1 by protein transfer blot (Fig. 8B). Wnt4 is precipitated by anti-Wnt4 and by anti-WIF-1, but not by an irrelevant primary antibody (anti-β-galactosidase). In addition, WIF-1 is precipitated by anti-Wnt4. This coprecipitation of Wnt4 and WIF-1 by an antibody against either component demonstrates the presence of a Wnt4/WIF-1 complex in the retina; this complex is also present in the newborn kidney. However, anti-WIF-1 does not precipitate all proteins: fzd4, which is precipitated by anti-fzd4, is not precipitated by anti-WIF-1, suggesting that fzd4 is not tightly associated with the Wnt4/WIF-1 complex (Fig. 8B). The coprecipitation of Wnt4 with WIF-1 is the first direct demonstration of a biochemical interaction between WIF-1 and a Wnt in tissue.
Fig. 8.
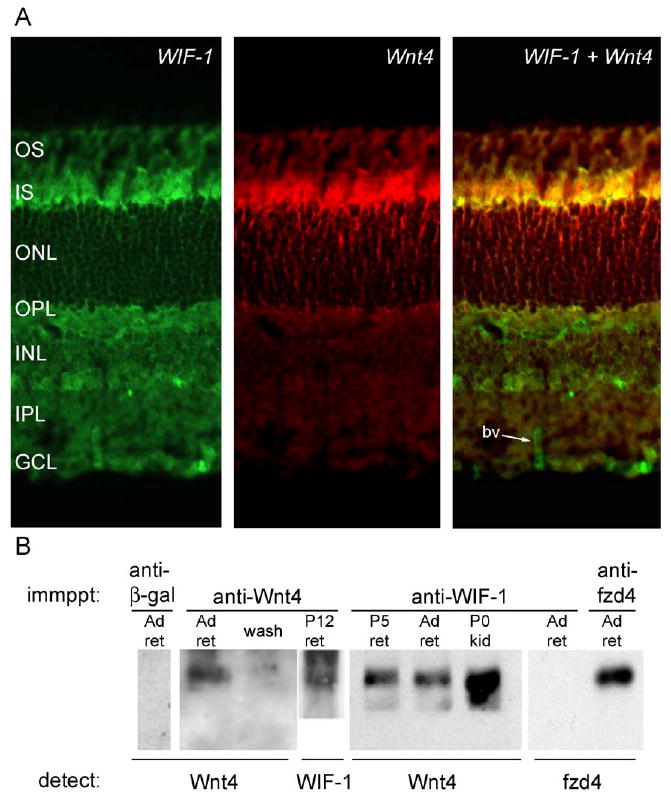
WIF-1 and Wnt4 are coexpressed and are physically linked. (A) Simultaneous double immunohistochemical analysis of adult mouse retina demonstrates WIF-1 (green) and Wnt4 (red) coexpressed in the outer retina (yellow), particularly in the region of the photoreceptor inner segments (IS). WIF-1 is also expressed in the OPL, portions of the INL, and in blood vessels (bv). Panels are reproduced from Figs. 3 and 5. (B) Co-immunoprecipitation of a retinal extract demonstrates a WIF-1/Wnt4 complex in the retina. Immunoprecipitations performed with antibodies to Wnt4 (anti-Wnt4) and WIF-1 (anti-WIF-1) both contain Wnt4. Immunoprecipitations with anti-WIF-1 contain Wnt4. Immunoprecipitation performed with antibodies to β-galactosidase (anti-β-gal) do not contain Wnt4. Fzd4 is present in immunoprecipitations performed with antibodies to fzd4 (anti-fzd4), but not with immunoprecipitations performed with antibodies to WIF-1.
WIF-1 and Wnt4 modulate retinal differentiation in vitro
In many other tissues, Wnts are morphogens that can stimulate proliferation, guide axis determination, and even control synapse formation. The in vitro differentiation capacity of retinal progenitor cells provides a unique opportunity for experimental analysis of protein function during cell commitment and differentiation stages. One of the major advantages of these systems is that, in a rapid, reproducible manner, all retinal cell types develop with a time course similar to that seen in vivo, allowing for easy manipulation of the progenitor's environment over time. We used our established method of in vitro retinal development (Hunter and Brunken, 1997) to begin to study the function of WIF-1 and its potential binding partners in retinal neurogenesis.
We used two approaches to study the function of WIF-1 in vitro. First, we cultured dissociated retinal cells from newborn rats at high density on glass coverslips, a condition that encourages rod photoreceptor development (Hunter and Brunken, 1997), and analyzed the activity of WIF-1 in this system.
Because changes in WIF-1 and Wnt4 protein distribution follow a time course similar to that for photoreceptor differentiation, we analyzed the effect of WIF-1 on the differentiation of rod photoreceptors in vitro. Rod photoreceptor production (as assessed by rhodopsin production) was inhibited in a dose-dependent manner when we cultured dissociated retinal cells in the presence of WIF-1 protein (Fig. 9A). In contrast, addition of anti-WIF-1 antiserum (thereby inhibiting endogenous WIF-1 activity) increased rod production approximately 125% (Fig. 9B). These data suggest an intrinsic interaction between WIF-1 and Wnts in the retina and that WIF-1 and Wnts act together to modulate the rod photoreceptor genesis.
Fig. 9.
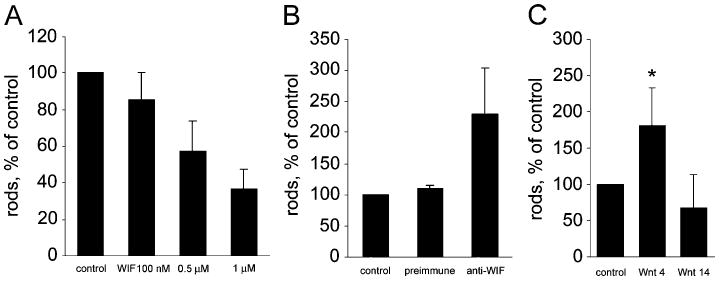
WIF-1 inhibits and Wnt4 enhances, the production of presumed photoreceptors in dissociated cultures of mouse retinae. (A) Synthetic WIF-1 inhibits the number of cells that express rhodopsin (rods) present in dissociated culture in a dose-dependent manner. (B) An antiserum raised against WIF-1 (anti-WIF; approximately 1 μg/ml) increases the number of rods produced in dissociated culture. (C) Conditioned medium containing Wnt4, but not Wnt14, increases the number of rods produced in dissociated culture. Mean ± SEM; *P < 0.05 vs. untreated.
In a second approach, we used a coculture system in which developing cells from similarly dissociated mouse retinae were cocultured with Wnt-producing chicken fibroblasts. Retinal cells developing from retinal neuroblasts in mixed cultures are thereby exposed to additional Wnts secreted by the transfected fibroblasts. In these mixed cultures, the exogenous secretion of Wnt4 into the culture medium significantly increased rod photoreceptor production (*P < 0.05), consistent with our hypothesis that Wnt4 is a proficient modulator of retinal development (Fig. 9C). Several other Wnts, including Wnt14 (Fig. 9) and Wnts 3 and 7 (not shown), had no significant effect on photoreceptor production.
These in vitro approaches jointly demonstrate that exogenous WIF-1 and Wnt4 have negative and positive effects, respectively, on rod photoreceptor differentiation. These data further support a modulatory interaction between the two molecules that affects rod photoreceptor development.
Together, these data are the first to suggest that members of the Wnt family of morphogens are essential regulators of mammalian retinal development. Moreover, these data suggest that Wnt activity in the developing retina, transduced through a fzd4/LRP6 complex, is regulated by elements of the retinal ECM, specifically, by WIF-1.
Discussion
Components of the ECM guide diverse aspects of differentiation and stabilization in multiple tissues, including the nervous system. We previously demonstrated that ECM components, including several laminins, are required for stabilization in at least one part of the nervous system, the retina (Libby et al., 1999, 2000b). Here, we examined the expression and activity of an additional potential ECM molecule, WIF-1, during retinal development. We also demonstrated the presence of a potential WIF-1 ligand, Wnt4, and a potential Wnt4 receptor, fzd4, during the period of photoreceptor differentiation. We postulate that WIF-1 functions, in part, by binding to and inhibiting Wnt4 in the extracellular matrix. The binding of Wnt4 by WIF-1 would reduce the level of Wnt4 available for activation of Wnt receptors including fzd4 and LRP6 (Fig. 10), leading to lowered activation of Wnt signaling through fzd4 and LRP6. This minimal scenario is undoubtedly further modified by (1) the presence of additional extracellular inhibitors of Wnts in the retina, (2) the presence of additional Wnts in the retina, and (3) the presence of additional Wnt receptors in the retina. We deal with each of these potential modifiers in turn.
Fig. 10.
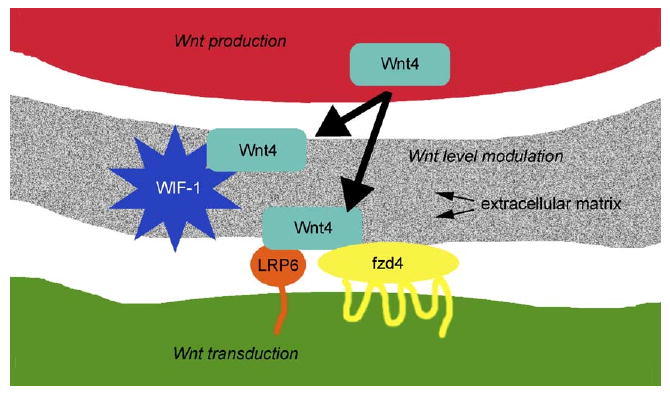
WIF-1 likely acts by inhibiting Wnt signaling during retinal development. Wnt signaling may be modulated at multiple levels. Free Wnt levels can be controlled by timing and location of production and secretion, binding to extracellular matrix, and sequestration by WIF-1 and other extracellular binding proteins. Wnt transduction can be controlled by timing and location of expression of Wnt receptors including fzds and coreceptors including LRP6. Modulation at any one of these levels could control the effective level of Wnt signaling.
Wnts are secreted into the extracellular space, where they become components of the ECM (Bradley and Brown, 1990; Papkoff and Schryver, 1990) and are bound to, and can be inhibited by, other extracellular components. Among these are the soluble frizzled-related proteins or sfrps. Several sfrps are present in the eye, in particular, sfrp2 (Chang et al., 1999; Jones et al., 2000) and sfrp1 (Esteve et al., 2003), are expressed in the neural retina, suggesting that there is at least one retinal Wnt with which these sfrps would interact.
Intriguingly, injection of sfrp2 into the developing Xenopus eye results in a dramatic alteration in the retina, disrupting lamination and causing rosette formation (Ladher et al., 2000), suggesting that sfrp2 can block the normal, Wnt-induced differentiation. The Wnt that interacts with sfrp2 in the retina has not been determined; however, in dermomyotome induction, sfrp2 can inhibit the action of Wnt4 (CS Lee et al., 2000). We have demonstrated the presence of Wnt4 in the retina and thus hypothesized that at least some of the effects of sfrp2 in the retina are due to inhibition of Wnt4 signaling.
Sfrp1 has been suggested to influence retinal differentiation in the cone-dominated chick retina by a mechanism that may be independent of canonical Wnt signaling (Esteve et al., 2003). When sfrp1 is overexpressed in early chick retina, early-born neurons (cones and retinal ganglion cells) are overproduced at the expense of the production of the later-born amacrine cells via a mechanism that does not seem to involve the classic Wnt–β-catenin signaling pathway. Although these results do not exclude interactions with endogenous Wnts, they do demonstrate that sfrps can influence retinal neuron differentiation, perhaps via a pathway that does not include β-catenin. It remains to be determined whether similar mechanisms are active in the cone-dominated chick retina (where most photoreceptors differentiate early) and rod-dominated mouse retina (where most photoreceptors differentiate late).
Nearly two dozen vertebrate Wnts have been isolated to date. Many of the members of this family are involved in early development of vertebrate and invertebrate nervous systems; in addition, several Wnts have been associated with tumorigenesis. Many of the vertebrate Wnts have been deleted by homologous recombination; in several instances, the loss of Wnt activity results in malformations of the nervous system. These include the loss of Wnt1, which results in malformed midbrain and cerebellum (McMahon and Bradley, 1990; Thomas and Capecchi, 1990), the loss of Wnt3a, which results in a malformed hippocampus (SM Lee et al., 2000), and the loss of Wnt7a, which results in malformed synapses within the cerebellum (Hall et al., 2000). In these cases, the effects of loss of Wnt activity on mouse retinal development have not been analyzed. Unfortunately, Wnt4-null animals (Stark et al., 1994) die at birth and therefore never undergo retinal development.
Which additional Wnts are present in the developing retina? Several studies have reported the presence of RNA encoding several Wnts during retinal development (Jin et al., 2002; Kubo et al., 2003; Liu et al., 2003). Together, these data are somewhat conflicting: for example, Wnt11 is either present (Jin et al., 2002) or absent (Liu et al., 2003); similarly, Wnt7a is present (Kubo et al., 2003) or absent (Liu et al., 2003). Some of this variation could be due to species differences but could also be due to the different stages of retinal differentiation that were analyzed. Nevertheless, it has become clear that several Wnts are present during differentiation of the neural retina and that levels of the different Wnts vary with time of differentiation of the retina. This timing corresponds to embryonic day 18 through birth and into the adult in the mouse. Although we have focused on Wnt4 during this time period, other Wnts are likely present as well.
Wnts elicit their responses in target cells by binding to transmembrane receptors. In Drosophila, one Wnt (Wnt5) can elicit a response through interactions with Derailed, a receptor tyrosine kinase-like molecule (Yoshikawa et al., 2003), presumably by binding to the extracellular WIF domain of Derailed. Transduction through the mammalian orthologue of Derailed, ryk, may involve interaction with the ephrin receptors EphB2 and EphB3 (Halford et al., 2000). Interestingly, deletion of ryk results in microophthalmia (Halford et al., 2000), suggesting that ryk may be involved in retinal development.
Most Wnt signaling is thought to occur via a class of transmembrane receptors known as frizzleds (fzds). RNAs encoding fzd4 and fzd5 are present during development of the chicken retina and have been postulated to interact with Wnt2b during differentiation of the neural retina (Kubo et al., 2003). RNAs encoding fzds 2, 3, 4, 5, 6, and 7 are present in the adult eye (Wang et al., 1996), and the presence of some fzds has been studied during several stages of development of the mouse retina (Liu et al., 2003; Wang et al., 1996).
Which of these fzds might act as a Wnt4 receptor during retinal development? (1) fzd3: Wnt4 can act via fzd3 during guidance of commissural axons in the developing mouse spinal cord (Lyuksyutova et al., 2003), and RNA encoding fzd3 is present in the P1 mouse eye (Wang et al., 1996). However, disruption of the mouse fzd3 gene does not produce obvious disruptions in the retina (Wang et al., 2002). (2) fzd4: RNA encoding Wnt4 colocalizes with that encoding fzd4 in some tissues of the developing chicken (Stark et al., 2000) and is present in the developing chicken retina (Kubo et al., 2003). Fzd4 has been suggested as the likely receptor for Wnt signaling during differentiation of the chicken retina (Kubo et al., 2003), and fzd4 protein is present in the appropriate location and the appropriate time for guidance of retinal development in the mouse (our data), suggesting that fzd4 can act as a Wnt receptor during retinal development. However, disruption of the mouse fzd4 gene does not produce obvious disruptions in the retina (Wang et al., 2001). (3) fzd5: Wnt4 does not synergize with fzd5 in Xenopus secondary axis induction (Ishikawa et al., 2001), suggesting that Wnt4 does not interact with fzd5. However, RNA encoding fzd5 is present in the E18 and P1 mouse eye (Wang et al., 1996). Disruption of the mouse fzd5 gene leads to embryonic lethality, long before the retina develops; therefore, the function of the receptor during retinal development has not been directly tested.
Our data, combined with others' (Kubo et al., 2003), suggest that fzd4 is likely important for Wnt signaling during retinal differentiation. Nevertheless, the most likely scenario for Wnt signal transduction in the developing retina is an overlapping set of several fzd receptors for each Wnt. Therefore, it may be necessary to eliminate transduction through several fzds simultaneously to effect a change in retinal development.
Several coreceptors are also important in Wnt signaling; of these, one, known as Dally (Lin and Perrimon, 1999; Tsuda et al., 1999), is a proteoglycan and, therefore, a likely component of the extracellular matrix (reviewed in Baeg and Perrimon, 2000). A mammalian homologue of Dally has not yet been identified, although it is related to a family of proteoglycans that play important roles in the nervous system, the glypicans (reviewed in Bandtlow and Zimmermann, 2000). Another Wnt coreceptor is the LDL receptor-related protein, LRP6, a transmembrane protein with extracellular EGF-like repeats. Mice lacking the LRP6 gene exhibit retinal malformations (Pinson et al., 2000), suggesting that this coreceptor may be involved in Wnt signaling during retinal differentiation. These LRP6-deficient mice also have defects in production and formation of the hippocampus (Zhou et al., 2004), suggesting that Wnt signaling through LRP6 plays multiple roles in central nervous system differentiation. Consistent with this, retinae from mice lacking LRP6 are grossly malformed, suggesting that this coreceptor is directly involved in retinal development. This gross malformation is in stark contrast to the minor disruptions seen with individual fzd deletions and suggests that LRP6 is a critical component of the Wnt signaling pathway during retinal development.
Here, we have demonstrated the presence of WIF-1 in particular regions of the retina during photoreceptor differentiation. Taken together, our biochemical, anatomical, and functional data suggest that Wnt4 stimulates photoreceptor production and that WIF-1 can bind to and block the action of Wnt4 in the production of photoreceptors. The levels of varying combinations of multiple Wnts, Wnt receptors (fzds), and extracellular Wnt inhibitors (sfrps, WIF-1) during development may represent a tunable system by which the production of different cell types can be controlled in a temporal and spatial manner.
Experimental methods
WIF-1 identification
A screen using a comparison of laminin chain amino acid sequences (SWISS-PROT) with the dbEST database (Boguski et al., 1993; NCBI) using the program BLAST (Altschul et al., 1990) yielded several novel laminin-like sequences; one, β-netrin, we previously reported (Koch et al., 2000). Another was isolated at the same time, and full-length mouse and human cDNAs were isolated. The full-length cDNAs are identical to those identified by others and named WIF-1 (Hsieh et al., 1999).
Analysis of tissue RNA
Reverse transcription–polymerase chain reaction (RT–PCR)
WIF-1 RNA expression was initially examined in human and mouse tissues as previously described for β-netrin (Koch et al., 2000). RNA from human tissues was either purchased from Clontech (Palo Alto, CA) or was obtained through reagent sharing with other laboratories at the Cutaneous Biology Research Center (CBRC) in accordance with protocols established for Massachusetts General Hospital (MGH) and Harvard University. RNA from mouse tissues was extracted using RNeasy (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Total RNA was reverse-transcribed using reverse transcriptase (Clontech). The amount of template to be used for comparison of WIF-1 RNA expression was first normalized by assessing the level of GAPDH in each sample using PCR with GAPDH primers ([human/mouse] forward: 5′-TGAAGGTCGG[A/T]GT[C/G]AACGGA-3′; reverse: 5′-GATGGCATGGACTGTGGTCA-3′) and Expand polymerase (Roche, Indianapolis, IN). PCR for WIF-1 was performed with the normalized cDNA templates using gene-specific primers for human WIF-1 (forward 5′-GCAACAGAGAATGCCAGCTATTCC-3′ reverse 5′-AATTGGATTCAGGTGGATCCC-3′) and mouse WIF-1 (forward: 5′-GAATTTTACCTGGCAAGCTGCGG-3′; reverse: 5′-GACGGGCTTAGAGCACAGGTCTCC-3′). PCR was performed as follows: denaturation, 94°C, 2 min; 10 cycles of 94°C for 30 s, 65°C (−0.5°C per cycle) for 30 s, and 68°C for 2 min; 22 cycles of 94°C for 30 s, 60°C for 30 s, 68°C for 2 min (+10 s per cycle), and a final extension period at 68°C for 5 min. PCR products were confirmed by sequencing.
For additional analyses of WIF-1 and Wnt4 RNA expression, RNA was extracted from mouse retinae with TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Total RNA was reverse-transcribed using random nonamer primers (Amersham, Piscataway, NJ) and Superscript II (Invitrogen) according to the manufacturer's instructions. The amount of template to be used for comparison of WIF-1 RNA expression was first normalized by assessing the level of 18S RNA in each sample using PCR with QuantumRNA (now Classic II) 18S primers (Ambion, Austin, TX) according to the manufacturer's instructions. PCR for WIF-1 and Wnt4 was then performed using normalized cDNA with Taq DNA Polymerase High Fidelity (Roche) and the following primers: WIF-1, 5′-TGCTTTAATGGAGGGACCTG-3′ (forward) and 5′-TCTTATTGCAGTGTCTGCCG-3′ (reverse); Wnt4, 5′-CTGGAGAAGTGTGGCTGTGA-3′ (forward) and 5′-CAGCCTCGTTGTTGTGAAGA-3′ (reverse). PCR was performed as follows: denaturation, 94°C, 10 min; 34 cycles of 94°C for 45 s, 60°C for 45 s, 72°C for 2 min, and a final extension period at 72°C for 10 min.
In situ hybridizations
In situ hybridization of retina tissue sections were performed as previously described (Libby et al., 1997). cRNA probes for WIF-1 and Wnt4 were labeled during transcription by the incorporation of digoxigenin-UTP (Roche); approximately 1 μg/ml cRNA was used for hybridization.
Protein transfer blots, immunohistochemistry, and immunoprecipitations
Antibodies
An anti-WIF-1 antiserum was generated in our laboratories against synthetic WIF-1 protein produced using a mammalian expression system (Koch et al., 2000; see below for details of protein production). Synthetic WIF-1 protein was injected intradermally into a rabbit for antibody production following standard procedures (Harlow and Lane, 1988). The collected antiserum was purified over a protein G Sepharose column (Amersham) and eluted with triethylamine (pH 10.2). The neutralized eluate was affinity-purified over a WIF-1 protein column, prepared by coupling synthetic WIF-1 protein to activated CNBr-Sepharose. Antibodies that bound to this WIF-1 column were eluted with triethylamine and immediately neutralized. Goat antisera against Wnt4 were purchased from several sources: anti-Wnt4 (AF475) from R and D Systems (Minneapolis, MN), several lots of anti-Wnt4 (C-14) from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-Wnt4 (Wnt4-GT) from Neuromics (Northfield, MN). Although all provided qualitatively similar results, the most consistent results were obtained with the Neuromics antiserum. A goat anti-mouse fzd4 antiserum was purchased from R and D Systems. A rabbit anti-β-galactosidase antiserum was purchased from Abcam (Cambridge, MA). An antibody against rhodopsin (Ret-P1) was a gift from Dr. C. Barnstable. Anti-rabbit and anti-goat antibodies conjugated to horseradish peroxidase (HRP) were from Roche, and those conjugated to cy3 and TRITC were from Jackson Immunoresearch (West Grove, PA).
Protein transfer blots
Retina, kidney cortex, or skin membrane-matrix extracts (20 μg; prepared as in Libby et al., 1997) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to immobilon P membranes (Millipore, Billerica, MA). Blots were blocked with 10 mg/ml bovine serum albumin (BSA) and 3% (wt/vol) nonfat dry milk in phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4), then incubated overnight at 4°C with primary antibodies diluted in 0.5% (vol/vol) Tween 20 in PBS, washed, and incubated for 1 h at ambient temperature with horseradish peroxidase-conjugated secondary antibodies (1:2–20,000). Chemiluminescent detection was performed using the SuperSignal kit (Pierce, Evansville, IL) and documented on BioMax Light films (Kodak, Rochester, NY).
Immunohistochemistry
Immunohistochemistry was performed essentially as previously described (Libby et al., 1996, 1997). Mouse retinae were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS then sequentially equilibrated with 10%, 20%, and 30% (wt/vol) sucrose in PBS. Tissue was rinsed in PBS, embedded in OCT (Miles, Elkhart, IN), and frozen by immersion in liquid nitrogen-cooled isopentane. Twelve-micrometer-thick sections were cut and placed onto Superfrost Plus slides (Fisher, Pittsburgh, PA) and were stored at −70°C. For use, slides were brought to ambient temperature, and sections were blocked with 20 mg/ml BSA and 2% donkey serum in PBS. Slides were then incubated overnight with affinity-purified rabbit anti-WIF-1, goat anti-Wnt4, goat anti-fzd4, or rabbit anti-β-galactosidase antibodies at 4°C. Sections were washed in PBS, incubated with appropriate secondary antibodies conjugated to a fluorochrome diluted in PBS containing 20 mg/ml BSA and 2% donkey serum for 60 min at room temperature, and washed in PBS. Tissue not incubated with a primary antibody but otherwise treated identically served as controls. Digital images were captured with a Zeiss Axioplan equipped with an Apogee cooled CCD camera.
Immunoprecipitations
Co-immunoprecipitations were performed using a modification of methods previously described for other ECM molecules (laminins; Green et al., 1992). Briefly, retinal tissue was solubilized in a lysing solution (150 mM NaCl, 0.1% SDS, 1% sodium deoxycholate, 1% Nonidet P-40, 10 mM N-ethylmaleimide, 1.5 mM phenylmethylsulfonyl fluoride, 1% aprotinin, 20 mM EDTA, and 20 mM Tris, pH 8). The lysate was sonicated briefly and centrifuged at 500 × g for 5 min to remove nuclei. Supernatants were incubated with anti-Wnt4 (1:200), anti-WIF-1 (1:200), or anti-fzd4 (1:100) in a binding solution (150 mM NaCl, 1% Nonidet P-40, 1% Triton X-100, 0.05% SDS, 20 mM EDTA, and 20 mM Tris, pH 8) for 1 h at 4°C. Complexes were then incubated with protein G-Sepharose beads at 4°C for 2 h, centrifuged at 10,000 × g for 10 min, then washed twice with binding solution and once with 0.02× binding solution. The precipitated complex was solubilized in SDS gel sample solution. The presence of Wnt4, WIF-1, or fzd4 in the immunoprecipitate was detected with the appropriate antiserum by protein transfer blot.
Expression of WIF-1 protein
WIF-1 protein was expressed in mammalian cells essentially as previously described for β-netrin (Koch et al., 2000). A 1056-bp, full-length WIF-1 PCR product (forward primer: 5′-CACGCTAGCGGGCAGCCACCTGAGGAGAGCTTG-3′; reverse primer: 5′-CACAGATCTTCACCAGATGTAATTGGATTCAG-3′) was purified (QIAGEN) from an agarose gel and subcloned (rapid DNA ligation; Roche) into a modified PCEP-4 expression vector (gift of Ernst Poeschl, Munich, Germany). For convenience, a six-histidine tag followed by a thrombin cleavage site was included. The ligated DNA was transformed into TOP 10 cells (Invitrogen), and plasmids were isolated (QIAGEN) and sequenced using Thermo Sequenase (Amersham). The expression construct was transfected into 293-EBNA cells (Invitrogen) using Fu-Gene (Roche) and selected after 2 d with puromycin (Sigma).
Stably transfected 293-EBNA cells were subcloned, and the highest protein-producing clones were expanded. Supernatant from these cells (2 l) was collected, and phenylmethylsulfonylfluoride was added to a final concentration of 0.5 mM. After ammonium sulfate precipitation (45%), the precipitate was collected by centrifugation and dialyzed against a column binding solution (200 mM NaCl and 20 mM Tris–HCl, pH 8.0). The dialyzed protein was applied to a nickel-chelated Sepharose column (Amersham) and washed and eluted with binding solution containing increasing concentrations of imidazole (10–80 mM). In some cases, the histidine tag was digested with thrombin (isolated from bovine plasma; Sigma) according to the protocol from Novagen. The digested protein was again applied to a nickel-chelated Sepharose column and eluted with increasing imidazole concentration. The protein solution was dialyzed against PBS, and the protein concentration was determined using a standard protocol (Whitaker and Granum, 1980). WIF-1 protein was adjusted to a final concentration of 0.1 mg/ml (2 mM).
Cell culture systems
Cultures of dissociated mouse retinae were performed using a modification of a protocol for rat retinae (Hunter and Brunken, 1997; Hunter et al., 1992). Briefly, single cell suspensions were prepared by incubating P0 retinae in 1 mg/ml hyaluronidase type IV-S (Sigma), 0.2 mg/ml collagenase type I (Worthington, Freehold, NJ) and 2 mg/ml BSA in DMEM for 30 min at 37°C. Cells were dissociated by trituration through a flame-polished Pasteur pipette, centrifuged at 400 × g for 5 min, and resuspended in DMEM (BioWhittaker), 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin sulfate (Invitrogen). Cells were seeded at a density of 5–7 × 105 cells per well onto uncoated glass coverslips in 24-well plates and maintained at 37°C in a humidified atmosphere of 95% air/5% CO2. Culture medium was changed every 2 days.
To determine potential modulatory effects of Wnt4 and WIF-1 on undifferentiated retinal progenitors, dissociated cells were cultured in the presence of recombinant purified WIF-1 (see above) at 100 nM to 1 μM or in the presence of preimmune or anti-WIF-1 antisera (see above) at approximately 1 μg/ml. After 10 to 14 days, the rod photoreceptor production was determined by counting cells that expressed rhodopsin, using the anti-rhodopsin antibody RetP1 (see Hunter et al., 1992, and Hunter and Brunken, 1997 for details). Numbers of rods in treated cultures were normalized to numbers in untreated cultures and averaged. In other experiments, retinal progenitors were cocultured with chicken fibroblasts transiently transfected with Wnt cDNAs (Morgan laboratory; CBRC/MGH/Harvard). Experiments were performed in triplicate in three independent trials. The levels of Wnts secreted into the conditioned medium could not be directly determined.
Statement of animal use
All procedures involving animals were approved by Tufts University Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health Guide for the Care and Use of Animals, and the policies of the Society for Neuroscience and the Association for Research In Vision and Ophthalmology. Mice were killed by exposure to CO2.
Acknowledgments
We thank Bruce Morgan from the Cutaneous Biology Research Center at Massachusetts General Hospital/Harvard Medical School for Wnt-producing chicken fibroblasts.
Supported by EY12037 to DDH, NS39502 to DDH and WJB and EY13078 to the Tufts Center for Vision Research. The authors acknowledge the generous support of Robert Burgeson.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Perrimon N. Functional binding of secreted molecules to heparan sulfate proteoglycans in Drosophila. Curr Opin Cell Biol. 2000;12:575–580. doi: 10.1016/s0955-0674(00)00134-4. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Belliveau MJ, Cepko CL. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development. 1999;126:555–566. doi: 10.1242/dev.126.3.555. [DOI] [PubMed] [Google Scholar]
- Boguski MS, Lowe TM, Tolstoshev CM. dbEST: database for “expressed sequence tags”. Nat Genet. 1993;4:332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- Bradley R, Brown AM. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990;9:1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Chang JT, Esumi N, Moore K, Li Y, Zhang S, Chew C, Goodman B, Rattner A, Moody S, Stetten G, Campochiaro PA, Zack DJ. Cloning and characterization of a secreted frizzled-related protein that is expressed by the retinal pigment epithelium. Hum Mol Genet. 1999;8:575–583. doi: 10.1093/hmg/8.4.575. [DOI] [PubMed] [Google Scholar]
- Esteve P, Trousse F, Rodríguez J, Bovolenta P. SFRP1 modulates retina cell differentiation through a β-catenin-independent mechanism. J Cell Sci. 2003;116:2471–2481. doi: 10.1242/jcs.00452. [DOI] [PubMed] [Google Scholar]
- Green TL, Hunter DD, Chan W, Merlie JP, Sanes JR. Synthesis and assembly of the synaptic cleft protein s-laminin by cultured cells. J Biol Chem. 1992;267:2014–2022. [PubMed] [Google Scholar]
- Halford MM, Armes J, Buchert M, Meskenaite V, Grail D, Hibbs ML, Wilks AF, Farlie PG, Newgreen DF, Hovens CM, Stacker SA. Ryk-deficient mice exhibit craniofacial defects associated with perturbed Eph receptor crosstalk. Nat Genet. 2000;25:414–418. doi: 10.1038/78099. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. p. 726. [Google Scholar]
- Hicks D, Courtois Y. Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J Neurosci. 1992;12:2022–2033. doi: 10.1523/JNEUROSCI.12-06-02022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Brunken WJ. β2 laminins modulate neuronal phenotype in the rat retina. Mol Cell Neurosci. 1997;10:7–15. doi: 10.1006/mcne.1997.0632. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Murphy MD, Olsson CV, Brunken WJ. S-laminin expression in adult and developing retinae: a potential cue for photoreceptor morphogenesis. Neuron. 1992;8:399–413. doi: 10.1016/0896-6273(92)90269-j. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Burrus LW, Erickson CA. The expression patterns of Wnts and their antagonists during avian eye development. Mech Dev. 2002;116:173–176. doi: 10.1016/s0925-4773(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Modulated expression of secreted frizzled-related proteins in human retinal degeneration. NeuroReport. 2000;11:3963–3967. doi: 10.1097/00001756-200012180-00012. [DOI] [PubMed] [Google Scholar]
- Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, Keene DR, Brunken WJ, Burgeson RE. A novel member of the netrin family, β-netrin, shares homology with the β chain of laminin: identification, expression, and functional characterization. J Cell Biol. 2000;151:221–234. doi: 10.1083/jcb.151.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Church VL, Allen S, Robson L, Abdelfattah A, Brown NA, Hattersley G, Rosen V, Luyten FP, Dale L, Francis-West PH. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- Lee CS, Buttitta LA, May NR, Kispert A, Fan CM. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development. 2000;127:109–118. doi: 10.1242/dev.127.1.109. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Libby RT, Hunter DD, Brunken WJ. Developmental expression of laminin β2 in rat retina. Further support for a role in rod morphogenesis. Invest Ophthalmol Visual Sci. 1996;37:1651–1661. [PubMed] [Google Scholar]
- Libby RT, Xu Y, Selfors LM, Brunken WJ, Hunter DD. Identification of the cellular source of laminin β2 in adult and developing vertebrate retinae. J Comp Neurol. 1997;389:655–667. doi: 10.1002/(sici)1096-9861(19971229)389:4<655::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Libby RT, Lavalle C, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin β2 chain production causes alterations in morphology and function in the central nervous system. J Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Brunken WJ, Hunter DD. The roles of the extracellular matrix in retinal development and maintenance. In: Fini ME, editor. Vertebrate Eye Development, Results Probl Cell Differ. Vol. 31. Springer-Verlag; Berlin: 2000a. pp. 115–140. [DOI] [PubMed] [Google Scholar]
- Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000b;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature. 1995;377:158–162. doi: 10.1038/377158a0. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova A, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior–posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Belliveau MJ, Cepko CL. Two phases of rod photoreceptor differentiation during rat retinal development. J Neurosci. 1998;18:3738–3748. doi: 10.1523/JNEUROSCI.18-10-03738.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J, Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990;10:2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Hansen LA, Vogel JC, Udey MC. Characterization of Wnt gene expression in murine skin: possible involvement of epidermis-derived Wnt-4 in cutaneous epithelial–mesenchymal interactions. Exp Cell Res. 1998;243:150–160. doi: 10.1006/excr.1998.4152. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Stark MR, Rao MS, Schoenwolf GC, Yang G, Smith D, Mauch TJ. Frizzled-4 expression during chick kidney development. Mech Dev. 2000;98:121–125. doi: 10.1016/s0925-4773(00)00440-8. [DOI] [PubMed] [Google Scholar]
- Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol: Renal Physiol. 2002;282:F431–F441. doi: 10.1152/ajprenal.0009.2001. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, Siegfried E, Stam L, Selleck SB. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature. 1999;400:276–280. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem. 1996;271:4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J Neurosci. 2002;22:8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Wetts R, Serbedzija GN, Fraser SE. Cell lineage analysis reveals multipotent precursors in the ciliary margin of the frog retina. Dev Biol. 1989;136:254–263. doi: 10.1016/0012-1606(89)90146-2. [DOI] [PubMed] [Google Scholar]
- Whitaker JR, Granum PE. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980;109:156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Zhao C, Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


