Abstract
Purpose
Retinal diseases are often accompanied by changes in the structure of the multilayered extracellular matrix underlying the retina, Bruch's membrane (BrM). These structural revisions potentially lead to alterations in retinal pigment epithelium (RPE) adhesion, likely via modification of interactions with extracellular matrix (ECM) proteins including laminins in BrM. The purpose of this study was to identify specific laminins in BrM and their receptors in RPE cells.
Methods
The laminin composition of BrM was determined using biochemical, molecular biological, and immunohistochemical techniques of rat, bovine, and human tissue and cell lines. An adhesion assay was used to test RPE attachment to laminins and the receptors used for this attachment.
Results
BrM contained laminin chains that could form laminin heterotrimers including laminins 1, 5, 10, and 11. RPE cells synthesized these laminin chains in vitro. Therefore, RPE cells may synthesize BrM laminins. The RPE cells preferentially adhered to potential BrM laminins. Although the cells adhered to the BrM component collagen IV, these cells preferentially adhered to laminins. Of the laminins tested, the RPE cells adhered preferentially to laminin 5. The cells interacted with these laminins via specific integrins and attained a different morphology on each laminin. In particular, the RPE cells rapidly attached and flattened on laminin 5.
Conclusions
BrM contains specific laminins, and RPE cells express integrin receptors for those laminins. The interaction of these specific laminins and integrins most likely leads to differential behavior of RPE cells.
The interface between the neural retina and retinal pigment epithelium (RPE) is formed during the unusual juxtaposition of two epithelial apical surfaces as the optic cup folds in from the neural tube. The inner limit of the retina is formed by the epithelial basal surface of the neural retina, the structured basement membrane known as the inner limiting membrane. Because the apical surface of the neural retina is juxtaposed to the apical surface of the RPE, the outer limit of the retina is also formed by an epithelial basal surface: that of the RPE, Bruch's membrane (BrM). BrM serves functions analogous to basement membranes in other tissues, including anchoring subjacent cells, acting as a barrier and a filter, and stabilizing the structure of the tissue.1
Epithelial basement membranes are minimally composed of a structural framework built from a combination of members of several families of glycoproteins.2,3 These families include fibronectins, the polymer-forming collagens (collagens type IV), and the laminins. Together, this complex forms the electrondense structure visible in electron micrographs apposed to the basal surface of epithelial cells.
BrM is a five-layered structure that consists of two basement membranes bordering an inner core of two collagenous layers (composed largely of collagen type I) and an elastic layer (composed largely of elastin). The choroidal border of BrM is formed by the basement membrane of the choriocapillaris. Subjacent to the RPE itself is another classic basement membrane, the RPE basement membrane, which, like other basement membranes, contains fibronectin, collagen type IV, and laminins.4–6
One of the many changes associated with the RPE basement membrane during disease is potentially critical: a splitting of Bruch's membrane between the basement membrane of RPE cells and the inner collagenous layer of BrM. This splitting occurs in retinas of patients with protein and lipid deposits known as drusen, during the development of choroidal neovascularization and, most obviously, in pigment epithelium detachment (either serous or fibrovascular). Changes in the composition and distribution of BrM extracellular matrix (ECM) components, including proteins promoting adhesion such as collagen IV, fibronectin, and laminins, can promote this splitting of BrM. Changes in these BrM ECM components also lead to changes in BrM hydroconductivity, thereby promoting pigment epithelium detachment.7,8 However, it is not known which of these ECM components—in particular, which laminins—are present and functional in the RPE basement membrane.
Laminins are large heterotrimeric glycoproteins consisting of an α, a β, and a γ chain.2,9 Vertebrates produce five α chains, three β chains, and three γ chains; these chains combine to form at least 15 different laminins.10,11 The distinct biological activity of each of these laminins is the result of combined properties of the individual chains. Of the 15 reported laminins, several have quite restricted tissue distribution and elicit distinct biological responses in cells with which they interact. One of the most distinctive is laminin 5, a critical component of skin stability.12
Although it is possible to purify heterotrimeric laminins from some tissues, the presence of a given laminin heterotrimer is usually inferred by the presence of its component chains. Most chains are components of several heterotrimers; however, the notable exceptions are the β3 and γ2 chains, which are thought only to exist as components of laminin 5. Thus, the presence of the β3 or γ2 chains infers the presence of the laminin 5 heterotrimer.
Epithelial cells adhere to their basement membranes via myriad membrane-associated receptors including dystroglycan13 and, critically for many laminins, a large family of transmembrane receptors, the integrins. Individual integrin heterodimers show some ligand specificity but also can be somewhat promiscuous in their binding.14
Integrins are composed of one each of the 18 α and 8 β subunits.14 Integrin subunits that are present on the basal side of the RPE include α3, α5, α6, and β1.15–17 Of these, potential heterodimeric laminin receptors are α3β1 and α6β1. Antibodies against the β1 subunit block attachment of RPE cells on isolated BrM18 and inhibit migration of RPE cells on isolated BrM,19 suggesting that β1-containing integrins can function in RPE interactions with laminins of Bruch's membrane.
Genetic disruptions demonstrate that interactions between integrins on epithelial cells and laminins in their subjacent basement membranes are critical for tissue function (see the references in the Discussion section). We propose that similar interactions between integrins in RPE and specific laminins in the RPE-basement membrane are vital for RPE stability and RPE-BrM interface integrity.
Methods
Animals and Tissue Preparation
All procedures involving animals were approved by the Tufts University animal care committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Rats and mice were killed by exposure to CO2. Bovine eyes were obtained from a local abattoir. Human retinal sections, unfixed and fixed, were provided by Ann Milam (Scheie Eye Institute, University of Pennsylvania, Philadelphia, PA) and from tissue sharing at Tufts-NEMC. All human tissue was obtained in accordance with the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry was performed as previously described,20,21 with the exception that autofluorescence of human sections was reduced with a proprietary commercial autofluorescence reduction reagent (Autofluorescence Eliminator Reagent, catalog no. 2160; Chemicon, Temecula. CA), according to the manufacturer's instructions. Adult rat or bovine eye cups were embedded in optimal cutting temperature (OCT) compound (Miles, Elkhart, IN) and frozen by immersion in liquid nitrogen-cooled isopentane. Transverse, 10-μm-thick sections were cut with a cryostat (Leica, Bannockburn, IL) and placed on slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA). Slides were stored at −20°C (or, for long-term storage, at −80°C) until use. For use, slides were returned to room temperature, immersed briefly in acetone (or, interchangeably, for all but the α5, β3, and γ2 chains, MeOH) at −20°C, washed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KC1, 10 mM Na2HPO4, and 1.76 mM KH2PO4, pH 7.4), and then incubated in primary antibodies for 2 hours at room temperature or overnight at 4°C. Primary antibodies (Table 1) were diluted in PBS containing 2% goat serum, 2% bovine serum albumin, or both. Sections were washed in PBS and incubated in species-appropriate, affinity-purified, fluorescently labeled secondary antibodies diluted in 2% goat serum in PBS for 1 hour at room temperature. Some sections were counterstained with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI). After washes in PBS, slides were mounted in 90% glycerol and 10% water, containing para-phenylenediamine (1 mg/mL; Sigma-Aldrich, St. Louis, MO), to reduce photobleaching, or in an antifade reagent (Prolong; Invitrogen-Molecular Probes, Eugene, OR). Sections were examined with epifluorescence on an upright microscope (Carl Zeiss Meditec, Inc. Oberkochen, Germany) and photographed with a cooled charge-coupled device (CCD) camera (Apogee Instruments, Auburn, CA) driven by microscopy imaging and processing software (MicroCCD; Diffraction Limited, Ottowa, ON, Canada), or with a scanning confocal system on an upright microscope (TCS SP2 AOBS; Leica) driven by the manufacturer's software. Images were adjusted for contrast and cropped (Photoshop; Adobe Systems, San Jose, CA).
Table 1.
Antibodies Used for Laminin Chains
| Laminin Chain | Antibody | Species Reactivity | ||||
|---|---|---|---|---|---|---|
| Name | Source | Reference | Bovine | Human | Rat | |
| α1 | AL-4 | Chemicon | − | + | + | NT |
| α2 | 5H2 | Chemicon | − | − | + | NT |
| α3 | BM2 | Hunter* | 22 | + | + | + |
| α4 | JS4 | Sanes | 23 | NT | + | + |
| α5 | 4C7 | Engvall | 24, 25 | + | + | + |
| α5 | 405 | Sorokin | 26 | + | + | + |
| β1 | LT3 | Chemicon | − | + | + | NT |
| β1 | C21 | Sanes | 27 | + | − | + |
| β2 | C4 | Hunter† | 27 | + | + | + |
| β2 | D5 | Hunter | 28 | + | − | + |
| β3 | 6F12 | Hunter* | 22 | + | + | + |
| γ1 | D18 | Hunter | 29 | + | + | + |
| γ2 | GB3 | Harlan | − | + | + | + |
| γ3 | R96 | Hunter* | ‡ | + | + | + |
| α1β1γ1 (laminin 1) | Anti-lam | Invitrogen | − | + | + | + |
| α3β3γ2 (laminin 5) | 9LN5 | Hunter* | 11 | + | + | + |
All antibodies function for immunohistochemistry; all but C21 function for protein transfer (Western) blots analysis. NT, not tested.
From collaboration with the Burgeson and Brunken laboratories.
From cells obtained from ATCC.
Li, Brunken, Koch, and Hunter, manuscript In preparation.
Cell Culture
Two widely used and extensively described RPE cell lines, rat RPE-J30 and human ARPE-19,31 and the rat Müller cell line RMC,32 were grown as described in the original publications. Cells were split every week or, for adhesion assays, the day before the assay.
Reverse Transcription–Polymerase Chain Reaction
RNA was isolated from mouse retina and skin and from the rat RPE-J and human ARPE-19 RPE cell lines (Trizol; Invitrogen, Carlsbad, CA), according to the manufacturer's procedures. RNA was reverse-transcribed (Superscript II; Invitrogen) with the reverse transcriptase primed with random hexamer or oligonucleotide primers. The resultant cDNAs were amplified in reactions with PCR master mix (High Fidelity; Roche, Indianapolis, IN) and the use of primers chosen in the laboratory for sequences of high similarity among species for each laminin chain. These sequences were designed by identifying regions of amino acid identity, comparing sequences manually and sequentially among the species for which sequences have been reported, and choosing regions of the highest sequence identity. Degenerate primers were used when necessary (see Table 2). The reaction product was analyzed on a 1% agarose gel, stained with ethidium bromide, and photographed.
Table 2.
ARPE-19 Cells Produce Laminin RNAs
| Laminin Chain | Primer (5′–3′) | ARPE-19 Cells | Controls | |||
|---|---|---|---|---|---|---|
| Forward | Reverse | Skin | Ret | RMC | ||
| α1 | TGTAGATGGCAAGGTCTTATTTCA | CTCAGGCAGTTCTGTTTGATGT | + | ND | + | + |
| α2 | CAAAGAYATTGAAATTTCAAG | GAGTTAATAACAAGRTTCCA | − | + | + | − |
| α3 | CTCATGGTGAGATACAAACT | TTGAAAGGTCTGAAACCCAAA | + | + | ND | ND |
| α4 | GGATGCGCCTTCATGGG | GTCAGGCTGTGGGACAGGA | − | ND | + | + |
| α5 | GAAGCTGGCTCTTGTCATCC | GCATAATCCAGGCCAAAGAA | + | + | + | + |
| β1 | TAACGAGGTGGAGTCCGGTTA | AAGGCCCGTCTGGTGAATCAA | + | ND | ND | + |
| β2 | CTTCGCTTGGGCCTACTTCT | GGATGGTGACCATCGGAACA | + | ND | + | + |
| β3 | AATGTAGTGGGCCCCAAATG | CTCCTTCTTGCTTCGGTAC | + | + | ND | + |
| γ1 | ACGGCTACTTTGGAGACCCT | GTCCAAACCCAAAGTGGTTG | + | ND | ND | ND |
| γ2 | ACCCCCGCAGCTGCAAGCCRTGT | GCAAGCTCGACACTTGTCTGCT | + | + | + | + |
| γ3 | TACGGTAACGCCTTCTCAGG | CAAGTGGGTGACATTTGCAG | − | ND | + | ND |
| γ3* | TATGGCAACCCTTTCGCGGG | CCAGTGGGTGACACTTGCAG | − | ND | ND | ND |
The human RPE cell line ARPE-19 and several other cell types were analyzed for expression of RNA encoding all 11 laminin chains. ND, not determined.
“Humanized” primers.
Protein-Transfer (Western) Blot Analysis
Protein-transfer blot analyses were performed as previously described.20,21 Briefly, RPE was extracted from rat or bovine eyes or RPE cells were scraped from dishes. Extracts of ECM protein were made in 25 mM Tris-HCl (pH 7.4) containing 0.2 mg/mL α2-macroglobulin and 1% phenylmethylsulfonyl fluoride. Proteins were analyzed by protein-transfer (Western) blot with anti-laminin antibodies (Table 1).
Adhesion Assays
Four spots of substrates (2 μL each) were allowed to adhere to 12-well or 24-well tissue culture plates for 60 minutes at ambient temperature. The wells were blocked with 10 mg/mL bovine serum albumin (BSA; Sigma-Aldrich) in PBS for 30 minutes and washed twice with PBS. ARPE-19 cells were dissociated from monolayer cultures using an enzyme-free dissociation solution (Specialty Media, Phillipsburg, NJ) to preserve cell surface receptors and resuspended in Hanks' balanced salt solution (Cambrex, East Rutherford, NJ). The cells (105, 12-well; and 5 × 104, 24-well in DMEM/F12; Cambrex) were added to each well and were allowed to adhere for 60 to 90 minutes. Wells were washed with PBS and adherent cells were fixed with 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS. Cells were stained with 10 μg/mL acridine orange in PBS, washed in PBS, photographed and counted (ImageJ software; available by ftp at zippy.nimh.nih.gov/ or at http://rsb.info.nih.gov/ij; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD). Nonspecific binding (to the BSA surrounding the adhesive substrate) was zero (e.g., Fig. 7). For assessment of the blocking of adhesion with anti-integrin antibodies, cells were preincubated with antibody for 20 minutes before addition to wells, and adhesion was continued in the presence of antibody for 60 minutes at ambient temperature. Laminin 1 from mouse EHS tumor was obtained from Invitrogen. Laminin 5 was a generous gift of Manuel Koch (University of Cologne, Germany); laminin 10/11 from human placenta was from Chemicon. Adhesion assays were performed in at least two independent experiments, each of which contained eight independent cell counts per assay condition.
Figure 7.
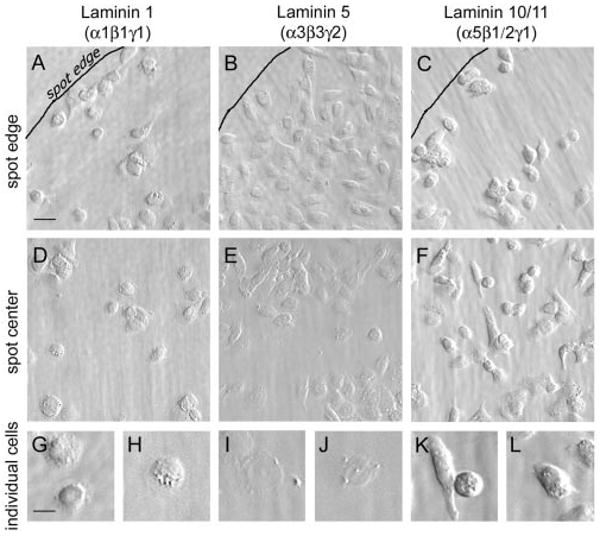
The morphology of the RPE cells was influenced by laminin type. Cells were plated in wells on spots of substrates. (A–C) Outlined edges of spots; (D–F) central areas of the spots. (G–L) Different cell morphology on various laminins, shown at a higher power. The cells attained a much flatter morphology on laminin 5 than on laminin 1 or 10/11, and they elongated on laminin 10/11. The cells were allowed to adhere for 60 minutes and then were rinsed and fixed. Bar: (A–F) 20 μm; (G–L) 10 μm.
Antibodies
Anti-laminin antibodies used are listed in Table 1. Rabbit anti-nidogen-1 was a gift of Rupert Timpl (Max-Planck-Institut für Biochemie, Martinsried, Germany). Function-blocking anti-integrin antibodies were obtained from Chemicon.
Results
To detect the presence of particular laminins in BrM, we analyzed the presence of heterotrimeric laminins and their component chains, using several antibodies (Table 1) on cryostat sections of human, rat, and bovine retinas. Laminin immunoreactivity nearly always does not survive aldehyde fixation; therefore, these immunohistochemical studies were performed on fresh-frozen cryostat sections.
Several examples of laminin immunoreactivity in bovine BrM are shown in Figure 1. Laminin immunoreactivity present at the basal surface included the previously demonstrated laminin 1 (α1β1γ1) as well as the individual chains of laminin 1: α1, β1, and γ1 (Fig. 1). The additional component chains (α5, β2) of laminin 10 (α5β1γ1) and 11 (α5β21) were also expressed in BrM (Fig. 1). Laminin chains including laminin β2 colocalized with the basement membrane component nidogen-1, demonstrating the presence of laminins in basement membranes (Fig. 1). Additional laminins are potentially present in BrM: laminin 5 and its component chains, including α3, and laminins, including laminins 13 (α3β2γ3) and 15 (α5β2γ3), that contain the laminin γ3 chain (Fig. 1). Similar results were obtained in human and rat BrM (not shown).
Figure 1.

BrM contained several potential laminins. Fluorescent images of bovine sections reacted with antibodies to laminins and laminin chains—in one case, doubly with anti-laminin β2 and an antibody to the basement membrane component, nidogen-1. Bottom right: nuclei of RPE cells counterstained with DAPI. BrM contained several laminin chains, consistent with the presence of multiple laminin trimers. bv, blood vessel; no 1°, no primary antibody.
To probe biochemically the presence of laminin chains in BrM, we performed protein transfer blots of ECM extracts from purified RPE/BrM. BrM was stripped from bovine neural retina, matrix fractions were isolated, run on denaturing gels, and transferred to blots. Blots were then probed with laminin chain-specific antibodies.
Several laminin chains, including α3, α5, β2, β3, γ1, γ2, and γ3, were present in matrix fractions of bovine RPE/BrM extracts (Fig. 2). Similar results were obtained in rat RPE/BrM extracts (not shown). These biochemical data, together with the immunohistochemical data presented earlier, are consistent with the presence of the heterotrimeric laminins 5 (α3β3γ2), 11 (α5β2γ1), 13 (α3β2γ3), and 15 (α5β2γ3) in BrM.
Figure 2.

Laminins were present in the RPE matrix. ECM extracts from bovine BrM were denatured, electrophoresed, transferred, and reacted with antibodies to laminin chains. Bruch's membrane contains several laminin chains, consistent with the presence of multiple laminin trimers.
Laminins present in the RPE basement membrane are probably secreted into the basement membrane from RPE cells themselves. To determine whether RPE cells produce laminins, we performed RT-PCR for laminin chains on RNA isolated from the human RPE cell line ARPE-19.31 As positive controls for laminin chains that did not amplify from some sources, we used tissues in which we knew those chains were expressed: for the chains of laminin 5, skin, and for other chains, Müller cells.18 As a source of purified Müller cell RNA, we used the rat Müller cell line RMC.32
Primers used were either already in our laboratory or were designed for regions of extensive homology among mouse, human, and rat (when available) sequences for individual chains. Degenerate primers were synthesized when necessary. Positive control experiments for laminin chains for which we had no product (e.g., α4 in ARPE-19) were run in parallel. Products were obtained for 8 (α1,3,5; β1,2,3; γ1,2) of the 11 laminin chains in ARPE-19 cells (Table 2). RNAs encoding the laminin α2, α4, and γ3 chains were not detectable in ARPE-19 cells, although they were present in the control tissues (Table 2): muscle and skin (α2), RMC cells (α4), and whole retina (γ3).
These data are consistent with the synthesis of at least laminins 1 (α1β1γ1), 5 (α3β3γ2), 10 (α5β1γ1), and 11 (α5β2γ1) by RPE cells. Therefore, RPE cells are competent to synthesize specific laminins potentially present in native BrM.
To determine whether ARPE-19 cells translate laminin RNAs into laminin proteins, we performed protein transfer blots on extracts of ARPE-19 cells. Cells were grown on uncoated plastic dishes for 3 to 6 days, during which they synthesized their own matrix, and then were scraped from the dishes. Extracts were made and run on denaturing gels, and transferred to blots. Blots were then probed with laminin chain-specific antibodies. Several laminin chains were present in ARPE-19 extracts, including α1, α3, α5, β1, β2, γ1, and γ2 (Fig. 3). ARPE-19 cells appeared to make both the α3A and α3B chains, and both chains were processed at both their N and C termini, at least by molecular weight (Fig. 3). ARPE-19 cells did not produce α2 or α4 chain protein (by protein transfer blot, not shown), consistent with the lack of α2 and α4 chain RNA. These biochemical data are consistent with the synthesis of laminins 1 (α1β1γ1), 5 (α3β3γ2), 10 (α5β1γ1), and 11 (α5β2γ1) by human RPE (ARPE-19) cells.
Figure 3.
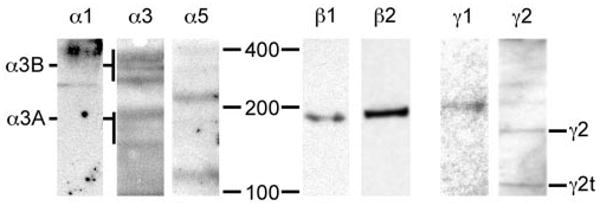
The human RPE cells produced laminin proteins. ECM extracts from ARPE-19 cells were denatured, electrophoresed, transferred, and reacted with antibodies to laminin chains. ARPE-19 matrix contained several laminin chains, consistent with the expression of multiple laminin trimers by RPE cells. The sizes of the α3A and α3B chains, and their processed forms, are shown (left). The truncated form of the γ2 chain (γ2t) is also noted.
To determine whether ARPE-19 cells assemble laminin heterotrimers and secrete them, we grew ARPE-19 cells on uncoated plastic and allowed them to synthesize their own ECM. After 3 to 6 days, cells grew to confluence and began to attain their differentiated hexagonal shape (Fig. 4). Cells were then examined for laminin chain expression. The presence of individual chains in the ECM necessarily reflects the presence of laminin heterotrimers, as secretion requires assembly of the trimer.
Figure 4.

The human RPE cells secreted laminins. ARPE-19 cells grown on uncoated glass coverslips were reacted with antibodies against individual laminin chains and an antiserum against laminin 5 The presence of flocculent extracellular immunoreactivity for laminin chains demonstrates the secretion of laminins by RPE cells. Bar, 20 μm.
Flocculent laminin immunoreactivity was present surrounding the ARPE-19 cells, consistent with secretion of laminins into ARPE-19 ECM. The laminin chains α3, α5, β1, β3, and γ2 and heterotrimeric laminin 5 are shown in Figure 4. We also tested the rat cell line RPE-J, and these cells also produced laminin RNAs and laminin proteins (not shown). These data (and data not shown) are consistent with the synthesis and secretion of several laminins, potentially including laminins 1 (α1β1γ1), 5 (α3β3γ2), 10 (α5β1γ1), and 11 (α5β2γ1) by RPE cells regardless of species.
Together, our immunohistochemical and biochemical data suggest that RPE cells make and secrete laminins of differing functional activities—in particular, laminins 1, 5, 10, and 11 — into BrM (Figs. 1-4). To determine whether RPE express receptors for particular laminins, we used a short-term in vitro binding assay to ascertain whether RPE cells bind to specific laminin isoforms: laminin 1 from mouse EHS tumor, affinity-purified laminin 5 from a squamous carcinoma cell line, and laminin 10/11 (contains a mixture of laminins 10 and 1133,34) from human placenta. ARPE-19 cells bound to laminins 1, 5, and 10/11 in a dose-dependent manner (Fig. 4). Under these conditions, RPE cells bound to laminins rapidly and avidly—preferentially to laminins rather than another component of BrM, collagen type IV (not shown). In addition, a dramatically higher number of cells bound to laminin 5 than to laminin-1 or -10/11 (Fig. 5). These data suggest that RPE cells preferentially bind to laminin 5.
Figure 5.

The RPE cells bound to laminins. Laminins 1, 5, and 10/11 were spotted onto plastic at increasing concentrations. ARPE-19 cells were allowed to bind, and adherent cells were fixed and counted. RPE cells bound saturably to all three substrates, but more cells bound to laminin 5 than to laminin 1 or 10/11. Data are from results ± SD of two independent experiments.
Binding of cells to laminins is often mediated by integrins. For some cell types, the particular integrins used as receptors for specific laminins has been elucidated. We began to investigate the interactions of RPE cells with laminins by asking whether the binding of ARPE-19 cells to laminins could be inhibited by antibodies to integrins expressed by RPE cells. RPE cells express the integrin subunits α3, α6, and β1 on their basal surface, apposed to the laminin-containing RPE basement membrane.15–17
ARPE-19 cell binding to laminin 1 was markedly inhibited by antibodies against the α6 and β1 subunits, whereas anti-α3 did not inhibit binding (Fig. 6). These data demonstrate that RPE cells, like other cells, bind to laminin 1 via α6β1 and not α3β1.
Figure 6.
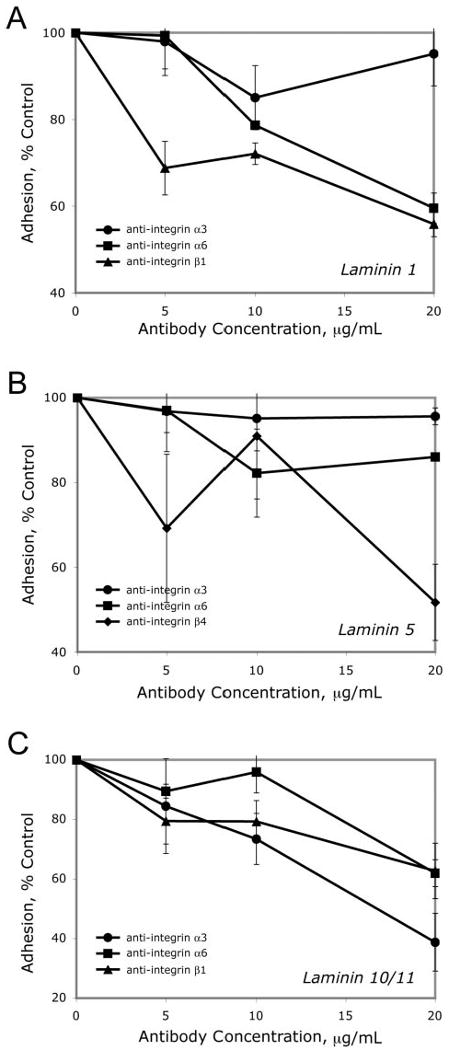
RPE binding to laminins 1, 5, and 10/11 was blocked by specific anti-integrins. ARPE-19 cells bound to laminins (10 μg/mL); integrin antibodies modulate binding. Cells were allowed to adhere for 60 minutes in the presence of increasing concentrations of anti-integrin antibodies and then were rinsed, fixed, and counted. Data shown are results ± SEM of at least three independent experiments.
ARPE-19 cell-binding to laminin 5 was partially inhibited by antibodies against the α6 subunit and markedly by anti-β4, whereas anti-α3 did not inhibit binding (Fig. 6). These data demonstrate that RPE cells, like other cells, bind to laminin 5 via the laminin 5 receptor, α6β4.
Finally, ARPE-19 cell binding to laminin 10/11 was partially inhibited by antibodies against α3 and α6 subunits and markedly by anti-β1 (Fig. 6). These data demonstrate that RPE cells, like other cells, bind to laminin 10/11 via α3β1 and α6β1.
RPE cells express other integrins in vivo: α5β1 (fibronectin receptors) on the basal surface,16,17 and αvβ5 (vitronectin receptors) on the apical surface.16 As expected, given the apical location of αvβ5 in vivo, where there are no basement membrane laminins, antibodies against αv did not inhibit binding to the basement membrane components laminins 1, 5, or 10/11 (not shown).
Epithelial cells respond to different laminins in two seemingly opposite ways: adhesion and migration. As a first step in determining the functional activity of individual laminins for RPE cells, we examined the morphology of RPE cells on different laminin isoforms. Laminins 1, 5, and 10/11—all potential components of BrM—were adhesive for ARPE-19 cells (Figs. 5,6). However, the morphology of the cells was remarkably different on the three substrates, even at short times of adherence (60 minutes; Fig. 7). Laminin 5 promoted the most epithelial phenotype; that is, cells were flattest on laminin 5, whereas they were rounded and had the appearance of migrating cells on laminin 10/11 (Fig. 7). Thus, we propose that laminin 5 preserves the stability of the mature RPE by promoting an adherent state.
Discussion
The data presented herein demonstrate that, in our study, RPE cells produced specific laminins including laminins 1, 5, and 10/11. RPE cells bound to these laminins using an integrin-mediated mechanism. Adhesion of RPE cells to laminins 1 and 10/11 is robust and mediated, in part, by integrin receptors for these laminins used by other epithelial cells. In addition, RPE cells synthesize and preferentially bind to laminin 5, a laminin previously thought to be largely associated with the dermis. In the skin, laminin 5 is a critical mediator of attachment. We propose that interactions between integrins in the RPE and laminins including laminin 5 in the RPE-BM are vital for RPE stability.
In other cell types, the highest affinity integrin receptor for laminin 1 is α6β1, and α3β1 is not a receptor for laminin 1.35 The best-characterized receptor for laminin 5 is α6β4,36 and laminin 10/11 binds to many integrins, including α3β1 and α6β1.34 Our data suggest that RPE cells use all these mechanisms as well.
Some of the retinal degenerative diseases (RDDs) are produced by mutations in proteins intrinsic to the photoreceptors and RPE cells. For example, several genetic RDDs are the result of mutations in the signal transduction, structural, or metabolic components of the photoreceptor, including those that cause autosomal dominant retinitis pigmentosa (e.g., mutations in rhodopsin37 and in peripherin38), Stargardt disease (mutations in ABCA439), and some forms of Leber congenital amaurosis (e.g., mutations in CRX40,41). Other RDDs result from mutations in genes of the RPE, including Best disease (mutations in VMD2 [bestrophin]42) and some forms of Leber congenital amaurosis (e.g., mutations in RPE6543). In addition, some RDDs are produced by mutations that affect the structure and function of BrM. These include Doyne's honeycomb retinal dystrophy (mutation in the ECM component, fibulin-3, also known as EFEMP144,45) and Sorsby fundus dystrophy (mutation in the matrix metalloproteinase inhibitor, TIMP-346). Thus, disruptions in the structure and function of BrM can lead to RDD.
Other mutations correlate with predisposition for age-related macular degeneration (AMD). The most recently characterized of these are in complement factor H and point to an inflammatory component as a resolute (and perhaps necessary) feature in the multifactorial AMD.47–49 However, mutations in several ECM components also correlate with a predisposition to AMD; these include mutations in the ECM components fibulin-550 and -6, also known as hemicentin.51,52
In addition, there are ocular defects associated with mutations in several other ECM-related components, including a gross ocular defect in Marfan syndrome (mutation in the ECM component, fibrillin-153) and muscle-eye-brain disease, in which there is defective glycosylation in the ECM receptor, β-dystroglycan.54 Deficiencies in several laminin chains also lead to ocular defects, including a recently characterized mutation in the laminin β2 chain.55
Together, these observations point to an intimate relationship between changes in BrM and RPE function. Diminishing of this function can lead to RDD, and changes in BrM are linked to predisposition to AMD. Alterations in the basic biological interplay between the RPE and BrM can affect the health of the retina; in humans, these changes can be particularly disturbing in the foveal and parafoveal areas because of our reliance on these regions for high-acuity vision.
Defining the function of each integrin and laminin present in RPE cells and BrM is critical in developing a model for stability of the RPE based on interactions between integrins and laminins at the RPE-BrM interface. The composition must maintain RPE quiescence, adherence, polarity, and health, yet be capable of supporting proliferation after damage and migration into a wound. Our data predict that laminins including 1, 5, and 10/11 and integrins including α3β1, α6β1, and α6β4 are differential players in these phenomena.
Acknowledgments
Supported by the Köln Fortune Program (SA), National Institute of Neurological Disorders and Stroke Grant R01-NS39502 (WJB, DDH), and National Eye Institute Grants R01-EY12676 (WJB, DDH) and P30-EY13078.
Footnotes
Disclosure: S. Aisenbrey, None; M. Zhang, None; D. Bacher, None; J. Yee, None; W.J. Brunken, P; D.D. Hunter, P
References
- 1.Libby RT, Hunter DD, Brunken WJ. The roles of the extracellular matrix in retinal development and maintenance. In: Fini E, editor. Vertebrate Eye Development. Berlin: Springer-Verlag; 2000. pp. 115–140. [DOI] [PubMed] [Google Scholar]
- 2.Tunggal P, Smyth N, Paulsson M, Ott MC. Laminins: structure and genetic regulation. Microsc Res Tech. 2000;51:214–227. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Turksen K, Aubin JE, Sodek J, Kalnins VI. Localization of laminin, type IV collagen, fibronectin, and heparan sulfate proteoglycan in chick retinal pigment epithelium basement membrane during embryonic development. J Histochem Cytochem. 1985;33:665–671. doi: 10.1177/33.7.3159787. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA, Jerdon JA, Glaser BM. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci. 1986;27:1615–1621. [PubMed] [Google Scholar]
- 6.Das A, Frank RN, Zhang NL, Turczyn TJ. Ultrastructural localization of extracellular matrix components in human retinal vessels and Bruch's membrane. Arch Ophthalmol. 1990;108:421–429. doi: 10.1001/archopht.1990.01070050119045. [DOI] [PubMed] [Google Scholar]
- 7.Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol. 2004;137:504–510. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 9.Burgeson RE, Chiquet M, Deutzmann R, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 10.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Libby RT, Champliaud MF, Claudepierre T, et al. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aumailley M, El Khal A, Knoss N, Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biol. 2003;22:49–54. doi: 10.1016/s0945-053x(03)00013-1. [DOI] [PubMed] [Google Scholar]
- 13.Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol. 1999;11:602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 14.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 15.Rizzolo LJ, Zhou S, Li ZQ. The neural retina maintains integrins in the apical membrane of the RPE early in development. Invest Ophthalmol Vis Sci. 1994;35:2567–2576. [PubMed] [Google Scholar]
- 16.Anderson DH, Johnson LV, Hageman GS. Vitronectin receptor expression and distribution at the photoreceptor-retinal pigment epithelial interface. J Comp Neurol. 1995;360:1–16. doi: 10.1002/cne.903600102. [DOI] [PubMed] [Google Scholar]
- 17.Proulx S, Guérin SL, Salesse C. Effect of quiescence on integrin α5β1 expression in human retinal pigment epithelium. Mol Vis. 2003;9:473–481. [PubMed] [Google Scholar]
- 18.Ho TC, Del Priore LV. Reattachment of cultured human retinal pigment epithelium to extracellular matrix and human Bruch's membrane. Invest Ophthalmol Vis Sci. 1997;38:1110–1118. [PubMed] [Google Scholar]
- 19.Hergott GJ, Nagai H, Kalnins VI. Inhibition of retinal pigment epithelial cell migration and proliferation with monoclonal antibodies against the beta 1 integrin subunit during wound healing in organ culture. Invest Ophthalmol Vis Sci. 1993;34:2761–2768. [PubMed] [Google Scholar]
- 20.Libby RT, Hunter DD, Brunken WJ. Developmental expression of laminin β2 in rat retina: further support for a role in rod morphogenesis. Invest Ophthalmol Vis Sci. 1996;37:1651–1661. [PubMed] [Google Scholar]
- 21.Libby RT, Xu Y, Selfors LM, Brunken WJ, Hunter DD. Identification of the cellular source of laminin beta2 in adult and developing vertebrate retinae. J Comp Neurol. 1997;389:655–667. doi: 10.1002/(sici)1096-9861(19971229)389:4<655::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miner JH, Patton BL, Lentz SI, et al. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of 1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel 3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engvall E, Davis GE, Dickerson K, Ruoslahti E, Varon S, Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986;103:2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiger CF, Champliaud MF, Pedrosa-Domellof F, Thornell LE, Ekblom P, Gullberg D. Presence of laminin 5 chain and lack of laminin 1 chain during human muscle development and in muscular dystrophies. J Biol Chem. 1997;272:28590–28595. doi: 10.1074/jbc.272.45.28590. [DOI] [PubMed] [Google Scholar]
- 26.Sorokin LM, Maley M, Moch H, et al. Laminin alpha4 and integrin alpha6 are upregulated in regenerating dy/dy skeletal muscle: comparative expression of laminin and integrin isoforms in muscles regenerating after crush injury. Exp Cell Res. 2000;256:500–514. doi: 10.1006/excr.2000.4842. [DOI] [PubMed] [Google Scholar]
- 27.Sanes JR, Chiu AY. The basal lamina of the neuromuscular junction. Cold Spring Harb Symp Quant Biol. 1983;48:667–678. doi: 10.1101/sqb.1983.048.01.070. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- 29.Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990;111:1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci. 1993;104:37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 32.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- 33.Ferletta M, Ekblom P. Identification of laminin-10/11 as a strong cell adhesive complex for a normal and a malignant human epithelial cell line. J Cell Sci. 1999;112:1–10. doi: 10.1242/jcs.112.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113:869–876. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 35.Delwel GO, de Melker AA, Hogervorst F, et al. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- 37.Sung CH, Davenport CM, Nathans J. Rhodopsin mutations responsible for autosomal dominant retinitis pigmentosa: clustering of functional classes along the polypeptide chain. J Biol Chem. 1993;268:26645–26649. [PubMed] [Google Scholar]
- 38.Keen TJ, Inglehearn CF. Mutations and polymorphisms in the human peripherin-RDS gene and their involvement in inherited retinal degeneration. Hum Mutat. 1996;8:297–303. doi: 10.1002/(SICI)1098-1004(1996)8:4<297::AID-HUMU1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 40.Freund CL, Gregory-Evans CY, Furukawa T, et al. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997;91:543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 41.Swain PK, Chen S, Wang QL, et al. Mutations in the cone-rod homeobox gene are associated with the cone-rod dystrophy photoreceptor degeneration. Neuron. 1997;19:1329–1336. doi: 10.1016/s0896-6273(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 42.Petrukhin K, Koisti MJ, Bakall B, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19:241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- 43.Gu S, Thompson DA, Srikumari CRS, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 44.Stone EM, Lotery AJ, Munier FL, et al. A single EFEMP1 mutation associated with both malattia leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- 45.Marmorstein LY, Munier FL, Arsenijevic Y, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in malattia leventinese and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:13067–13072. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber BHF, Vogt G, Pruett RC, Stohr H, Felbor U. Mutations in the tissue inhibitor metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994;8:352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- 47.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 48.Haines JL, Hauser MA, Schmid S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 49.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone EM, Braun TA, Russell SR, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. New Engl J Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 51.Klein ML, Schultz DW, Edwards A, et al. Age-related macular degeneration: clinical features in a large family and linkage to chromosome 1q. Arch Ophthalmol. 1998;116:1082–1088. doi: 10.1001/archopht.116.8.1082. [DOI] [PubMed] [Google Scholar]
- 52.Schultz DW, Klein ML, Humpert AJ, et al. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet. 2003;12:3315–3323. doi: 10.1093/hmg/ddg348. [DOI] [PubMed] [Google Scholar]
- 53.Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 54.Michele DE, Barresi R, Kanagawa M, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 55.Zenker M, Aigner T, Wendler O, et al. Human laminin beta-2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]


