Abstract
Neuronal precursors generated in the subventricular zone (SVZ) migrate through the rostral migratory stream (RMS) to the olfactory bulb (OB). Although, the mechanisms regulating this migration remain largely unknown, studies have suggested that molecular factors, such as Brain-Derived Neurotrophic Factor (BDNF) emanating from the OB, may function as chemoattractants drawing neuroblasts toward their target. To better understand the role of BDNF in RMS migration, we used an acute slice preparation from early postnatal mice to track the tangential migration of GAD65-GFP labeled RMS neuroblasts with confocal time-lapse imaging. By quantifying the cell dynamics using specific directional and motility criteria, our results showed that removal of the OB did not alter the overall directional trajectory of neuroblasts, but did reduce their motility. This suggested that additional guidance factors may be present locally within the RMS. Thus, we next demonstrated that BDNF and its high affinity receptor, TrkB, are indeed heterogeneously expressed within the RMS at postnatal day 7, and by altering BDNF levels within the entire pathway, showed that reduced BDNF signaling changes both neuroblast motility and direction, while increased BDNF levels changes only motility. Together these data reveal that during this early postnatal period BDNF plays a complex role in regulating both the motility and direction of RMS flow, and that it arises from within the RMS itself, as well as from the olfactory bulb.
Introduction
The migration of RMS neurons from the SVZ to the OB continues throughout life (Altman, 1969, Luskin, 1993, Lois and Alvarez-Buylla, 1994, Wichterle et al., 2001), but the elements controlling this process are not fully understood. Studies have shown that the vascular network within the RMS may act as a source of molecular factors (Leventhal et al., 1999, Snapyan et al., 2009), and as a scaffold for migration (Whitman et al., 2009). Many molecular signals influencing RMS migration have been identified, including slits (Wu et al., 1999, Nguyen-Ba-Charvet et al., 2004), semaphorins (Ito et al., 2008, Melendez-Herrera et al., 2008), netrins (Hamasaki et al., 2001, Murase and Horwitz, 2002), ephrins (Conover et al., 2000), and growth factors (Paratcha et al., 2006, Chiaramello et al., 2007, Garzotto et al., 2008). However, it is not clear where all these signals originate, how they relate to one another, or whether they distinctly modulate direction or motility. Recent studies demonstrated that molecules, such as the inhibitory neurotransmitter GABA (Bolteus and Bordey, 2004), metalloproteases (Bovetti et al., 2007), and the adhesion molecule PSA-NCAM (Tomasiewicz et al., 1993, Chazal et al., 2000), can indeed alter neuroblast motility without affecting the direction of migration. These findings suggest that separate mechanisms may be used to control different aspects of the migration process.
One candidate molecule implicated in regulating migration through the RMS is Brain-Derived Neurotrophic Factor (BDNF), which is present in the OB from late embryonic periods through adulthood (Maisonpierre et al., 1990). BDNF is a pleiotropic signaling molecule involved in the survival, proliferation and differention of many neural cells types (Ernfors et al., 1994, Jones et al., 1994, Lindsay, 1996). Studies using explant culture assays have also shown that BDNF acts as a chemoattractant for RMS neuroblasts derived from early postnatal tissue (Chiaramello et al., 2007). Together, these findings have suggested that BDNF secreted by the OB may provide a targeting signal for migrating neuroblasts. Interestingly, studies in adult mice have also shown that RMS neuroblasts can migrate in the absence of the OB (Kirschenbaum et al., 1999), and that BDNF is present within the RMS itself (Bath et al., 2008, Galvao et al., 2008, Snapyan et al., 2009) These findings raise questions about the role of the OB as a source of migration factors, the specific effect BDNF exerts on migrating cells, and the function of BDNF arising within the RMS.
Our study examines the role BDNF plays in regulating RMS neuroblast migration during the early postnatal period using time-lapse imaging to track and quantify distinct aspects of neuroblast motility. Our results show that removal of the OB does not alter the direction of migrating cells but does reduce neuroblast motility. By contrast, altering BDNF concentration or disrupting its signaling can affect both the direction and motility of migrating neuroblasts. Our data indicate that BDNF signaling likely occurs through the TrkB receptor, which we find is expressed within the RMS in a non-homogeneous pattern similar to BDNF. In addition, we show that reducing BDNF levels affects motility in a similar fashion to inhibition of TrkB activity.
Materials and Methods
Animals
Wildtype C57BL/6 mice were used for in vivo injections. Time-lapse imaging used GAD65-GFP transgenic mice which express green fluorescent protein (GFP) under the control of the glutamic acid decarboxylase 65kDa promoter (GAD65) as described previously (Lopez-Bendito et al., 2004). All procedures conformed to National Institute of Neurological Disorders and Stroke’s Animal Care and Use Committee guidelines.
In vivo Injections
Wildtype mice, 7-9 days old, were initially anesthetized with an intraperitoneal injection of ketamine (0.04 mg/kg) /xylazene (0.0008 mg/kg) mixture for craniotomy, and then maintained with isofluorane (1-2%) during injection. Mice were then recovered in a heated incubator for 4-5 hours before being sacrificed and processed for histology. Dextran-tetramethylrhodamine (TMR) 3000 molecular weight (Invitrogen, CA) was loaded into a quartz micropipette (10 μm tip diameter) and injected using iontophoresis (+10μA, 200 ms duration, 0.4Hz, 120 pulses) into the RMS at stereotaxic coordinates 0.8mm lateral to midline, 1.5 mm anterior to bregma, and depth 2.5 mm.
Acute Brain Slice Experiments
Transgenic GAD65-GFP mice, 6-8 days old, were decapitated, and their brains were dissected in ice cold artificial cerebral spinal fluid (ACSF) containing (in mM): NaCl 124, KCl 3, MgSO4 1.3, CaCl2 2, NaHCO3 26, NaH2PO4 1.25, dextrose 10, while oxygenated with 95% O2 5% CO2. Tissue was then sectioned sagittally (300 μm thick) using a Vibratome (3000, Vibratome, MO) in ice cold oxygenated ACSF. Fluorescent sections containing an intact SVZ-RMS-OB pathway were selected and maintained in oxygenated ACSF at RT for no longer than 30 minutes until imaged. Removal of the OB was performed on acute slice sections bathed in ice-cold, oxygenated ACSF, under a dissecting microscope (Leica, IL) using a scalpel. Signaling pathways were perturbed using bath application of either BDNF-IgG (10 μg/mL, Millipore, MA), BDNF (100 ng/mL, R&D, MN), or k-252a (2 μM, Tocris, MO). In all experiments, the sections were incubated with these solutions for 1 hour prior to image acquisition.
Immunohistochemistry
All animals were sacrificed with an overdose intraperitoneal injection of ketamine (0.25 mg/kg), and perfused intracardially with cold 1X PBS followed by 4% paraformaldehyde (PFA) in PBS. Dissected brains were post-fixed in 4% PFA, followed by cryoprotection in 30% sucrose overnight at 4°C. Tissue was sectioned on a freezing microtome (SM 2000R, Leica, IL) at 40μm. Antibody staining was carried out as described previously (Marks et al., 2006) using the following primary antibodies: rabbit anti-BDNF, 1:1000 (Novus, CO); rabbit anti-TrkB, 1:1000 (Millipore, MA); or rabbit anti-p75NTR, 1:1000 (Millipore, MA). Secondary and tertiary antibodies included biotin conjugated donkey anti-rabbit, 1.75 mg/mL, and Cy3 conjugated streptavidin, 1.75 mg/mL (Jackson Immunoresearch, PA) which were applied at RT for 2 hours. Sections were then rinsed in PBS and mounted on Superfrost slides (VWR, PA) using Vectashield-DAPI mounting medium (Vector Labs, CA).
In situ Hybridization
Procedure was carried out as described in (Schaeren-Wiemers and Gerfin-Moser, 1993). Briefly, brains from P7 mice were dissected and immediately frozen in O.C.T. Compound (Sakura, Ca). Fresh frozen sections (16μm) were cut on a cryostat then fixed in 4% PFA for 20 minutes and probed for BDNF mRNA using a digoxigenin (Roche, In) labeled riboprobe that was generated from a portion of the mouse BDNF coding sequence (1-588 bp). Slides were hybridized at 65°C; washed at 68°C. Signal was detected following overnight incubation at 4°C with anti-digoxygenin antibody (Roche, In.) and NBT/BCIP (Promega, Wi) solutions at room temperature.
Imaging fixed sections
Slides containing sections either labeled with antibody or injected with rhodamine were imaged at low and high magnification. Low magnification fluorescent images were collected on an Axiovert 200 microscope with a halogen lamp (Carl Zeiss, NY) using a 10x (NA=0.5) objective, Cy3 (570 nm) or FITC (520 nm) emission filters, and a Qcolor 5 camera (Olympus, PA). High magnification images were collected with an LSM 510 Axioskop 2 confocal microscope (Carl Zeiss, NY) using a 40x (NA=1.3) or 63x (NA=1.4) oil immersion lens, and either an argon (488nm) laser to excite GFP or a HeNe (543 nm) laser to excite Cy3 or Rhodamine. Confocal z-stacks (1 μm z-step) were collected in the RMS, midway between the SVZ and the OB, and co-localization of GFP and immunofluorescence was confirmed in three dimensions (although presented here as representative single slices).
In-vivo Time-Lapse Imaging
Acute brain slices were loaded into a heated perfusion chamber (RC22c, Warner, CT), maintained at 32°C and secured with a string weight, while being constantly perfused (approximately 0.5mL/min) with oxygenated ACSF. After a 1 hr pre-incubation period in the chamber, the central portion of the RMS midway between the SVZ and OB was imaged on a confocal microscope using a 488nm Argon laser to excite GFP fluorescence, and a 20x water immersion lens (NA=0.5, Zeiss, NY). A 40μm stack (6 z-slices) was collected every minute for 3 hours. Slices were also rotated 180° to control for direction of perfusion flow, which revealed no discernable difference.
Image Processing Cell Tracking and Analysis
Each image z-stack was flattened (Zeiss software) to create a tiff image sequence of 180 frames, which was transferred to MATLAB (Mathworks, MA) and filtered using noisecomp (Kovesi, 2000). Single migrating cells were automatically tracked using ImarisTrack module (Bitplane, MN). Non-migrating cells outside the RMS were identified by unchanging morphology and used as an internal reference point to correct for tissue motion artifacts (‘Drift Correction’ function). The soma of migrating neurons in the RMS, were tracked by assigning an x-y position at each time point based upon a unique track reference number. Tracks with a displacement greater than two standard deviations above the combined mean for all tracked cells in a given experiment were then selected for further analysis to reduce the number of non-migrating cells in our population. All tracks from 4 experiments within each experimental condition were then pooled and analyzed with custom MATLAB programs. For each track, displacement was determined by measuring the distance from the first position to the final position. Direction was then determined by classifying the displacement vector as either anterior (rostral toward the OB) or posterior (caudal toward the SVZ). Track length was calculated as the summation of distances traveled between each imaging time point. Average velocity was calculated by dividing the total track length of each cell by its total duration of imaging. Polar plots representing each track as a vector were created for each individual track by normalizing the final x,y-coordinate position by initial x,y-coordinate position. The overall track direction is represented by the angular coordinates relative to initial position, and the magnitude of displacement is represented by the radial coordinates of the plot.
Statistics
Individual tracks representing the migratory path of one cell were pooled from four different experiments, and grouped by experimental condition (control, OB removed, +BDNF, +k252a, +BDNF-IgG). Direction was classified as either Anterior or Posterior and assigned a numerical value (Anterior=2, Posterior=1). Each experimental group was then compared to the control group using the Mann-Whitney-Wilcoxon rank sum test to determine significant differences in direction. For easier comparison, direction data is presented as color coded bar graphs showing percent of total cells exhibiting anterior (green) or posterior (red) directions. Motility measures of two sub-populations of cells (anterior and posterior migrating cells) within control group animals were compared to each other using Student t-test. For motility measures between different experimental groups (control, OB-removed, BDNF, BDNF-IgG, and k252a) a one-way ANOVA was performed between all groups followed by a post-hoc Bonferroni/Dunn test to determine which differences were significant compared to control.
Results
RMS neuroblasts migrate bidirectionally in vivo
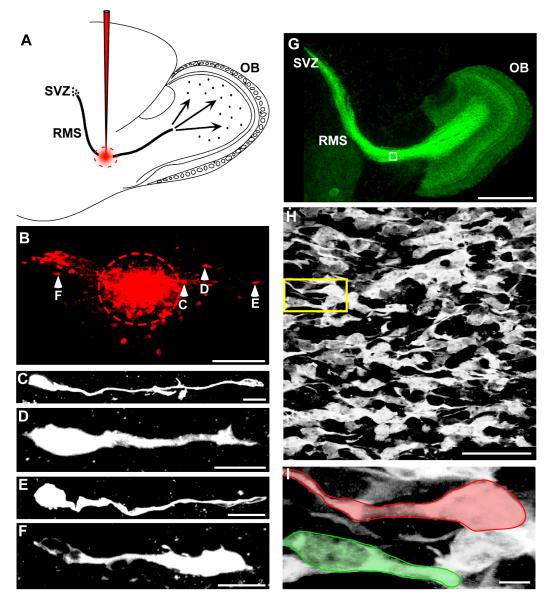
While RMS neuroblasts generally migrate anteriorly from the SVZ to the OB (Altman, 1969, Luskin, 1993, Lois and Alvarez-Buylla, 1994, Wichterle et al., 2001), some in vitro studies have observed neuroblasts in adult mice moving in a posterior direction (Anton et al., 2004, Nam et al., 2007, Zhao and Nam, 2007), and one in vivo study also in adult mice used viral labeling to show that RMS neuroblasts can be found oriented in many directions (Sawamoto et al., 2006). Thus, we sought to determine if RMS neuroblasts from early postnatal mice exhibit bidirectional migration in vivo. To address this, we performed in vivo tracer injections targeting dextran-rhodamine directly into the RMS of P6-P8 wildtype mice (Figure 1A). Consistent with previous in vitro results, we found rhodamine labeled cells located both anterior to and posterior to the injection site (Figure 1B) demonstrating migration in both directions. Higher magnification images also revealed cellular morphology consisting of elongated somas extending a single, long-leading process indicative of migrating neuroblasts (Figure 1C-F) (Rakic, 1971) with some cells extended in an anterior direction and others in a posterior direction. Together these data clearly indicate that RMS neuroblasts migrate bidirectionally in vivo.
Figure 1. RMS neuroblasts migrate bidirectionally in vivo and in GAD65-GFP mice.
(A) Sagittal schematic view of an in vivo dextran-rhodamine injection site, shown in red, targeted to the RMS. (B) Fluorescent image of a histological section 4 hours after RMS injection, showing cells (arrowheads) having moved both anterior and posterior to the injection site (dashed circle). (C-F) High magnification view of cells in B, showing a leading process in both anteriorly migrating cells (C-E), and a posteriorly migrating cell (F). (G) Sagittal fluorescent image showing GAD65-GFP expression throughout the entire SVZ-OB migratory pathway. (H) High magnification of boxed region in G showing RMS neuroblasts. (I) Higher magnification of yellow boxed region in H showing a pair of migrating cells outlined by colored boundaries tracing both the anterior (green) and posterior (red) leading processes. Scale bars: B, 150μm; C-F, 10μm; G, 1mm; H, 40μm; I, 5μm.
Time-lapse imaging of RMS neurons reveals complex migratory behavior
To assess RMS migration with both quantitative and qualitative measures, we performed confocal time-lapse imaging on acute brain slices prepared from P6-P8 GAD65-GFP mice, in which ~35% of the RMS neuroblasts are labeled with GFP (De Marchis et al., 2004b). This enabled us to simultaneously track the movement of an entire population of fluorescently labeled RMS neuroblasts. High magnification views of these GFP-labeled RMS neuroblasts in histological sections revealed a migratory morphology similar to the in vivo injections, with leading processes extending in both anterior and posterior directions (Figure 1H, I) suggesting that they also migrate bidirectionally. Utilizing these mice, we focused our experiments on a central portion of the RMS immediately caudal to the ascending limb entering the OB and collected fluorescent images as 3D stacks every minute for three hours.
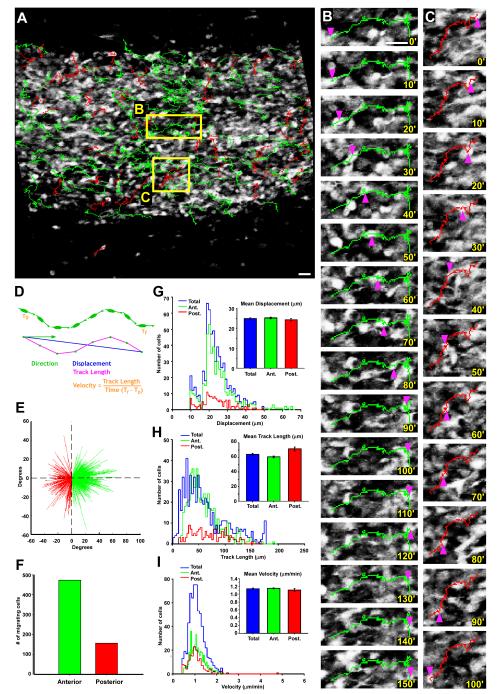
We imaged 4538 individual GAD65-GFP positive neuroblasts, of which 629 were actively migrating, and analyzed them based upon four measures: direction, displacement, track length, and velocity. We first observed that most migration paths contained brief pauses, consistent with a saltatory motion pattern (Figure 2A-C, and Supplemental Movies). As a population, we found that a larger proportion of cells migrates in an anterior direction (mean 74.71% ± 4.37; S.E.) in each slice (n=4) than in a posterior direction (mean 25.29%± 4.37; S.E.; Figure 2E, F), without a discernable relationship between the location of a migrating cell and the direction of its migration (Figure 2A). These proportions are similar, but slightly higher than a previously published report showing that 19 % of virally labeled RMS cells in histological sections were oriented away from the bulb (Sawamoto et al., 2006). This variation may be due to the different methods used to label RMS neuroblasts.
Figure 2. Dynamic imaging shows broad bidirectional migration in the RMS neuroblast population.
(A) Image of the RMS from an acute slice with overlaid tracks showing cells moving in an anterior (green) or posterior (red) direction. (B-C) Close-up views of the boxed regions in A, showing the time-lapse sequence and tracks of single cells moving in an anterior direction (B) and a posterior direction (C). Pink arrowheads point to the location of the cell bodies in each panel, with the time (minutes) indicated in the lower right corner. (D) Schematic representation of the method used to quantify the mobility characteristics of each cell. (E-I) Time lapse analysis of 629 migrating GAD65-GFP neuroblasts (n=4 experiments). (E) Vector plot showing the displacement and direction of each neuroblast plotted from a common origin (initial position). Green vectors represent neuroblasts with a net anterior displacement. Red vectors represent neuroblasts with a net posterior displacement. (F) Bar graph of migrating cells shown in E, presenting a total of 473 RMS neuroblasts moving anteriorly (75.20% of total cells) and 156 moving posteriorly (24.80% of total cells). (G-I) Distribution plots of motility measures for displacement (G), track length (H) and velocity (I) of migrating neuroblasts showing anterior (green), posterior (red), and combined total migrating neuroblasts (blue). Comparisons between anterior and posterior sub-populations reveal no significant differences in average motility characteristics using Student t-test (inset bar graphs). Scale bar: 10μm.
With regard to motility, GAD65-GFP neuroblasts showed a mean displacement distance of 25.04μm ± 0.36, S.E., traveled a mean total track length of 63.35μm ± 1.22, S.E., and migrated with a mean velocity of 1.14μm/min ± 0.015, S.E. These motility results are similar to previously reported findings from migratory studies of various RMS neuroblast subtypes (De Marchis et al., 2001, Anton et al., 2004, Bolteus and Bordey, 2004, Nam et al., 2007, Zhao and Nam, 2007), indicating that the migration GAD65-GFP expressing RMS neuroblasts is representative of the neuroblast population as a whole. Furthermore, analysis of anterior and posterior migrating cells as separate groups revealed no significant differences between these two sub-populations when compared to each other in either displacement, track length, or velocity measures (Figure 2G-I).
Removal of the OB alters motility but not direction of migrating neuroblasts
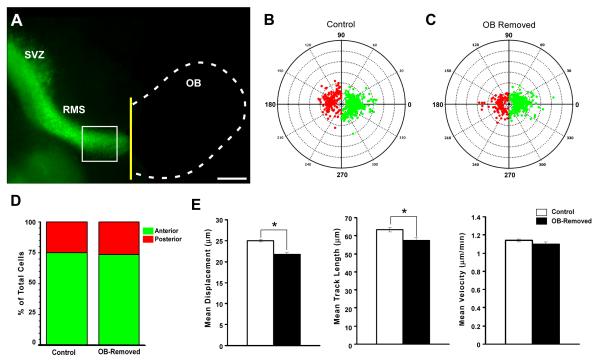
Experiments using in vitro explant assays demonstrated that the OB is an important source of factors, such as BDNF, which function as chemoattractants for SVZ and RMS neuroblasts (Liu and Rao, 2003, Chiaramello et al., 2007). By contrast, other studies using BrdU showed that SVZ neuroblasts continue to proliferate and migrate in mice even after excision of the olfactory bulbs (Jankovski et al., 1998, Kirschenbaum et al., 1999). To determine the role that the olfactory bulb plays in RMS migration, we performed time-lapse imaging experiments in acute slices after removal of the OB. Our results showed that in the absence of the OB, no significant change occured in the proportion of neuroblasts migrating in an anterior direction (73.25% of total cell, n=486 cells from 4 experiments; p=0.568) compared with controls (75.20% of total cells, n=629 cells from 4 experiments) (Figure 3B-D). Interestingly, while direction was unaffected by removing the OB, motility was clearly reduced such that both the average neuroblast displacement (21.81 ± 0.42 μm, S.E.; n=486 cells from 4 experiments) and average track length (57.31± 0.42 μm, S.E; n=486 cells from 4 experiments) were significantly reduced compared to controls (mean displacement, 25.04 ± 0.36 μm, S.E. p<0.0001; mean track length, 63.35 ± 1.22 μm, S.E., n=629 cells from 4 experiments, p<0.01) with mean velocity slightly decreased (OB-removed; 1.1 ± 0.023 μm/min, S.E. n=486 cells from 4 experiments; control; 1.14 ± 0.015 μm/min, S.E. n=629 cells from 4 experiments, p=0.141) (Figure 3E). These data suggest that substances within the OB, such as BDNF, (Supplemental Figures 1 and 2) may be secreted into the RMS and affect neuroblast migration. However, since directional control was not affected by OB removal, there appears to be another source for the factors that regulate this aspect of migration.
Figure 3. Removal of the olfactory bulb alters cell motility but not direction of migration.
(A) Fluorescent sagittal image of an acute brain slice from a GAD65-GFP mouse in which the OB (dotted line) has been removed (yellow line) before time-lapse imaging. (B, C) Polar plots illustrating the final position of each tracked cell with respect to its initial starting position plotted from a common origin (center). Cells migrating in an anterior direction are plotted in green and those migrating in a posterior direction are plotted in red. The control condition is shown in (B) (n=4 experiments; 629 cells tracked), and following OB removal (n=4 experiments; 486 cells tracked) is shown in (C). (D) Graph summarizing the population data from B and C, revealing no significant difference between the two groups (control, 75.20% of cells migrated anteriorly; 24,80% migrated posteriorly, n=629 from 4 experiments; OB removed, 73.25% of cells migrated anteriorly, 26.75% of cells moved posteriorly; p=0.568 using the Mann-Whitney-Wilcoxon rank sum test.). (E) Graphs showing mean motility measures of cells in control (white bars) and OB-removed (black bars) groups, revealing a significant reduction in displacement (Left graph: control, 25.04 ± 0.36 μm, S.E.; OB-removed, 21.81 ± 0.42 μm, S.E.; n=486 cells from 4 experiments; p<0.0001), and Track Length (Middle graph: control, 63.35 ± 1.22 μm, S.E., n=629 cells from 4 experiments; OB-removed, 57.31± 0.42 μm, S.E; n=486 cells from 4 experiments; p<0.01) but not Velocity (Right graph: control; 1.14 ± 0.015 μm/min, S.E. n=629 cells from 4 experiments; OB-removed, 1.1 ± 0.023 μm/min, S.E. n=486 cells from 4 experiments; p=0.141) when the OB is removed. Significance for motility measures determined using ANOVA followed by Bonferroni/Dunn post-hoc test. Scale bar: 500 μm
Patterned expression of BDNF, TrkB and p75NTR within the RMS region
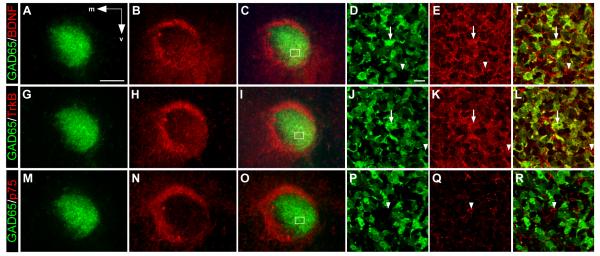
Since removal of the OB only partially altered RMS migration, we explored the possibility that a more proximal source of guidance cues may also be involved. Previous work has indicated that BDNF, its high affinity receptor, TrkB, and its low affinity receptor, p75NTR, are all present within the RMS region (Bath et al., 2008, Snapyan et al., 2009). However, the precise distribution of these different components has not been defined. Recent studies have also indicated that neuroblasts within the RMS are heterogeneous, (Merkle et al., 2007, Nam et al., 2007, Young et al., 2007, Lledo et al., 2008), suggesting that the molecular factors guiding them may also be present in heterogeneous patterns. Thus, we sought to better define the expression patterns for BDNF, TrkB, and p75NTR within the RMS region, in particular with respect to GAD65-GFP neuroblasts. Our results show that BDNF, TrkB and p75NTR are all expressed in the RMS region with the highest levels found in the anterior olfactory nucleus which surrounds the RMS and forms a ring-like pattern (Figure 4A-C, G-I, M-O). Interestingly, our data indicate that within the RMS, BDNF and TrkB both show some regional specificity with slightly more elevated expression levels in the ventrolateral portion of the RMS (Figure 4B, H). High magnification views further reveal that both BDNF and TrkB expression partially co-localize with GAD65-GFP neuroblasts (Figure 4D-F, J-L), whereas p75NTR was undetectable in GAD65-GFP cells (Figure 4P-R). These results indicate that GAD65-GFP cells are heterogeneous with respect to BDNF and TrkB expression, but do not express p75NTR.
Figure 4. Differential expression of BDNF, TrkB and p75NTR within the RMS region.
Coronal sections through the RMS of a P7 mouse showing GAD65-GFP (green) and antibody staining (red) against BDNF (A-F), TrkB (G-L), and p75NTR (M-R). Low power images show expression within the RMS is non-uniform for all three proteins with more elevated staining for BDNF (A-C), and TrkB (G-I), compared to p75NTR (M-O). High power images of boxed region in C and I indicating the presence of GFP+ neuroblasts that co-localize (arrows) with BDNF (D-F), and TrkB (J-L), as well as those that do not co-localize (arrowheads). (P-R) High power image of boxed region in O, showing that p75NTR is poorly expressed by RMS cells (arrowhead) and does not co-localize with GFP. Arrows in A indicate medial (m) and ventral (v) orientation. Scale bars: (in A) A-C, G-I, M-O, 100μm; (in D) D-F, J-L, P-R, 10μm.
In situ hybridization experiments in adult mice have shown that mRNA for BDNF, TrkB and p75NTR is present in the RMS (Supplemental Figure 3). However, it is unclear if this expression is also present in early postnatal mice. Therefore, to determine if the source of BDNF protein that we detect originates within the RMS we performed in situ hybridization experiments in P7 mice. The data revealed that BDNF mRNA is indeed present within the RMS and in the surrounding tissue (Figure 5) as well as in the OB. Together, these data illustrate that BDNF protein and mRNA are present within the RMS region and that multiple sources of BDNF, including the OB and the RMS itself, likely contribute to its heterogeneous pattern in the RMS.
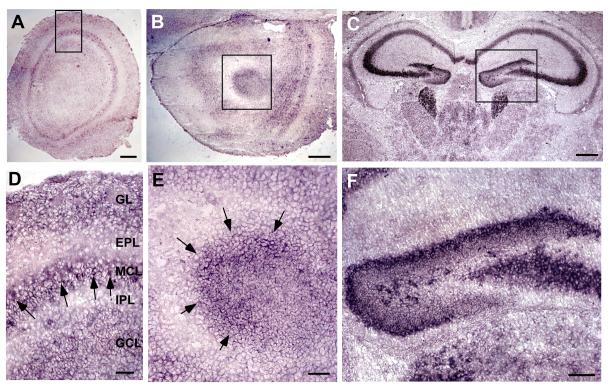
Figure 5. BDNF mRNA expression in the Olfactory Bulb and RMS.
In situ hybridization experiments of P7 mouse brain showing BDNF expression in the olfactory bulb (A), anterior olfactory nucleus (AON) region (B), and hippocampal area (C). (D) Close-up of boxed area in A, shows BDNF mRNA expression is highest in the mitral cell layer of the OB (Arrows). (E) Close-up of boxed area in B, showing BDNF mRNA expressed in a semicircular pattern in the RMS region. (F) Close-up of boxed area in C, showing high levels of BDNF produced in the dentate gyrus region of the hippocampus. Scale bars: A, B, 200μm; C, 400μm; D, E, 50μm; F, 100μm.
Elevated Levels of BDNF Increase Neuroblast Motility
The finding that BDNF and TrkB co-localize with GAD65-GFP neuroblasts, combined with the continued migration of these cells in the absence of the OB, raised the possibility that local gradients of BDNF may be involved in regulating RMS flow. One study has also shown that the RMS vasculature and glia are another potential local source of BDNF (Snapyan et al. 2009). Thus, to explore this possibility we applied a high concentration of BDNF (100 ng/ml) to our slice preparation in order to homogenize any pre-existing BDNF gradients. We then used time-lapse imaging to measure the effects of this perturbation on four aspects of neuroblast motility. Our results reveal that under these conditions, the direction of migration was only slightly affected (+BDNF, 77.86 % of total cell migrating anterior, n=569 cells from 4 experiments; Control, 75.20% of total cells migrating anterior, n=629 cells from 4 experiments; p=0.413) (Figure 6A, B). However, motility was significantly increased compared to controls, both in average displacement (+BDNF, 29.55 ± 0.49 μm, S.E., n=569 cells from 4 experiments; controls, 25.04 ± 0.36 μm, S.E., n=629 cells from 4 experiments, p<0.0001), and velocity (+BDNF, 1.52 ± 0.02 μm/min, S.E., n=569 cells from 4 experiments; control, 1.14 ± 0.015 μm/min, S.E. n=629 cells from 4 experiments; p<0.0001), but not in track length (+BDNF, 65.86 ± 1.55 μm, S.E., n=569 cells from 4 experiments; control, 63.35 ± 1.22 μm, S.E., n=629 cells from 4 experiments, p=0.189) (Figure 6C). These data indicate that elevating BDNF levels increases neuroblast motility without affecting the direction of migration. They are therefore consistent with the results of the earlier experiment in which OB removal likely decreased total BDNF levels, resulting in decreased RMS mobility without changing direction of migration.
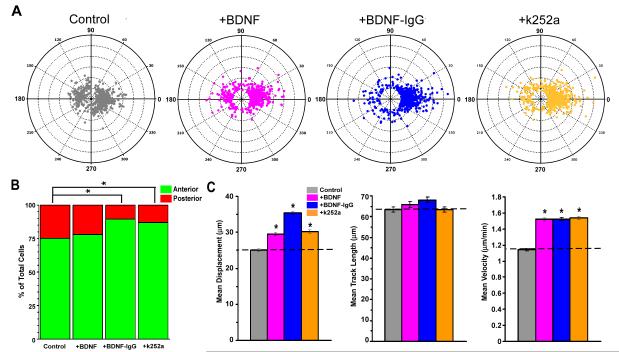
Figure 6. Changes in BDNF signaling alter RMS migration.
(A) Polar plots showing the final position of each tracked cell with respect to its initial starting point plotted from a common origin (center) comparing control (gray), with the addition of BDNF (pink), BDNF-IgG (blue), , and k252a (orange) illustrating the directional distribution of the migrating cell populations. (B) Bar graph showing the percent of total cells from four experiments in each group migrating in an anterior (green) and posterior (red) direction under three experimental conditions revealing a significant difference in the direction of migrating cells following +BDNF-IgG (89.71% of total cells migrate anterior, 10.29% migrate posterior; n=651 cells from 4 experiments; p<0.0001), and +k252a (86.85% of total cells migrate anterior, 13.15% migrate posterior; n=707 cells from 4 experiments; p<0.0001); but not +BDNF (77.86% of total cells migrate anterior; 22.14% migrate posterior; n=569 cells from 4 experiments; p=0.413), when compared to control (75.20% of total cells migrate anterior; 24.80% migrate posterior; n=629 cells from 4 experiments) using the Mann-Whitney-Wilcoxon rank sum test. (C) Bar graphs showing motility measures for each experimental condition compared to control (dotted line in each graph). Left graph, shows significant increases in mean displacement for +BDNF (29.55 ± 0.49 μm, n=569 cells from 4 experiments), +BDNF-IgG (35.35 ± 0.5 μm, n=651 cells from 4 experiments) and +k252a (30.25 ± 0.47 μm, n=707 cells from 4 experiments) compared to control (25.04 ± 0.36 μm, n=629 cells from 4 experiments). Middle graph, shows no significant change in mean track length between experimental groups and control (+BDNF, 65.86 ± 1.55 μm, n=569 cells from 4 experiments, p=0.189; +BDNF-IgG, 68.02 ± 1.23 μm, n=651 cells from 4 experiments, p=0.011; +k252a, 63.3 ± 1.25 μm, n=707 cells from 4 experiments, p=0.977; control, 63.35 ± 1.22 μm, n=629 cells from 4 experiments) Right graph, shows significant increases in velocity for each experimental group compared to control (+BDNF, 1.52 ± 0.02 μm/min, n=569 cells from 4 experiments; +BDNF-IgG, 1.52 ± 0.02 μm/min, n=651 cells from 4 experiments; +k252a, 1.54 ± 0.02 μm/min, n=707 cells from 4 experiments; control, 1.14 ± 0.015 μm/min, n=629 cells from 4 experiments). All error ± S.E. Significance for motility measures determined using ANOVA followed by Bonferroni/Dunn post-hoc test, *p<0.0001.
Inhibition of BDNF activity alters both motility and direction of migration
We sought to confirm the role of BDNF in regulating motility by directly decreasing its activity within the slice. Thus, we applied anti-BDNF-IgG (10 μg/ml) to the bath both prior to and during the imaging process. Surprisingly, in addition to an increase in motility, this perturbation produced a striking increase in the proportion of GAD65-GFP neuroblasts migrating toward the OB (89.71 % of total cells, n=651 cells from 4 experiments; p<0.001) compared to controls (75.20% of total cells, n=629 cells from 4 experiments; Figure 6A, B). This directional change can clearly be seen in the distribution of track directions recorded in polar plots (Figure 6A). Each of the three separate measures of motility showed a marked increase as well: displacement increased 41.16 % ± 2.02 S.E. over control (+BDNF-IgG, 35.35 ± 0.5 μm, S.E.; n=651 cells from 4 experiments; control, 25.04 ± 0.36 μm, S.E.; n=629 cells from 4 experiments, p<0.0001); track length increased 7.37 % ± 1.95 S.E, (+BDNF-IgG, 68.02 ± 1.23 μm, S.E., n=651 cells from 4 experiments; control, 63.35 ± 1.22 μm, S.E., n=629 cells from 4 experiments; p=0.011); and velocity increased 33.74 % ± 1.60 S.E., (+BDNF-IgG, 1.52 ± 0.02 μm/min, S.E.; n=651 cells from 4 experiments; control, 1.14 ± 0.015 μm/min, S.E. n=629 cells from 4 experiments, p<0.0001)(Figure 6C). These data reveal the presence of other factors that influence RMS migration, such that in the absence of BDNF signaling, anterior migration is enhanced.
BDNF affects migration through the TrkB receptor
To further understand the signaling mechanism by which BDNF modulates RMS migration, we sought to determine which of the two BDNF receptors (TrkB or p75NTR) mediates its effects. While studies have shown that p75NTR is expressed in the RMS region (Snapyan et al., 2009), our immunostaining of the GAD65-GFP slices revealed that TrkB, but not p75NTR, co-localizes with the GAD65-GFP neuroblasts (Figure 4). We therefore tested the role of TrkB by bath-applying the specific inhibitor, k252a (2 μM), to RMS slices, and then performing time-lapse imaging. The results were strikingly similar to those in which BDNF activity was decreased with BDNF-IgG, in that both direction and motility were affected (Figure 6). Inhibition of TrkB receptor signaling produced an increase in the proportion of neuroblasts migrating toward the OB (86.85% of total cells, n=707 cells from 4 experiments; p<0.05, Figure 6A, B) compared to controls (75.20% of total cells migrating anterior, n=629 cells from 4 experiments). With regard to motility, application of k252a caused a 20.81% ± 1.88, S.E increase in displacement (+k252a, 30.25 ± 0..47 μm, S.E.; n=707 cells from 4 experiments; controls, 25.04 ± 0.36 μm, S.E.; n=629 cells from 4 experiments, p<0.0001), and a 35.09 % ± 1.49 S.E. increase in velocity (+k252a, 1.54 ± 0.02 μm/min, S.E.; n=707 cells from 4 experiments; control; 1.14 ± 0.015 μm/min, S.E. n=629 cells from 4 experiments; p<0.0001), compared to controls. However, there was no significant change in track length (+k252a, 63.3 ± 1.25 μm, S.E., n=707 cells from 4 experiments; control, 63.35 ± 1.22 μm, S.E., n=629 cells from 4 experiments; p=0.977; Figure 6C). Thus, similar to the BDNF-IgG result, the k252a data suggest that neuroblasts are likely taking a more direct path with less meandering, Together, these data indicate that BDNF is modulating neuroblast migration within the RMS primarily through TrkB receptor signaling.
Discussion
RMS migration is a multidirectional dynamic process involving many factors. We have shown that bidirectional migration occurs at a broad level within the RMS both in vitro and in vivo during the early postnatal period. Using an acute slice preparation in P6-P8 mice, we performed dynamic imaging to explore the complex role that BDNF plays in regulating neuroblast migration. We showed that changes in BDNF signaling can affect both RMS motility and migrational direction. However, migrational direction does not appear to be controlled by factors secreted from the olfactory bulb, as its removal affects only motility. It is therefore likely that the direction of migration is regulated by factors local to the RMS, one of which is BDNF. Since blocking BDNF signals caused an increase in anterior migration we postulate the presence of other factors that also promote anterior migration and that the integration of all these signals regulates bidirectional RMS flow.
Obviously, one concern associated with most in vitro studies is their relevance to the in vivo condition. In general, in vitro preparations are used to model in vivo events in a manner that usually allows easier access and better control of experimental variables to produce more precise measurements. These advantages are the reason that we and others have taken an in vitro approach to study RMS migration. At a practical level, the location of the RMS deep in the brain also presents a significant obstacle to acquiring high temporal and spatial resolution images of neuroblast migration in intact animals. Therefore, while bidirectional migration is inferred from static images of in vivo tracer injections the precise short term dynamics of the process are difficult to measure with a purely in vivo approach. Nevertheless, as ours is an in vitro system, RMS neurons may migrate with different motility values in vivo and despite the fact that both in vivo and in vitro data show evidence of bidirectional migration, the proportions of anterior and posterior migrating cells may differ. Indeed, one study uses an in vivo viral labeling approach to report that 19% of labeled RMS neurons are oriented in a posterior direction following immunostaining (Sawamoto et al., 2006), while we observe 25% of GAD65-GFP labeled neuroblasts actually migrating posteriorly. While there are many differences between these studies, including which cell types are labeled and the methods used,to label them, it is possible that part of the discrepancy is due to differences in the in vivo and in vitro environment experienced by the RMS neurons in the two studies. Our results also indicate a possible regulatory role of BDNF in bidirectional migration, which provides further evidence that the flow of the RMS is a highly regulated process. These data support the idea that the specific dynamics of RMS migration are accomplished through the interplay of multiple signaling mechanisms, and that BDNF contributes to the regulation of both motility and direction. Further in vivo characterization of this mechanism will be very informative.
Bidirectional movement is a significant component of RMS migration
While the presence of posteriorly migrating RMS cells has been reported in adult mice (Zhao and Nam, 2007), the degree to which bidirectional migration occurs during early postnatal period has not been fully appreciated. One in vivo study used viral labeling to visualize migrating neuroblasts in the SVZ, and alluded to the presence of posteriorly migrating RMS neuroblasts based upon the direction of their leading processes observed in fixed sections (Sawamoto et al., 2006). However, this study was performed in adult mice, and did not capture the actual movement of neuroblasts in vivo. In the present study we demonstrated through time-lapse imaging that, during a three hour period, ~ 30% of migrating RMS neuroblasts travel away from their OB target. Interestingly, this percentage is similar to the percentages reported in the Sawamoto study, suggesting that the migration of GAD65-GFP labeled neuroblasts in our study adequately represents the RMS population as a whole. This finding raises several questions, including: what role does bidirectional migration play in the RMS, and what is the fate of neuroblasts migrating in a posterior direction? One possibility is that not all RMS neuroblasts migrate to the OB, but that a portion is diverted to surrounding brain regions. Although in early postnatal mice cells have been shown to branch off the ventrocaudal portion of the RMS and migrate toward the basal forebrain (De Marchis et al., 2004a), they are relatively few in number compared to the total population within the RMS, and therefore unlikely to account for such a large subset of neuroblasts moving away from the OB. A second possibility is that neuroblasts moving in a posterior direction fail in their migration and are lost through cell death. While there is evidence for cell death occurring in the developing RMS (Brunjes and Armstrong, 1996), it is also infrequent compared to the large numbers of posteriorly migrating RMS neuroblasts. A third possibility is that RMS neuroblasts do not migrate in a single direction, but rather alternate between anterior and posterior directions, perhaps changing directions during the pauses that are characteristic of the saltatory movement observed in virtually all of the cells that we tracked (Figure 2). This is consistent with the timing and pattern of directional change seen in other studies, in which cells traveled in one direction for several hours and then reversed course (Tanaka et al., 2009). Since we have also identified neuroblasts that appeared to switch directions (Supplemental Movies), we propose that for most neuroblasts, posterior migration is relatively short-lived and thus only a temporary diversion from their eventual migration to the OB.
What is the role of the OB in RMS migration?
Experiments in early postnatal animals using in vitro explant assays demonstrated that the OB is an important source of chemoattractants for SVZ and RMS neuroblasts (Liu and Rao, 2003, Chiaramello et al., 2007). By contrast, other studies using BrdU showed that in adult mice SVZ neuroblasts continue to proliferate and migrate even after their olfactory bulbs were excised (Jankovski et al., 1998, Kirschenbaum et al., 1999). While these studies were carried out at different ages yielding scenarios that are not necessarily mutually exclusive, they do illustrate that the role of the OB in RMS migration remains unclear. Interestingly, our data lend support to both scenarios, since removal of the OB did not affect the direction of neuroblast migration within the RMS, but did alter motility. By analyzing the dynamic aspects of RMS neuroblast migration, we detected a decrease in cell displacement, track length, and velocity that had not been previously reported. This suggests that in early postnatal development, RMS migration may continue in absence of the OB, but that substances secreted by the OB still affect migration, although through a more complex mechanism than simple point-source chemoattraction.
BDNF affects RMS neuroblast motility
Among the many factors associated with RMS migration is BDNF, which is present in the OB (Bath et al., 2008; Maisonpierre et al., 1990; Figure 4 and Supplemental Figures 1 and 2). Since removal of the OB produced a decrease in RMS motility, it is likely that this is, in part, due to a reduction in BDNF levels derived from the OB. If so, one might predict that an increase in BDNF levels would have the opposite effect. Indeed, we showed through our imaging assay that when BDNF levels were elevated, RMS neuroblast motility increased by every measure. Previous in vitro studies using cultured explant assays from early postnatal mice have presented similar data, suggesting that BDNF functions as a chemoattractant for RMS neuroblasts (Chiaramello et al., 2007). However given the nature of explant experiments, in which pieces of the RMS are removed from surrounding tissue and placed adjacent to a BDNF source, it is difficult to clearly distinguish whether BDNF was acting as a guidance factor or as a motility factor. Since our preparation preserves the integrity of the RMS within the context of its surrounding tissue, RMS neuroblasts maintain their orientation and thus direction and motility can be assessed independently. Although it is important to note that there still may be differences from the in vivo state. Nevertheless, our data demonstrate a clear role for BDNF in RMS neuroblast motility using our slice preparation such that average displacement, track length, and velocity all increase in response to elevated levels of BDNF. Interestingly, we find that the direction of RMS migration is not affected when BDNF levels are raised, suggesting that during the early postnatal period BDNF functions primarily as a motility factor for RMS migration.
BDNF controls RMS migration in concert with other regulatory factors
RMS migration involves many factors, from several potential sources including the local vasculature, glia, the OB, as well as neuroblasts themselves. This suggests that the direction and pattern of motility for an individual RMS neuroblast is determined through a complex process of integration. The resulting cellular dynamics of a given neuroblast would therefore be the net product of many attractive and repulsive cues, in conjunction with factors that modulate motility. If so, then removal of any one factor or any one source would simply produce a new equilibrium causing neuroblasts to adjust their migration based upon the remaining factors and sources. Previous in vitro studies have shown that TrkB signaling can mediate chemotaxis of RMS neuroblasts in early postnatal mice (Chiaramello et al., 2007, Bath et al., 2008) as well as neuronal precursors in other brain regions (Zhou et al., 2007). In the present study we have sought to broadly disrupt BDNF signaling from any source: first, by bath applying anti-BDNF-IgG, to uniformly decrease the levels of BDNF throughout the RMS; and second, by introducing k252a, an inhibitor of TrkB signaling. Strikingly, both manipulations revealed identical changes in motility and migrational direction of RMS neuroblasts, demonstrating that BDNF exerts it effects on GAD65-GFP neuroblasts primarily through TrkB signaling. It is possible that p75NTR signaling is also affected by our experimental manipulations, although it would likely play an indirect role in our assay since we do not see expression of p75NTR in GAD65-GFP neuroblasts (Figure 4). Interestingly, disruption of BDNF/TrkB signaling produces an increase in RMS motility, and more neurons migrating in an anterior direction. One explanation of this finding is that, by blocking BDNF signaling, we have revealed the migratory effects of the many other factors within the RMS that have been shown to influence neuroblast migration. Thus, the changes in motility and direction that we observe upon application of either BDNF IgG or k252a are likely the net result of other RMS regulatory compounds exerting their effects in the absence of competing BDNF signals. Another important consideration is that BDNF is a pleiotopic signaling molecule, and has the potential to affect such broad developmental processes as cell survival, proliferation, and differentiation (Ernfors et al., 1994, Jones et al., 1994, Lindsay, 1996), which in turn may also influence RMS migration.
Regulating migration in a diverse RMS population
The population of migrating GAD65-GFP neuroblasts within the RMS is heterogeneous (Merkle et al., 2007, Nam et al., 2007, Young et al., 2007, Lledo et al., 2008). Therefore, the response of these neuroblasts to available chemoattractants or signaling molecules is also likely to be heterogeneous. We have shown that BDNF levels within the RMS are probably derived from multiple sources including the OB and the RMS region, with neither TrkB nor BDNF expressed uniformly among all RMS neuroblasts (Figure 3). As a result, it is likely that the changes in population dynamics that we observed in response to changes in BDNF signaling are derived from alteration of only a subset of RMS neurons. One recent study also showed that p75NTR signaling plays a role in the migration of GAD67 positive RMS neuroblasts in adult mice (Snapyan et al., 2009). While our study did not follow these same neuroblasts and used pups instead of adult mice, it is possible that indirect effects from other p75NTR positive GAD67 neuroblasts may have influenced GAD65-GFP neuroblast migration as well. A second possibility is that these two neuroblast populations function separately with TrkB-mediated BDNF signaling regulating the migration of GAD65 positive neuroblasts, and p75NTR signaling regulating GAD67 positive neuroblasts with only a small overlapping portion of the two populations influenced by both signaling pathways. If so, then BDNF could regulate the migration of each subset independently based simply upon which receptor is expressed. Thus, in conclusion, we have shown that BDNF clearly plays an important role in regulating both motility and direction of RMS migration, although future work will be necessary to elucidate how other factors work in concert with BDNF to precisely control the process which determines the distribution of cell types populating the OB.
Supplementary Material
Supplemental Figure 1. BDNF Protein in the Immature Mouse Olfactory Bulb. (A) Coronal section from a P7 mouse olfactory bulb stained with anti-BDNF antibody reveals broad protein expression with highest levels in external plexiform layer (EPL), and internal plexiform layer (IPL) as well as scattered expression in the granule cell layer (GCL). (B and C) Close-up of boxed region in A shows differential BDNF expression within the layers of the olfactory bulb. (D) Close-up of upper boxed region in C shows BDNF protein scattered within the glomerular region. (D) Close-up of lower boxed region in C shows some BDNF protein in the granule cell layer. Scale bar: 200μm.
Supplemental Figure 2. BDNF protein expression in the adult mouse olfactory bulb. (A) Coronal section of the mouse olfactory bulbs stained with anti-BDNF antibody showing a broad distribution of BDNF protein with highest levels in the outer nerve layer. (B) Same section as in A without DAPI stain shows differential levels of BDNF protein within the leaders of the olfactory bulb. (C) Close-up of boxed region in B showing higher levels of BDNF expression in the external plexiform layer (EPL) with clear expression of BDNF protein by mitral cells (arrowheads). (D) Close-up of boxed region and C shows discrete puncta of BDNF protein scattered within the superficial granule cell layer (GCL) immediately adjacent to the internal plexiform layer (IPL). Scale bar: 200 μm.
Supplemental Figure 3. Expression of BDNF, p75NTR and TrkB mRNA within the RMS region of the adult mouse brain. Coronal section images downloaded from the Allen Brain Atlas (http://www.brain-map.org/) corresponding to the central portion of the RMS region (specific section designation listed on each panel). In situ hybridization images show broad distribution of mRNA signal for: (A) BDNF, (B) p75NTR and (C) TrkB. (D -E) Close-up of boxed regions in A-C respectively, show the presence of scattered cells throughout the RMS region which express different levels of message for BDNF (D), p75NTR (E) and TrkB (F). Dotted circles within each panel depict the RMS showing differential positive signals for all three genes, with F clearly showing a robust TrkB signal within discrete portions of the RMS.
Supplemental Movie 1. Anterior Migrating Neuroblast
Supplemental Movie 2. Posterior Migrating Neuroblast
Supplemental Movie 3. Posterior Migrating Neuroblast Changing Direction
Acknowledgments
The authors wish to thank Stefano Vicini for the Gad65-GFP mice and Kai Cheng for help with in-situ hybridizations. We would also like to thank Beth Belluscio, Ron McKay and the members of the Belluscio lab for helpful discussions and critical review of the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders, and Stroke.
Abbreviations
- OB
olfactory bulb
- SVZ
subventricular zone
- RMS
rostral migratory stream
- BDNF
brain derived neurotrophic factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S, Bovolin P, Perroteau I, Puche AC. Subventricular zone-derived neuroblast migration to the olfactory bulb is modulated by matrix remodelling. Eur J Neurosci. 2007;25:2021–2033. doi: 10.1111/j.1460-9568.2007.05441.x. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Armstrong AM. Apoptosis in the rostral migratory stream of the developing rat. Brain Res Dev Brain Res. 1996;92:219–222. doi: 10.1016/0165-3806(96)00006-5. [DOI] [PubMed] [Google Scholar]
- Chazal G, Durbec P, Jankovski A, Rougon G, Cremer H. Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse. J Neurosci. 2000;20:1446–1457. doi: 10.1523/JNEUROSCI.20-04-01446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/ TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Fasolo A, Puche AC. Subventricular zone-derived neuronal progenitors migrate into the subcortical forebrain of postnatal mice. J Comp Neurol. 2004a;476:290–300. doi: 10.1002/cne.20217. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Fasolo A, Shipley M, Puche A. Unique neuronal tracers show migration and differentiation of SVZ progenitors in organotypic slices. J Neurobiol. 2001;49:326–338. doi: 10.1002/neu.10012. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, Szabo G, Puche AC. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur J Neurosci. 2004b;20:1307–1317. doi: 10.1111/j.1460-9568.2004.03584.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzotto D, Giacobini P, Crepaldi T, Fasolo A, De Marchis S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. J Neurosci. 2008;28:5901–5909. doi: 10.1523/JNEUROSCI.1083-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Goto S, Nishikawa S, Ushio Y. A role of netrin-1 in the formation of the subcortical structure striatum: repulsive action on the migration of late-born striatal neurons. J Neurosci. 2001;21:4272–4280. doi: 10.1523/JNEUROSCI.21-12-04272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Kawasaki T, Takashima S, Matsuda I, Aiba A, Hirata T. Semaphorin 3F confines ventral tangential migration of lateral olfactory tract neurons onto the telencephalon surface. J Neurosci. 2008;28:4414–4422. doi: 10.1523/JNEUROSCI.0372-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, Garcia C, Soriano E, Sotelo C. Proliferation, migration and differentiation of neuronal progenitor cells in the adult mouse subventricular zone surgically separated from its olfactory bulb. Eur J Neurosci. 1998;10:3853–3868. doi: 10.1046/j.1460-9568.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci. 1999;19:2171–2180. doi: 10.1523/JNEUROSCI.19-06-02171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesi PD. MATLAB and Octave Functions for Computer Vision and Image Processing. School of Computer Science & Software Engineering, The University of Western Australia; 2000. [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- Liu G, Rao Y. Neuronal migration from the forebrain to the olfactory bulb requires a new attractant persistent in the olfactory bulb. J Neurosci. 2003;23:6651–6659. doi: 10.1523/JNEUROSCI.23-16-06651.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008 doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sturgess K, Erdelyi F, Szabo G, Molnar Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14:1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Marks CA, Cheng K, Cummings DM, Belluscio L. Activity-dependent plasticity in the olfactory intrabulbar map. J Neurosci. 2006;26:11257–11266. doi: 10.1523/JNEUROSCI.2805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Herrera E, Colin-Castelan D, Varela-Echavarria A, Gutierrez-Ospina G. Semaphorin-3A and its receptor neuropilin-1 are predominantly expressed in endothelial cells along the rostral migratory stream of young and adult mice. Cell Tissue Res. 2008 doi: 10.1007/s00441-008-0643-3. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SC, Kim Y, Dryanovski D, Walker A, Goings G, Woolfrey K, Kang SS, Chu C, Chenn A, Erdelyi F, Szabo G, Hockberger P, Szele FG. Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. J Comp Neurol. 2007;505:190–208. doi: 10.1002/cne.21473. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24:1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratcha G, Ibanez CF, Ledda F. GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Mol Cell Neurosci. 2006;31:505–514. doi: 10.1016/j.mcn.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka DH, Yanagida M, Zhu Y, Mikami S, Nagasawa T, Miyazaki J, Yanagawa Y, Obata K, Murakami F. Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex. J Neurosci. 2009;29:1300–1311. doi: 10.1523/JNEUROSCI.5446-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993;11:1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol. 2009;516:94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LR, Nam SC. Multiphoton microscope imaging: the behavior of neural progenitor cells in the rostral migratory stream. Neurosci Lett. 2007;425:83–88. doi: 10.1016/j.neulet.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55:53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. BDNF Protein in the Immature Mouse Olfactory Bulb. (A) Coronal section from a P7 mouse olfactory bulb stained with anti-BDNF antibody reveals broad protein expression with highest levels in external plexiform layer (EPL), and internal plexiform layer (IPL) as well as scattered expression in the granule cell layer (GCL). (B and C) Close-up of boxed region in A shows differential BDNF expression within the layers of the olfactory bulb. (D) Close-up of upper boxed region in C shows BDNF protein scattered within the glomerular region. (D) Close-up of lower boxed region in C shows some BDNF protein in the granule cell layer. Scale bar: 200μm.
Supplemental Figure 2. BDNF protein expression in the adult mouse olfactory bulb. (A) Coronal section of the mouse olfactory bulbs stained with anti-BDNF antibody showing a broad distribution of BDNF protein with highest levels in the outer nerve layer. (B) Same section as in A without DAPI stain shows differential levels of BDNF protein within the leaders of the olfactory bulb. (C) Close-up of boxed region in B showing higher levels of BDNF expression in the external plexiform layer (EPL) with clear expression of BDNF protein by mitral cells (arrowheads). (D) Close-up of boxed region and C shows discrete puncta of BDNF protein scattered within the superficial granule cell layer (GCL) immediately adjacent to the internal plexiform layer (IPL). Scale bar: 200 μm.
Supplemental Figure 3. Expression of BDNF, p75NTR and TrkB mRNA within the RMS region of the adult mouse brain. Coronal section images downloaded from the Allen Brain Atlas (http://www.brain-map.org/) corresponding to the central portion of the RMS region (specific section designation listed on each panel). In situ hybridization images show broad distribution of mRNA signal for: (A) BDNF, (B) p75NTR and (C) TrkB. (D -E) Close-up of boxed regions in A-C respectively, show the presence of scattered cells throughout the RMS region which express different levels of message for BDNF (D), p75NTR (E) and TrkB (F). Dotted circles within each panel depict the RMS showing differential positive signals for all three genes, with F clearly showing a robust TrkB signal within discrete portions of the RMS.
Supplemental Movie 1. Anterior Migrating Neuroblast
Supplemental Movie 2. Posterior Migrating Neuroblast
Supplemental Movie 3. Posterior Migrating Neuroblast Changing Direction